Introduction
Infection of the uterine cervix by an oncogenic,
high-risk (HR)-human papilloma virus (HPV) frequently results in
low-grade cervical intraepithelial neoplasia (CIN), a dysplastic
lesion that may progress into high-grade CIN and cervical carcinoma
(1). The prevalence and persistence
of HR-HPV, the incidence of CIN and the risk of CIN progression are
high in women infected with HR-HPV and human immunodeficiency virus
(HIV) (1). In these patients,
antiretroviral drugs, including HIV-protease inhibitors (PIs), have
reduced the rate of uterine CIN incidence and progression (2–4).
The progression of CIN to invasive cervical
carcinoma is initiated when HR-HPV+ keratinocytes invade
the basement membrane at the stromal/epithelial junction of the
lesion (1). A previous study
demonstrated that therapeutic concentrations of HIV-PIs, such as
saquinavir (SQV) and ritonavir (RTV), effectively inhibit the
invasive capabilities of HR-HPV+ human primary
keratinocytes obtained from low-grade CIN lesions (5). This inhibition of invasion was
associated with the downregulation of the pro-invasive, basement
membrane-degrading enzyme matrix metalloproteinase (MMP)-9 and, to
a lesser extent, with the downregulation of MMP-2 (5). As these results were obtained in an
in vitro experimental model devoid of HIV or immune cells,
they confirm preclinical and clinical work indicating that HIV-PIs
exert direct antitumour effects independently of their anti-HIV
and/or immune reconstituting activities (5–14).
The capability of SQV and RTV to inhibit the
expression of MMP-9 has important implications, since MMP-9 serves
a key role in the invasion and clinical progression of CIN
(15–18). A previous study identified that MMP-9
expression is induced in CIN cells by epidermal growth factor (EGF)
(5), which is a marker of CIN
progression (19). Another study
demonstrated that the induction of MMP-9 expression by EGF in
epithelial cells is preceded and/or accompanied by the
phosphorylation and activation of signalling molecules, including
the serine/threonine kinase AKT (20). EGF-induced phosphorylation of AKT
leads to the activation of members of the activator protein-1
transcriptional complex, such as Fos-related antigen (Fra)-1
(21), which is a potent activator of
MMP-9 gene expression (22–24). Notably, the E6 and E7 proteins of
HR-HPV have been shown to phosphorylate AKT (25,26) and to
promote MMP-9 expression (27,28). In
view of the inhibitory effect of SQV/RTV on CIN cell invasion and
MMP-9 expression, the present study investigated whether
therapeutic concentrations of these HIV-PIs would affect the
AKT/Fra-1 signalling pathway or the expression of E6/E7 in
HR-HPV+ human primary CIN cells.
Materials and methods
Reagents
SQV (Roche Diagnostics, Basel, Switzerland) and RTV
(National Institutes of Health, Bethesda, MD, USA) were diluted and
handled as previously described (5).
Cell growth medium (DMEM/Ham's F12 enriched with NaHCO3,
HEPES, HCl, penicillin and streptomycin), PBS and monoclonal
antibodies raised against the E7 protein of HPV16 (dilution, 1:200;
catalog no. MA5-15822) were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Sigma-Aldrich (Merck
Millipore, Darmstadt, Germany) was the source of growth media
supplements (fetal bovine serum, hydrocortisone, adenine, insulin,
transferrin, cholera enterotoxin and 3,3′,5-triiodo-L-thyronine),
anti β-actin monoclonal antibodies (dilution, 1:1,000; catalog no.
