Introduction
Tumor necrosis factor (TNF)-α is a cytokine with
numerous functions that serves an important role in cell survival,
apoptosis, inflammation and immunity (1). TNF-α has been demonstrated to possess
anti-tumor activity as it has cytostatic and cytotoxic effects on a
number of cancer cell lines (2).
However, previous studies have demonstrated that TNF-α may also
affect matrix degradation and mediate the development of tumor
metastases (3,4). Additionally, TNF-α regulates the
expression levels of cell adhesion proteins and therefore may serve
a role in determining the metastatic phenotype of tumor cells
(5,6).
Interleukin (IL)-17 is a proinflammatory cytokine
that is secreted by T helper 17 cells and it has been identified to
have an important role in the host defense through its involvement
in inflammatory and autoimmune diseases, including inflammatory
bowel disease (7), multiple sclerosis
(8) and rheumatoid arthritis
(9). Notably, IL-17 has a complex
role in tumor initiation, development and metastasis (10–12).
A number of previous studies have demonstrated that
the combination of IL-17 and TNF-α promote the upregulation of gene
expression (13,14), whereas treatment with only one of the
two cytokines does not affect gene expression. Other previous
studies have indicated that IL-17 augments the expression of
TNF-α-induced genes, including granulocyte-colony stimulating
factor (G-CSF), granulocyte macrophage-colony stimulating factor
(GM-CSF) (15), keratinocyte-derived
chemokine (KC), macrophage inflammatory protein (MIP)-2,
prostaglandin E2 (PGE2) and vascular endothelial growth factor
(VEGF) (16). A previous study has
suggested the potential hypothesis that IL-17 may promote the
stability of TNF-α-induced mRNA (17). However, previous studies investigating
the combination of IL-17 and TNF-α have focused on inflammatory
angiogenesis (16), investigating the
role of these cytokines in inflammation (14,15) and
autoimmune diseases, including rheumatoid arthritis (13,18) and
psoriasis (19). Therefore, the
current study focused on the combination of IL-17 and TNF-α and its
effect on tumor cells.
Vasodilator-stimulated phosphoprotein (VASP) is an
Ena/VASP protein family member that has been associated with the
microfilament system via promoting actin polymerization in a number
of cell types (20,21). VASP has also been associated with the
regulation of adherens junctions in epithelial cells (22). Clinical studies have revealed that
VASP may be involved in the invasive biological behavior of lung
adenocarcinomas, potentially through the regulation of focal
adhesions, intracellular actin filament formation and cell
migration (23). In previous studies,
TNF-α was established to inhibit VASP expression via the
TNF-α/hypoxia-inducible factor (HIF)-1α/VASP signaling pathway and
modulate the adhesive and proliferative ability of breast cancer
cells (24,25).
In the present study, the MDA-MB-231 breast cancer
cell line was used to investigate the effects of co-stimulation
with IL-17 and TNF-α on the levels of HIF-1α and VASP expression,
as well as the cell adhesive ability.
Materials and methods
Cell culture, transfection and
treatment
The MDA-MB-231 human breast cancer cell line was
obtained from the Department of Pathology and Pathophysiology,
School of Medicine, Wuhan University (Wuhan, China). MDA-MB-231
cells were cultured in RPMI-1640 medium (HyClone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine
serum (HyClone; GE Healthcare Life Sciences) and 1% penicillin and
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C in a humidified atmosphere containing 5%
CO2. The human HIF-1α CDS fragment (NM_001530.3) was
amplified using polymerase chain reaction (PCR) from the
pCGN-HAM-HIF-1α plasmid (24) using
the following primers: Forward,
5′-CCGGAATTCCATGGAGGGCGCCGGCGGCGCGAACG-3′ and reverse,
5′-CGCGGATCCGTTAACTTGATCCAAAGCTCTGAGT-3′. The PCR reaction system
consisted of 100 ng of pCGN-HAM-HIF-1α plasmid DNA, 2 µl of forward
primer (10 µmol/l), 2 µl of reverse primer (10 µmol/l), 25 µl of 2
GC buffer I (Takara Biotechnology Co., Ltd., Dalian, China) and 2
µl of Ex Taq DNA polymerase (cat. no. RRX001A; Takara Biotechnology
Co., Ltd.). Thermocycling conditions were as follows: An initial 5
min incubation at 95°C; followed by 30 cycles of 15 sec at 95°C, 20
sec at 57°C and 150 sec at 72°C; and lastly 10 min at 72°. The PCR
product was identified via agarose gel electrophoresis on a 1% gel.
The fragment was inserted in the pEGFP-C1 (Clontech Laboratories,
Inc., Mountainview, CA, USA) vector between the EcoRI and
BamHI restriction sites. All primers were purchased from
Sangon Biotech Co., Ltd. (Shanghai, China). The DNA sequence of all
constructs was verified by sequencing (Sangon Biotech Co., Ltd.).
Transfection of the empty pEGFP-C1 vector was used as the control.
Transient transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
cells were cultured in serum-free RPMI-1640 for >8 h prior to
treatment with IL-17 (Cell Signaling Technology, Inc., Danvers, MA,
USA) and TNF-α (Invitrogen; Thermo Fisher Scientific, Inc.), or
control medium (equal volumes of normal saline solution to IL-17
and/or TNF-α).