A5316), bovine serum albumin (BSA; fraction V) and the chemicals
employed for protein extraction, which included Tris HCl, NaCl,
MgCl2, KCl, Nonidet P (NP)-40, sodium deoxycholate,
phenylmethyl-sulfonyl fluoride (PMSF), dithiothreitol (DTT), EDTA,
glycerol, HEPES, leupeptin, aprotinin, or pepstatin. Monoclonal
antibodies directed against the E6 protein of HPV16 (dilution,
1:200; catalog no. sc-460) or human C23 (nucleolin; dilution,
1:250; catalog no. sc-515312) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Rabbit monoclonal antibodies
raised against Fra-1 (dilution, 1:250; catalog no. 5281) or
phosphorylated AKT (ser473; dilution, 1:250; catalog no. 4060) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The primers and probes employed for RNA analyses were purchased
from Applied Biosystems (Thermo Fisher Scientific, Inc.). Human
recombinant EGF was purchased from BD Biosciences (Franklin Lakes,
NJ, USA), and suspended in 0.1% BSA in PBS.
Cell culture
W12 (HPV16+) and CIN612-7E
(HPV31+) human primary keratinocyte cell lines, derived
from low-grade CIN in HIV-negative women were obtained,
characterised and cultured as previously described (29,30). In
all experiments, cells were cultured for 96 h in the absence or
presence of 10 µM SQV or RTV, which were added to the growth medium
on a daily basis (5). Subsequently,
cells were exposed to EGF or its suspension buffer (0.1% BSA in
PBS) for 15, 30, 60 or 90 min, or 3, 6, 12 or 96 h, in the presence
or absence of SQV or RTV.
Invasion assays and zymography
The effect of SQV and RTV on W12 cell invasion was
assayed using the Boyden chambers (5), as previously described. At the end of
the assay, invaded cells were fixed in ethanol (Sigma-Aldrich),
stained with toluidine blue (Sigma-Aldrich), and quantitated using
light microscopy, counting 5 fields/filter (5). With regard to zymography, W12 cells were
cultured overnight in EGF-supplemented, serum-free medium, in the
absence or presence of SQV or RTV. Cell supernatants were collected
and concentrated with the use of Centricon centrifugal filter
devices (Merck Millipore). Protein concentration was determined
with Bradford reagent (Bio-Rad, Hercules, CA, USA), using BSA as a
standard. To detect collagenolytic activity, 2 µg total proteins
from concentrated supernatants were electrophoresed using 9% SDS
PAGE embedded with 1 mg/ml gelatin (Sigma-Aldrich), as described
(31). Following staining with
Coomassie blue (Bio-Rad), the decrease in staining of each band due
to protease activity was quantified using densitometry (5).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells, purified,
and used to synthesize complementary DNA (cDNA), as previously
described (5). The
reverse-transcribed (RT) cDNA from repeated, independent
experiments was used for quantitative (q)PCR analysis of MMP-9
(Hs_MMP9_1_SG Quantitect Primer Assay; Qiagen, Inc., Valencia, CA,
USA). The reaction was normalized by amplifying samples for
glyceraldehyde-3-phosphate dehydrogenase as the reference gene
(Hs_GAPDH_2_SG Quantitect Primer Assay; Qiagen). RT-PCR was
performed, and the data were analyzed, as reported previously
(5).
In other experiments, cDNA was used for PCR analysis
of HPV-E6 or HPV-E7, according the TaqMan technique. Specifically,
2X TaqMan gene expression master mix (Thermo Fisher Scientific) was
employed, while primers or probes were used at 0.9 or 0.2 µM,
respectively. The following primers were used for qPCR: HPV16-E6
forward (F), 5′-AATGTTTCAGGACCCACAGG-3′ and reverse (R),
5′-TTGTTTGCAGCTCTGTGCAT-3′; HPV16-E7 F,
5′-CAAGTGTGACTCTACGCTTCGG-3′ and R, 5′-GTGGCCCATTAACAGGTCTTCCAA-3′;
HPV31-E6 F, 5′-ATTCCACAACATAGGAGGAAGGT-3′ and R,
5′-CACTTGGGTTTCAGTACGAGGTCT-3′; HPV31-E7 F,
5′-GGCAACTGACCTCCACTGTT-3′ and R, 5′-ATTGGATGTGTCCGGTTCTG-3′; and β
actin F, 5′-AAGAGCTACGAGCTGCCTGA3′ and R,
5′-TGGAGTTGAAGGTAGTTTCGTG-3′. The probes used were as follows:
HPV16-E6, 5′-AGCGACCCAGAAAGTTACCA-3′; HPV16-E7,
5′-TGCGTACAAAGCACACACGTAGACATTCGT-3′; HPV31-E6,
5′-ACAGGACGTTGCATAGCATGTTGGA-3′; HPV31-E7,
5′-ATGAGCAATTACCCGACAGC-3′; and β actin,
5′-CATCACCATTGGCAATGAGCGGT-3′.