Reverse transcription-quantitative PCR
(RT-qPCR)
MDA-MB-231 cells were incubated for 6 h with 100
ng/ml IL-17, and various concentrations of TNF-α (0.1, 1 and 10
ng/ml) or combination of doses of IL-17 (1, 10 and 100 ng/ml) and
TNF-α (0.1, 1 and 10 ng/ml). Subsequently, the cells were harvested
for RT-qPCR analysis of HIF-1α mRNA. To further investigate the
time course of HIF-1α induction following IL-17 and TNF-α
stimulation, the transcript levels of HIF-α were measured during a
12 h period. The quantity of HIF-1α mRNA in MDA-MB-231 cells was
determined at several time points (3, 6, 9 and 12 h) following
treatment with 100 ng/ml IL-17 or 1 ng/ml TNF-α alone or in
combination. Total RNA was extracted according to the
manufacturer's protocol. In order to lyse the cells, 1 ml/well
TRIzol® (Ambion; Thermo Fisher Scientific, Inc.) was
added to the 6-well plate, which was then incubated and agitated
for 5 min at room temperature. A total of 4 µg RNA was then
reverse-transcribed to synthesize first-strand cDNA (final volume
20 µl) using the RevertAid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.). RT-qPCR was performed in the presence of
SYBR green using a Bio-Rad IQ5 Real-Time PCR system (Bio-Rad
Laboratories Inc., Hercules, CA, USA). The primers for human HIF-1α
were as follows: Sense, 5′-GAAAGCGCAAGTCCTCAAAG-3′; antisense,
5′-TGGGTAGGAGATGGAGATGC-3′. The primers for β-actin were as
follows: Sense, 5′-CATTAAGGAGAAGCTGTGCT-3′; antisense,
5′-GTTGAAGGTAGTTTCGTGGA-3′. The total volume for the RT-qPCR
reaction was 20 µl. This consisted of 1 µl of a 1/5 water dilution
of cDNA, 1 µl of forward primer (10 µmol/l), 1 µl of reverse primer
(10 µmol/l), 10 µl of master mix (Bio-Rad Laboratories Inc.) and 7
µl nuclease-free water. RT-qPCR was performed using the following
thermal cycling program: An initial 2 min incubation at 95°C
followed by 40 cycles of 10 sec at 95°C, 15 sec at 52°C and 20 sec
at 72°C. Fluorescence readings were taken during the extension step
(72°C incubation). Following the cycling, melting was performed
from 72 to 95°C at 0.5°C/sec, to obtain a melting curve. PCR
reactions were run in triplicate within each experiment and the
experiments were repeated >3 times. Results were calculated
according to the 2−ΔΔCq relative quantization method
(26) using the β-actin gene for
calibration.
RNA interference
The MDA-MB-231 cells were cultured in 6-well culture
plates for 24 h at 37°C and the cells were cultured in
antibiotic-free and serum-free RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences) for >8 h at 37°C prior to TNF-α and
IL-17 treatment. The cells were transiently transfected using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturer's protocol. RNA
extraction and RT-qPCR were performed as previously described
(27). Briefly, the shRNA duplexes
targeting VASP (GenBank accession no. BC038224) had the following
sequence:
5′-TGCTGTAAAGCATCACAGTGGCCCGGGTTTTGGCCACTGACTGACCCGGGCCAGTGATGCTTTA-3′.
This sequence was inserted into the pcDNA6.2-GW/EmGFP vector
(Invitrogen; Thermo Fisher Scientific, Inc.) to produce
pcDNA6.2-GW/EmGFP-shR-VASP insert-containing vectors as has been
previously described (27). A
scrambled shRNA
(5′-GAAATGTACTGCGCGTGGAGACGTTTTGGCCACTGACTGACGTCTCCACGCAGTACATTT-3′)was
obtained from Invitrogen (Thermo Fisher Scientific, Inc.) and used
as the negative control in all experiments. The small interfering
(si) RNA duplex oligonucleotides targeting HIF-1α (GenBank
accession no. NM_001530) were synthesized by Shanghai GenePharma
Co., Ltd. (Shanghai, China). The sequence for HIF-1α-siRNA was:
5′-CUGAUGACCAGCAACUUGAdTdT-3′. A scrambled-siRNA, sequence
5′-AGUUCAACGACCAGUAGUCdTdT-3′, was utilized as a control. Following
incubation for 8–9 h at 37°C and 5% CO2, the
transfection reagent was removed. Medium with antibiotic and serum
was added to the plate and the cells were cultured for 24 h at
37°C.
Western blotting
Following treatment with 100 ng/ml IL-17, 1 ng/ml
TNF-α or the combination for 6 h, the total protein of the cells
was extracted. The MDA-MB-231 cells were washed three times with
ice-cold PBS and lysed in a modified radioimmunoprecipitation assay
buffer (Biyuntian, Shanghai, China) containing 50 mM Tris-HCl, 150
mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 2 mM sodium
fluoride, 2 mM Na3VO4, 1 mM EDTA, 1 mM EGTA
and 1x protease inhibitor cocktail. The protein concentration was
measured with the Bicinchoninic Acid Protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Equal quantities of total protein (10 µg) were separated
by 10% (v/v) SDS-PAGE and transferred to polyvinylidene fluoride
membranes (Roche Applied Science, Pleasanton, CA, USA). Membranes
were blocked for 1 h at room temperature with 5% powdered skimmed
milk in Tris-buffered saline with 0.05% Tween 20 (TBST) and probed
with VASP antibody (dilution, 1:1,000; cat. no. 3112; Cell
Signaling Technology, Inc.), HIF-1α antibody (dilution, 1:1,000;
cat. no. ab210073; Abcam, Cambridge, UK) or GAPDH antibody
(dilution, 1:5,000; cat. no. A0080, ABclonal Biotech Co., Ltd.,
Cambridge, MA, USA) at 4°C overnight and washed with TBST. This was
followed by incubation with horseradish peroxidase (HRP)-linked
secondary antibodies (dilutions 1:40,000; cat. nos. 7071 and 7072,
Cell Signaling Technology, Inc.) for 1 h at room temperature.
Washing was performed with TBST and detection occurred using
enhanced chemiluminescence (ECL) western blotting detection
reagents (Advansta, Inc., California, USA). The experiments were
repeated 3 times with similar results. The bands were visualized
using WesternBright ECL HRP substrate (Advansta, Inc.) and
developed using Kodak film (Kodak, Rochester, NY, USA) in a
darkroom. Quantification of band densities was performed using
Image J (version no., 1.6.0_20, National Institutes of Health,
Bethesda, MD, USA).
Cell adhesion assay
Following transfection, the MDA-MB-231 cells were
seeded at a density of 1×105 per ml (100 µl per well) in
96-well plates coated with fibronectin (100 mg/ml; BD Biosciences,
Franklin Lakes, NJ, USA). Following a 2 h incubation at 37°C in an
incubator containing 5% CO2, the cells were washed with
PBS to remove non-adherent cells. A total of 10 µl MTT (5 mg/ml,
Amresco, LCC, Solon, OH, USA) was added to each well. Following 4 h
of additional incubation, the supernatant was discarded and 100 µl
dimethyl sulfoxide was added to each well to dissolve the formazan
crystals and culture plates were agitated on a horizontal shaker
for 10 min. Absorbance values were determined by using an ELISA
reader (Infinite® 200 PRO; Tecan Group Ltd., Männedorf,
Switzerland) at a wavelength of 490 nm. The percentage of adhesive
cells was calculated according to the following formula: Percentage
of adhesion=[optical density (OD) 490 of cells treated/OD490 of
cells untreated]x100%. Three independent experiments were performed
in triplicate.
Statistical analysis
All data were expressed as the mean + standard
deviation. The statistical significance of the differences observed
between experimental groups was determined using the Student's
t-test. Data were analyzed using GraphPad Prism software version
6.0.2 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
IL-17 augments TNF-α-induced HIF-1 α
gene expression in MDA-MB-231 breast cancer cells
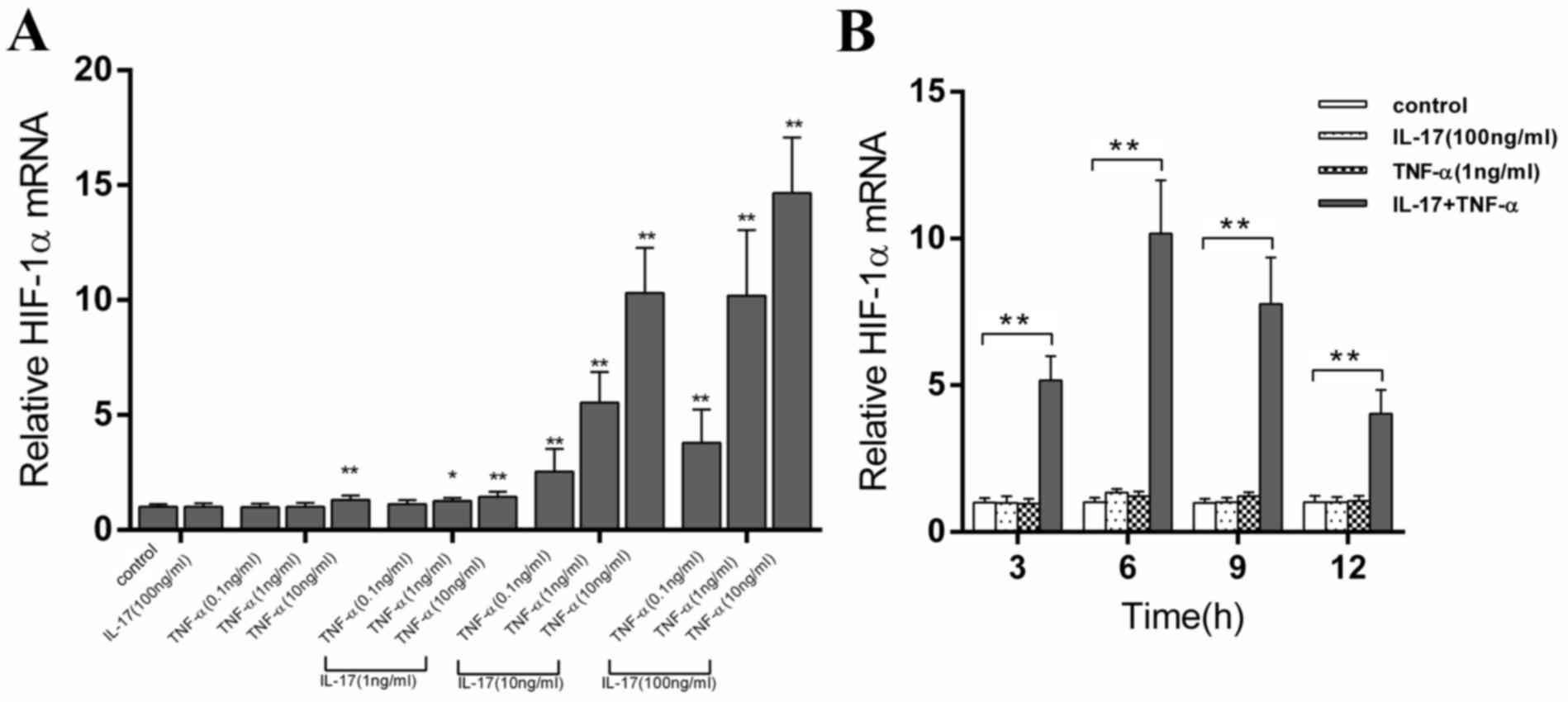
As presented in Fig.
1, compared with the control group, stimulation with IL-17
alone did not affect the expression of HIF-1α mRNA and the use of
TNF-α alone at various concentrations had a minimal effect on
HIF-1α mRNA levels. Similarly, the effects of treatment with low
dose (1 ng/ml) IL-17 and TNF-α on expression levels of the HIF-1α
gene were also mild. However, the moderate (10 ng/ml) and the high
(100 ng/ml) concentrations of IL-17 significantly increased
(P<0.01) the ability of TNF-α to induce HIF-1α mRNA expression
levels compared with TNF-α alone in a dose-dependent manner.
To further investigate the time course of HIF-1α
induction following IL-17 and TNF-α stimulation, the transcript
level of HIF-α was measured during a 12 h period. The results
presented in Fig. 1B demonstrate that
the expression levels of HIF-1α were not affected by IL-17 or TNF-α
alone. However, a rapid and sustained increase in HIF-1α mRNA
levels was identified following stimulation with the combination of
drugs compared with the control treatments. The highest level of
HIF-1α mRNA was observed at 6 h following treatment and this was
~10X higher compared with the control cells.