In order to visualize the probes, these were
conjugated to tetramethyl-rhodamine (Thermo Fisher Scientific,
Inc.). qPCR thermocycling conditions were as follows: 45 cycles of
20 sec at 50°C, 10 min at 95°C and 15 sec at 95°C, followed by 1
min at 58°C. PCR data were analyzed using the 7500 Fast System SDS
software (version 2.0.5; Applied Biosystems, Thermo Fisher
Scientific, Inc.), and the results were normalized to β-actin. The
complexes formed by PCR products and associated probes were
quantified by employing the 2−∆∆Cq method (32).
Western blot analysis
To extract total proteins, W12 or CIN612-7E cells
were lysed in 50 mM Tris (pH 7.5), 150 mM NaCl, 1% NP-40,
0.25/sodium deoxycholate, 1 mM PMSF, 2 mM ethyleneglycol-bis
(β-aminoethyl ether)-N,N, N'-tetraacetic acid, 30 µg/ml leupeptin
and 10 µg/ml aprotinin. To evaluate phosphorylated AKT levels, W12
or CIN612-7E cells were lysed in 40 mM Tris (pH 8.0), 120 mM NaCl
and 0.1% NP-40, and kept in ice for 30 min. Cellular lysate was
centrifuged at 13,000 × g for 15 min at 4°C, and the cleared
supernatant was then collected. To obtain nuclear protein, W12 or
CIN612-7E cells were lysed in 10 mM HEPES pH 7.9, 1.5 mM
MgCl2, 10 mM KCl, 0.5 mM PMSF, 0.5 DTT, 0.1% NP-40, 10
µg/ml leupeptin, 10 µg/ml pepstatin and 5 µg/ml aprotinin. Nuclei
were separated by centrifugation (13,000 × g for 15 min at 4°C),
suspended in 20 mM HEPES (pH 7.9), 0.42 M NaCl, 1.5 mM
MgCl2, 0.2 mM EDTA, 25% glycerol, 0.5 mM PMSF, 0.5 mM
DTT, 10 µg/ml leupeptin, 10 µg/ml pepstatin and 5 µg/ml aprotinin,
and kept in ice for 60 min. Nuclear lysate was sonicated,
centrifuged at 13,000 × g for 15 min at 4°C and the cleared
supernatant was then collected.
Protein content in cell lysates was assayed with
Bradford reagent (Bio-Rad). Proteins from each sample were
separated onto 10 or 12% SDS-PAGE and transferred onto Hybond
nitrocellulose membrane filters (GE Healthcare Life Sciences,
Pittsburgh, PA, USA), which were probed with primary antibodies and
the corresponding secondary horseradish peroxidase-conjugated
antibodies, as previously described (33). Bands were visualised using
LiteAblot® PLUS Enhanced Chemiluminescent Substrate
(Euroclone SpA, Milan, Italy), and the intensity of the bands was
quantified relative to β-actin using the ChemiDoc XRS+ system
(Bio-Rad Laboratories S.r.l., Segrate, Italy).
Statistical analysis
Data are expressed as the mean ± standard deviation
from three independent experiments. Statistical analysis was
performed using the SPSS 15.0 software (SPSS Inc., Chicago, IL,
USA). P-values were determined with Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Prior research indicated that EGF triggers the
invasion of HR-HPV31+ CIN cells through inducing MMP-9
expression, and that this effect is strongly inhibited by exposure
of CIN cells to 10 µM SQV or RTV for 72–96 h (5). This concentration of SQV/RTV corresponds
to the drugs' peak plasma levels in treated individuals (5). In the present study, initial experiments
investigated the molecular mechanisms underlying the inhibitory
effect exerted by SQV/RTV on MMP-9 expression. These experiments
were conducted in HR-HPV31+ human primary CIN612-7E
cells.