The combination of IL-17 and TNF-α
reduces VASP expression levels and suppresses cell adhesion
The adhesive ability of tumor cells to the
extracellular matrix (ECM) may be an important event in the
progression to metastasis (28). VASP
possesses an important role in the regulation of cell adhesion
(29). Previous studies have
established that HIF-1α binds to the VASP promoter and this
inhibits VASP transcription, which suppresses cell adhesion
(24). To investigate the potential
role of IL-17 and TNF-α in cell adhesion, the protein expression
levels of HIF-1α and VASP were examined in MDA-MB-231 cells treated
with IL-17, TNF-α or a combination of the two. The HIF-1α and VASP
expression levels were detected using western blotting. As
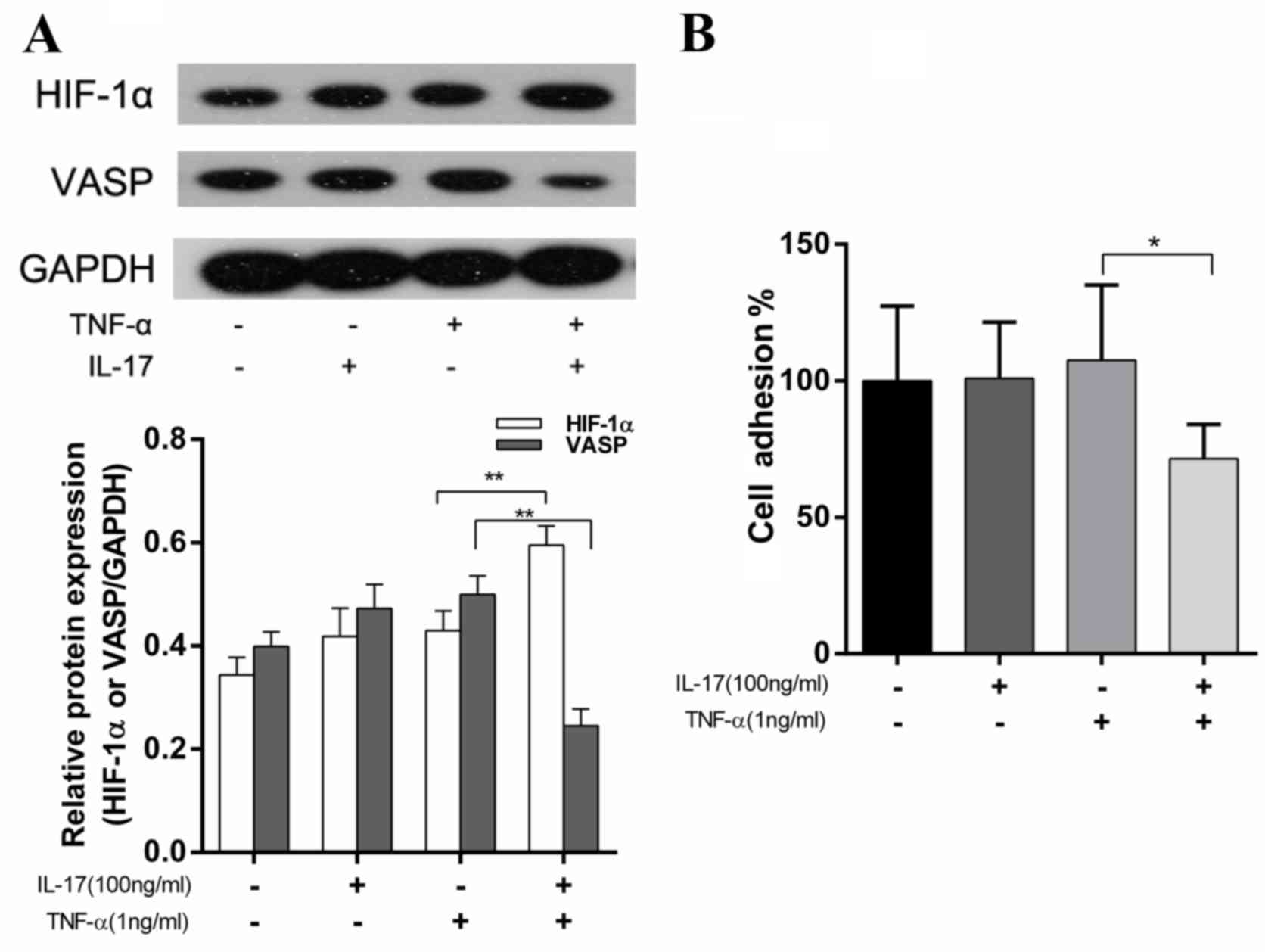
presented in Fig. 2A, stimulation
with IL-17 or TNF-α alone did not affect HIF-1α or VASP expression
levels. However, the combination of IL-17 and TNF-α significantly
increased HIF-1α expression levels by 38.5% (P<0.01) and
decreased VASP expression levels by 51% (P<0.01). These results
were significantly different compared with the TNF-α treatment
group. Additionally, the adhesive ability of cells was evaluated.
It was observed that the cell adhesion ability in the IL-17 and
TNF-α treatment group was reduced by 33.5% (P<0.05) compared
with the TNF-α treatment group (Fig.
2B). These results were in accordance with the effects on VASP
expression levels observed following this combination drug
treatment (Fig. 2A).