Consistent with previous data obtained in cells of
epithelial origin (20), the present
study identified that EGF promoted AKT phosphorylation in CIN cells
(Fig. 1A). This effect was
demonstrated to be dose and time dependent, with AKT
phosphorylation peaking at 30 min in the presence of 50 ng/ml EGF
(data not shown). Since SQV and RTV impair AKT phosphorylation
(12,13), the impact of these HIV-PIs on
EGF-induced AKT phosphorylation was assessed. A concentration of 10
µM SQV/RTV, a therapeutic concentration that inhibits EGF-induced
CIN cell invasion and MMP-9 expression (5), significantly reduced the phosphorylation
of AKT in CIN612-7E cells compared with the control group (SQV,
P=0.047; RTV, P=0.024; Fig. 1A).
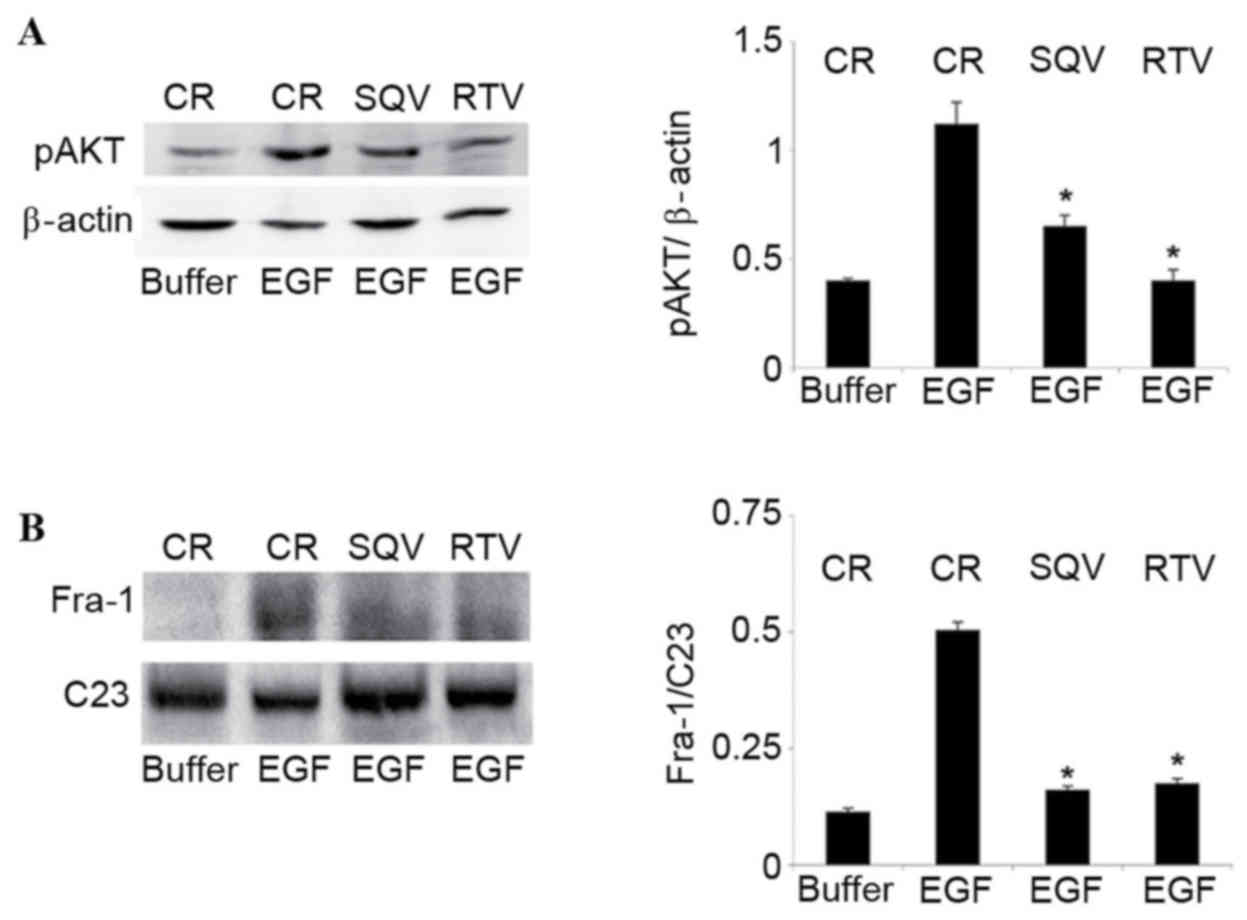 | Figure 1.SQV and RTV inhibit AKT
phosphorylation and reduce nuclear Fra-1 protein levels in
EGF-stimulated CIN612-7E cells. CIN612-7E cells were cultured for
96 h in the presence of 10 µM SQV or RTV. CIN612-7E cells cultured
without SQV/RTV were employed as the control. Cells were
subsequently exposed to 50 ng/ml human recombinant EGF, or to its
suspension buffer (0.1% bovine serum albumin in phosphate-buffered
saline). (A) Cells were lysed following a 30-min exposure to EGF,
and their total protein content was analysed by western blotting,
followed by quantification of pAKT protein expression (relative to
β-actin) by densitometry. (B) Cells were lysed following a 6-h
exposure to EGF, and their nuclear protein content was analysed via
western blotting, followed by quantification of nuclear Fra-1
protein levels (relative to C23) by densitometry. Results are
presented as the mean ± standard deviation from three experiments.
*P<0.05 vs. the control group. SQV, saquinavir; RTV, ritonavir;
Fra-1, Fos-related antigen 1; CR, control; EGF, epidermal growth
factor; p, phosphorylated. |