HIF-1α-siRNA knockdown in MDA-MB-231
cells reduces cell adhesive abilities and the inhibition of VASP
expression following IL-17 and TNF-α treatment
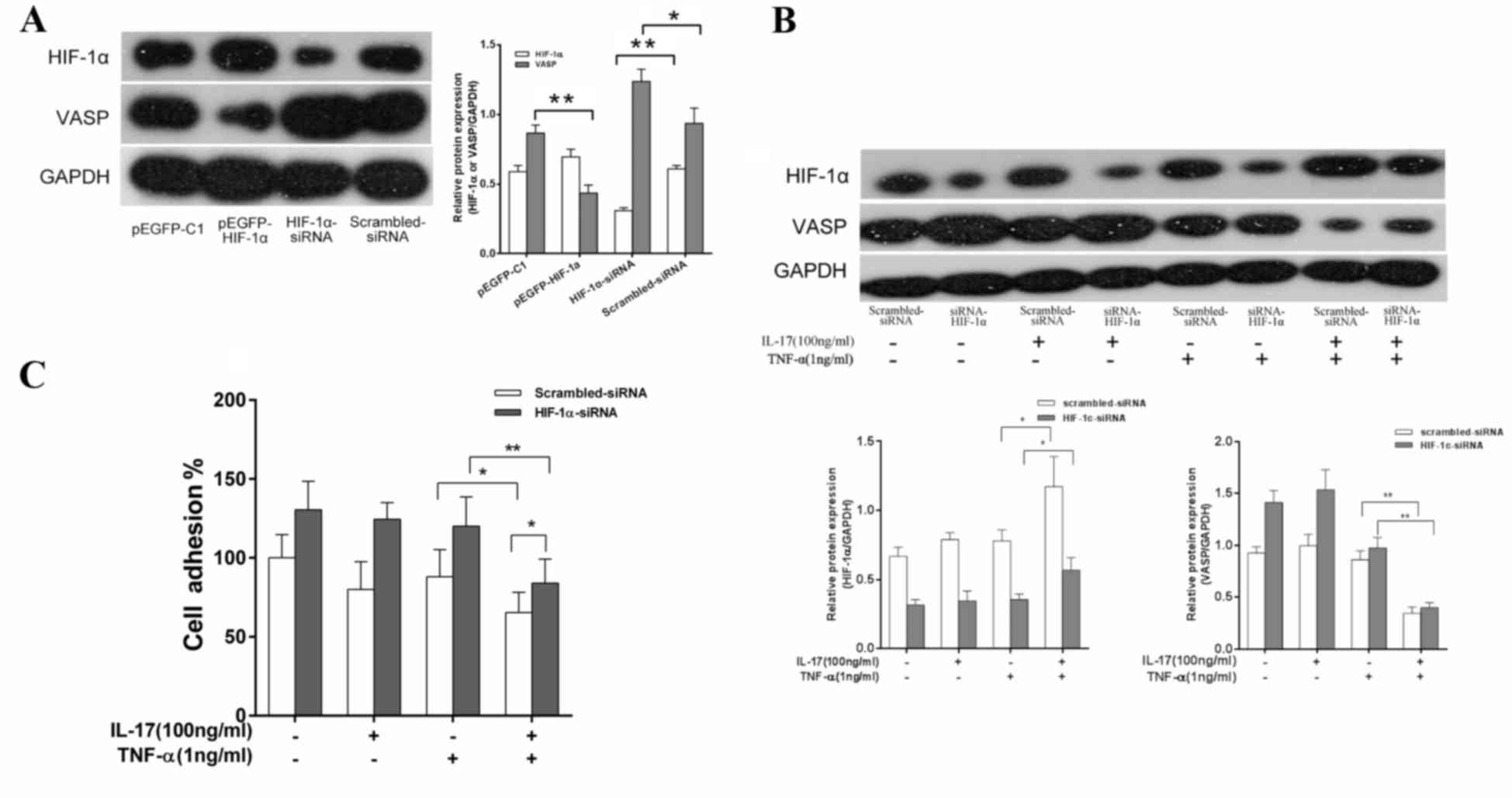
To evaluate the association between IL-17 and TNF-α
combination drug treatment and HIF-1α and VASP expression levels,
MDA-MB-231 cells were transfected with HIF-1α-siRNA. MDA-MB-231
cells were also transfected with scrambled-siRNA as a control. It
was observed that, following knockdown of HIF-1α expression levels,
VASP protein expression levels were significantly increased
(P<0.05) compared with the control group (Fig. 3A). By contrast, the cells transfected
with pEGFP-Cl-HIF-1α in order to overexpress HIF-1α exhibited a
significant decrease (P<0.01) in VASP protein compared with the
empty vector pEGFP-C1 control group (Fig.
3A). These results indicate that HIF-1α may be involved in the
regulation of VASP expression levels. The role of HIF-1α in the
effect of IL-17 and TNF-α combination treatment on VASP expression
levels was investigated. MDA-MB-231 cells were transfected with
HIF-1α-siRNA or scrambled-siRNA and treated with 100 ng/ml IL-17, 1
ng/ml TNF-α or IL-17 and TNF-α. Western blotting was used to detect
HIF-1α and VASP protein expression levels and the adhesive ability
of cells was also evaluated. As presented in Fig. 3B, there were no significant
differences in the protein levels of VASP or HIF-1α in the IL-17 or
TNF-α treatment groups compared with the control group. However,
compared with the TNF-α alone treatment group, in the IL-17 and
TNF-α combination treatment group, the HIF-1α expression levels in
cells transfected with scrambled-siRNA were significantly increased
(P<0.05) and the VASP protein levels were significantly
decreased (P<0.01). Additionally, the increase of HIF-1α levels
(P<0.05) and decrease in VASP expression levels (P<0.01)
following the combination treatment were reduced in the cells
transfected with HIF-1α-siRNA compared with those treated with
TNF-α alone. Similarly, to cells treated with a combination of
IL-17 and TNF-α, the adhesive ability of MDA-MB-231 cells
transfected with HIF-1α-siRNA was significantly increased
(P<0.05) compared with cells transfected scrambled-siRNA
(Fig. 3C).
IL-17 and TNF-α enhance the reduction
of VASP expression levels and decrease the adhesive ability of the
MDA-MB-231 cells transfected with shRNA-VASP
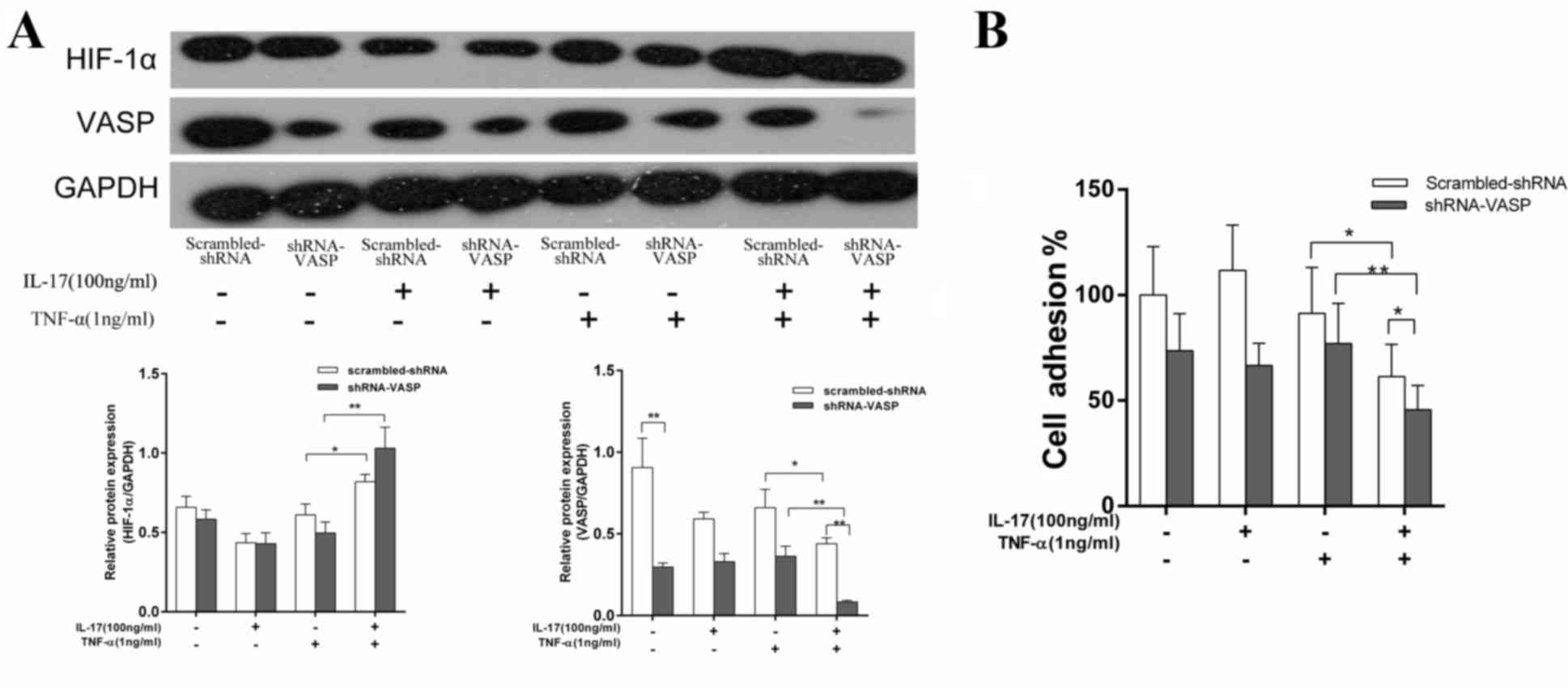
To investigate the association between VASP and
HIF-1α, MDA-MB-231 cells were transfected with shRNA-VASP
(pcDNA6.2-GW/EmGFP-shR-VASP vector) to inhibit VASP expression
levels. The cells were transfected with scrambled shRNA
(pcDNA6.2-GW/EmGFP-MIR) as a control. Following incubation for 6 h
with either the vehicle control, 100 ng/ml IL-17, 1 ng/ml TNF-α or
IL-17 and TNF-α, the HIF-1α and VASP expression levels in
MDA-MB-231 cells were evaluated using western blotting and the
adhesive ability of the cells was also analyzed. As presented in
Fig. 4A, VASP expression levels in
cells transfected with shRNA-VASP were decreased by 67.3% compared
with those transfected with scrambled-shRNA in the vehicle control
groups (P<0.01), which suggests that shRNA-VASP was effectively
transfected and exhibited a clear inhibitory effect on VASP
expression levels. HIF-1α protein expression levels in cells
transfected with scrambled-shRNA or shRNA-VASP in the vehicle
control groups were not significantly different. In the IL-17 and
TNF-α combination treatment group, VASP expression levels in the
shRNA-VASP and scrambled-shRNA transfected cells were reduced by
33.2 and 77.9%, respectively (P<0.05 and P<0.01), compared
with cells in the TNF-α treatment group. The variation in the
degree of protein reduction between the two methods of knockdown
indicates that treatment with shRNA-VASP and the combination of
IL-17 and TNF-α may provide more effective inhibition of VASP
expression compared with shRNA-VASP treatment alone. This indicates
that the combination of IL-17 and TNF-α may provide a robust method
for inhibiting VASP expression levels. The adhesive ability of
cells was decreased in the groups that also exhibited a reduction
in VASP expression levels and the strength of the effect was
concordant with the level of VASP reduction (Fig. 4B).
Discussion
IL-17 and TNF-α are associated and typically
observed in acute and chronic inflammation and the effects of
combined IL-17 and TNF-α treatment is, therefore, biologically
relevant to the interaction between inflammation and cancer
(30). A previous study has
established that the combination of IL-17 and TNF-α induces the
activation of certain genes, including HIF-1α (13), neutrophil gelatinase-associated
lipocalin (14), whereas IL-17 or
TNF-α alone did not produce a significant effect. Other previous
studies (15,16,18) have
also demonstrated that IL-17 augments TNF-α-induced gene
expression, including G-CSF, GM-CSF, KC, MIP-2, PGE2 and VEGF. A
potential underlying mechanism that has been hypothesized is that
IL-17 may promote the stability of TNF-α-induced mRNA (7).
In the current study, MDA-MB-231 cells were
incubated with a number of doses of TNF-α (0.1, 1, 10 ng/ml) and
the results demonstrate that HIF-1α mRNA expression levels were
increased in the high dose group (10 ng/ml). The low, moderate and
high doses of IL-17 (1, 10, 100 ng/ml, respectively) were also
investigated in combination with each of the TNF-α doses. These
results indicated that HIF-1α mRNA levels were increased in the
combined IL-17 and TNF-α groups in a dose-dependent manner. The
increase of HIF-1α mRNA levels was markedly increased in cells
treated with the moderate (1 ng/ml) and high (10 ng/ml) doses of
TNF-α in combination with IL-17. Therefore, these results suggest
that IL-17 may increase the effect of TNF-α on its mediation of
HIF-1α mRNA expression levels (Fig.
1A).
A number of distinct physiological and pathological
processes are dependent on the adhesive and migratory abilities of
cells, including the immune response, tissue morphogenesis and
cancer metastasis (31). Cell-to-cell
and cell-to-ECM interactions are important in the ability to
metastases and a number of families of adhesion molecules,
including cadherins, selectins, integrins and the immunoglobulin
superfamily (28,32,33)
mediate these interactions. Additionally, adhesion dynamics require
coordination with new protrusions containing F-actin being
assembled, which are controlled by various elongating and actin
nucleating molecules (34). It has
been demonstrated that a reduction in the adhesive ability of tumor
cells to attach to ECM proteins may provide novel targets for
therapeutic intervention (28). VASP
is a member of the Ena/VASP protein family, which is associated
with a number of diseases, including cancer, thrombosis,
cardiomyopathy, arteriosclerosis and nephritis (35). Ena/VASP proteins have been established
as regulators of actin-associated processes, including axon
outgrowth and guidance, epithelial cell adhesion, cell motility,
cell polarity and pathogen F-actin tail formation (36). In epithelial cells, VASP has also been
identified as contributing to cell-cell adhesion via the regulation
of actin polymerization and bundling (37). VASP affects F-actin filament
elongation and bundling by localizing to regions of dynamic actin
reorganization, including focal adhesions and filopodia at the
leading edge in motile cells (38,39).
In previous studies, high-dose TNF-α has been
indicated to mediate VASP expression via the TNF-α/HIF-1α/VASP
signaling pathway, promoting the alteration of cell adhesiveness,
whereas low-doses of TNF-α did not exhibit a significant effect
(24,25). In the present study, TNF-α (1 ng/ml)
was not able to significantly affect HIF-1α mRNA expression levels
compared with the control group; however, when used in combination
with IL-17 (100 ng/ml), breast cancer cells exhibited alterations
to the protein expression levels of HIF-1α and VASP and the ability
of cells to adhere. These results indicated that the combination
TNFα and IL-17 treatment significantly increased the protein
expression levels of HIF-1α and decreased the protein expression
levels of VASP and subsequently led to a decline in the adhesive
ability of cells.
To further investigate the distinct association
between HIF-1α and VASP following treatment with a combination of
IL-17 and TNF-α, MDA-MB-231 cells were transfected with
HIF-1α-siRNA and shRNA-VASP. The results indicated that
HIF-1α-siRNA decreased the level of HIF-1α and increased VASP
protein expression, which promoted MDA-MB-231 cell adhesion.
Following stimulation with IL-17 and TNF-α, HIF-1α expression was
increased and the VASP expression levels were decreased. However,
this inhibitory effect was altered by the knockdown of HIF-1α.
Additionally, it was also observed that the knockdown of VASP by
VASP shRNA, markedly reduced the adhesion of the MDA-MB-231 cells
and reduced the levels of VASP protein expression compared with the
scrambled shRNA control. The levels of HIF-1α were not altered
following transfection. These results indicate that HIF-1α may be
upstream of VASP in signaling pathways that control the adhesion of
MDA-MB-231 cells. The treatment of IL-17 and TNF-α was able to
inhibit VASP expression levels to a higher extent compared with
shRNA-VASP alone, which suggests that IL-17 and TNF-α may be used
to further inhibit VASP expression levels. These results
demonstrate that HIF-1α may mediate the repression of VASP
expression levels via IL-17 and TNF-α in MDA-MB-231 cells.
In conclusion, the current study has demonstrated
that IL-17 may enhance the TNF-α-induced increase HIF-1α inhibition
VASP expression, which reduces the adhesive ability of MDA-MB-231
breast cancer cells. Therefore, targeting IL-17 and TNF-α may
provide a novel insight into potential anti-tumor signaling
pathways.
Acknowledgements
This study was supported by The Fundamental Research
Funds for the Central Universities (grant nos. 2014301020204 and
2042014kf0186).
References
|
1
|
van Horssen R, Ten Hagen TL and Eggermont
AM: TNF-alpha in cancer treatment: Molecular insights, antitumor
effects, and clinical utility. Oncologist. 11:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilt SG, Milward E, Zhou JM, Nagasato K,
Patton H, Rusten R, Griffin DE, O'Connor M and Dubois-Dalcq M: In
vitro evidence for a dual role of tumor necrosis factor-alpha in
human immunodeficiency virus type 1 encephalopathy. Ann Neurol.
37:381–394. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han YP, Nien YD and Garner WL: Tumor
necrosis factor-alpha-induced proteolytic activation of pro-matrix
metalloproteinase-9 by human skin is controlled by down-regulating
tissue inhibitor of metalloproteinase-1 and mediated by
tissue-associated chymotrypsin-like proteinase. J Biol Chem.
277:27319–27327. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jammal MP, DA Silva AA, Filho AM, DE
Castro Côbo E, Adad SJ, Murta EF and Nomelini RS:
Immunohistochemical staining of tumor necrosis factor-α and
interleukin-10 in benign and malignant ovarian neoplasms. Oncol
Lett. 9:979–983. 2015.PubMed/NCBI
|
|
5
|
Zhu N, Lalla R, Eves P, Brown TL, King A,
Kemp EH, Haycock JW and MacNeil S: Melanoma cell migration is
upregulated by tumour necrosis factor-alpha and suppressed by
alpha-melanocyte-stimulating hormone. Br J Cancer. 90:1457–1463.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen M and Geng JG: P-selectin mediates
adhesion of leukocytes, platelets, and cancer cells in
inflammation, thrombosis, and cancer growth and metastasis. Arch
Immunol Ther Exp (Warsz). 54:75–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujino S, Andoh A, Bamba S, Ogawa A, Hata
K, Araki Y, Bamba T and Fujiyama Y: Increased expression of
interleukin 17 in inflammatory bowel disease. Gut. 52:65–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tzartos JS, Friese MA, Craner MJ, Palace
J, Newcombe J, Esiri MM and Fugger L: Interleukin-17 production in
central nervous system-infiltrating T cells and glial cells is
associated with active disease in multiple sclerosis. Am J Pathol.
172:146–155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chabaud M, Garnero P, Dayer JM, Guerne PA,
Fossiez F and Miossec P: Contribution of interleukin 17 to synovium
matrix destruction in rheumatoid arthritis. Cytokine. 12:1092–1099.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilke CM, Kryczek I, Wei S, Zhao E, Wu K,
Wang G and Zou W: Th17 cells in cancer: Help or hindrance?
Carcinogenesis. 32:643–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilke CM, Bishop K, Fox D and Zou W:
Deciphering the role of Th17 cells in human disease. Trends
Immunol. 32:603–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu J, Ye H, Zhang D, Liu W, Li M, Mao Y
and Lu Y: U87MG glioma cells overexpressing IL-17 acclerate
early-stage growth and cause a higher level of CD31 mRNA expression
in tumor tissues. Oncol Lett. 6:993–999. 2013.PubMed/NCBI
|
|
13
|
Hot A, Zrioual S, Lenief V and Miossec P:
IL-17 and tumour necrosis factor alpha combination induces a
HIF-1α-dependent invasive phenotype in synoviocytes. Ann Rheum Dis.
71:1393–1401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karlsen JR, Borregaard N and Cowland JB:
Induction of neutrophil gelatinase-associated lipocalin expression
by co-stimulation with interleukin-17 and tumor necrosis
factor-alpha is controlled by IkappaB-zeta but neither by
C/EBP-beta nor C/EBP-delta. J Biol Chem. 285:14088–14100. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andoh A, Yasui H, Inatomi O, Zhang Z,
Deguchi Y, Hata K, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S,
et al: Interleukin-17 augments tumor necrosis factor-alpha-induced
granulocyte and granulocyte/macrophage colony-stimulating factor
release from human colonic myofibroblasts. J Gastroenterol.
40:802–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Numasaki M, Lotze MT and Sasaki H:
Interleukin-17 augments tumor necrosis factor-alpha-induced
elaboration of proangiogenic factors from fibroblasts. Immunol
Lett. 93:39–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamilton T, Li X, Novotny M, Pavicic PG
Jr, Datta S, Zhao C, Hartupee J and Sun D: Cell type- and
stimulus-specific mechanisms for post-transcriptional control of
neutrophil chemokine gene expression. J Leukoc Biol. 91:377–383.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koenders MI, Marijnissen RJ, Devesa I,
Lubberts E, Joosten LA, Roth J, van Lent PL, van de Loo FA and van
den Berg WB: Tumor necrosis factor-interleukin-17 interplay induces
S100A8, interleukin-1β, and matrix metalloproteinases, and drives
irreversible cartilage destruction in murine arthritis: Rationale
for combination treatment during arthritis. Arthritis Rheum.
63:2329–2339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiricozzi A, Guttman-Yassky E,
Suárez-Fariñas M, Nograles KE, Tian S, Cardinale I, Chimenti S and
Krueger JG: Integrative responses to IL-17 and TNF-alpha in human
keratinocytes account for key inflammatory pathogenic circuits in
psoriasis. The Journal of investigative dermatology. 131:677–687.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barzik M, Kotova TI, Higgs HN, Hazelwood
L, Hanein D, Gertler FB and Schafer DA: Ena/VASP proteins enhance
actin polymerization in the presence of barbed end capping
proteins. J Biol Chem. 280:28653–28662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schirenbeck A, Arasada R, Bretschneider T,
Stradal TE, Schleicher M and Faix J: The bundling activity of
vasodilator-stimulated phosphoprotein is required for filopodium
formation. Proc Natl Acad Sci USA. 103:7694–7699. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krause M, Dent EW, Bear JE, Loureiro JJ
and Gertler FB: Ena/VASP proteins: Regulators of the actin
cytoskeleton and cell migration. Annu Rev Cell Dev Biol.
19:541–564. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dertsiz L, Ozbilim G, Kayisli Y, Gokhan
GA, Demircan A and Kayisli UA: Differential expression of VASP in
normal lung tissue and lung adenocarcinomas. Thorax. 60:576–581.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su K, Tian Y, Wang J, Shi W, Luo D, Liu J,
Tong Z, Wu J, Zhang J and Wei L: HIF-1alpha acts downstream of
TNF-α to inhibit vasodilator-stimulated phosphoprotein expression
and modulates the adhesion and proliferation of breast cancer
cells. DNA Cell Biol. 31:1078–1087. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang M, Tian Y, Li D, Lv J, Li Q, Kuang C,
Hu P, Wang Y, Wang J, Su K and Wei L: TNF-α mediated increase of
HIF-1α inhibits VASP expression, which reduces alveolar-capillary
barrier function during acute lung injury (ALI). PloS One.
9:e1029672014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Zhang J, Wu J, Luo D, Su K, Shi W,
Liu J, Tian Y and Wei L: MicroRNA-610 inhibits the migration and
invasion of gastric cancer cells by suppressing the expression of
vasodilator-stimulated phosphoprotein. Eur J Cancer. 48:1904–1913.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kato R, Ishikawa T, Kamiya S, Oguma F,
Ueki M, Goto S, Nakamura H, Katayama T and Fukai F: A new type of
antimetastatic peptide derived from fibronectin. Clin Cancer Res.
8:2455–2462. 2002.PubMed/NCBI
|
|
29
|
Galler AB, Arguinzonis Garcia MI,
Baumgartner W, Kuhn M, Smolenski A, Simm A and Reinhard M:
VASP-dependent regulation of actin cytoskeleton rigidity, cell
adhesion, and detachment. Histochem Cell Biol. 125:457–474. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Straus DS: TNFα and IL-17 cooperatively
stimulate glucose metabolism and growth factor production in human
colorectal cancer cells. Mol Cancer. 12:782013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stroka KM and Konstantopoulos K: Physical
biology in cancer. 4. Physical cues guide tumor cell adhesion and
migration. Am J Physiol Cell Physiol. 306:C98–C109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mareel M and Leroy A: Clinical, cellular,
and molecular aspects of cancer invasion. Physiol Rev. 83:337–376.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bhatt T, Rizvi A, Batta SP, Kataria S and
Jamora C: Signaling and mechanical roles of E-cadherin. Cell Commun
Adhes. 20:189–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Le Clainche C and Carlier MF: Regulation
of actin assembly associated with protrusion and adhesion in cell
migration. Physiol Rev. 88:489–513. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pula G and Krause M: Role of Ena/VASP
proteins in homeostasis and disease. Handb Exp Pharmacol. 39–65.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lebrand C, Dent EW, Strasser GA, Lanier
LM, Krause M, Svitkina TM, Borisy GG and Gertler FB: Critical role
of Ena/VASP proteins for filopodia formation in neurons and in
function downstream of netrin-1. Neuron. 42:37–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Doppler H and Storz P: Regulation of VASP
by phosphorylation: consequences for cell migration. Cell Adh Migr.
7:482–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bear JE and Gertler FB: Ena/VASP: Towards
resolving a pointed controversy at the barbed end. J Cell Sci.
122:1947–1953. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Trichet L, Sykes C and Plastino J:
Relaxing the actin cytoskeleton for adhesion and movement with
Ena/VASP. J Cell Biol. 181:19–25. 2008. View Article : Google Scholar : PubMed/NCBI
|