In agreement with a previous study in epithelial
cells (21), 50 ng/ml EGF was
observed to augment Fra-1 protein levels in CIN cell nuclei
(Fig. 1B). This effect was time
dependent, peaking at 6 h and declining thereafter (data not
shown). SQV/RTV significantly reduced Fra-1 content in the nuclei
of EGF-stimulated CIN612-7E cells compared with the control group
(SQV, P=0.048; RTV, P=0.035; Fig.
1B). The E6 or E7 proteins of HR-HPV are known to phosphorylate
AKT and promote MMP-9 expression (25–28). Thus,
experiments were performed in order to evaluate whether the
inhibitory effect that SQV/RTV exert on AKT phosphorylation and
MMP-9 expression paralleled a reduction in E6/E7 protein levels in
CIN612-7E cells. However, this was not assessed, as antibodies
directed against the E6 or E7 proteins of HPV31 were not
commercially available, and antibodies raised against the E6 or E7
proteins of HPV16 did not recognise the HPV31 E6 or E7 proteins
expressed by CIN612-7E cells.
Further experiments employing W12 cells,
HPV16+ human primary keratinocytes derived from
low-grade CIN lesions (29), were
performed. Confirmatory experiments indicated that, as for
HPV31+ CIN cells (5), a
96-h exposure to 10 µM SQV or RTV significantly inhibited cell
invasion compared with the control group (SQV, P=0.008; RTV,
P=0.006; Fig. 2A), markedly reduced
MMP-9 proteolytic activity (Fig. 2B)
and significantly downregulated MMP-9 expression (SQV, P=0.008;
RTV, P=0.010; Fig. 2C). Notably, SQV
and RTV produced these effects in low-passage, but not in
high-passage, W12 cells (data not shown). Additional experiments
demonstrated that, compared with control cells, SQV and RTV notably
reduced EGF-induced AKT phosphorylation in W12 cells (Fig. 2D). Furthermore, SQV/RTV reduced Fra-1
content in the nuclei of EGF-stimulated W12 cells (Fig. 2E), similarly to the results obtained
with HPV31+ CIN612-7E cells.
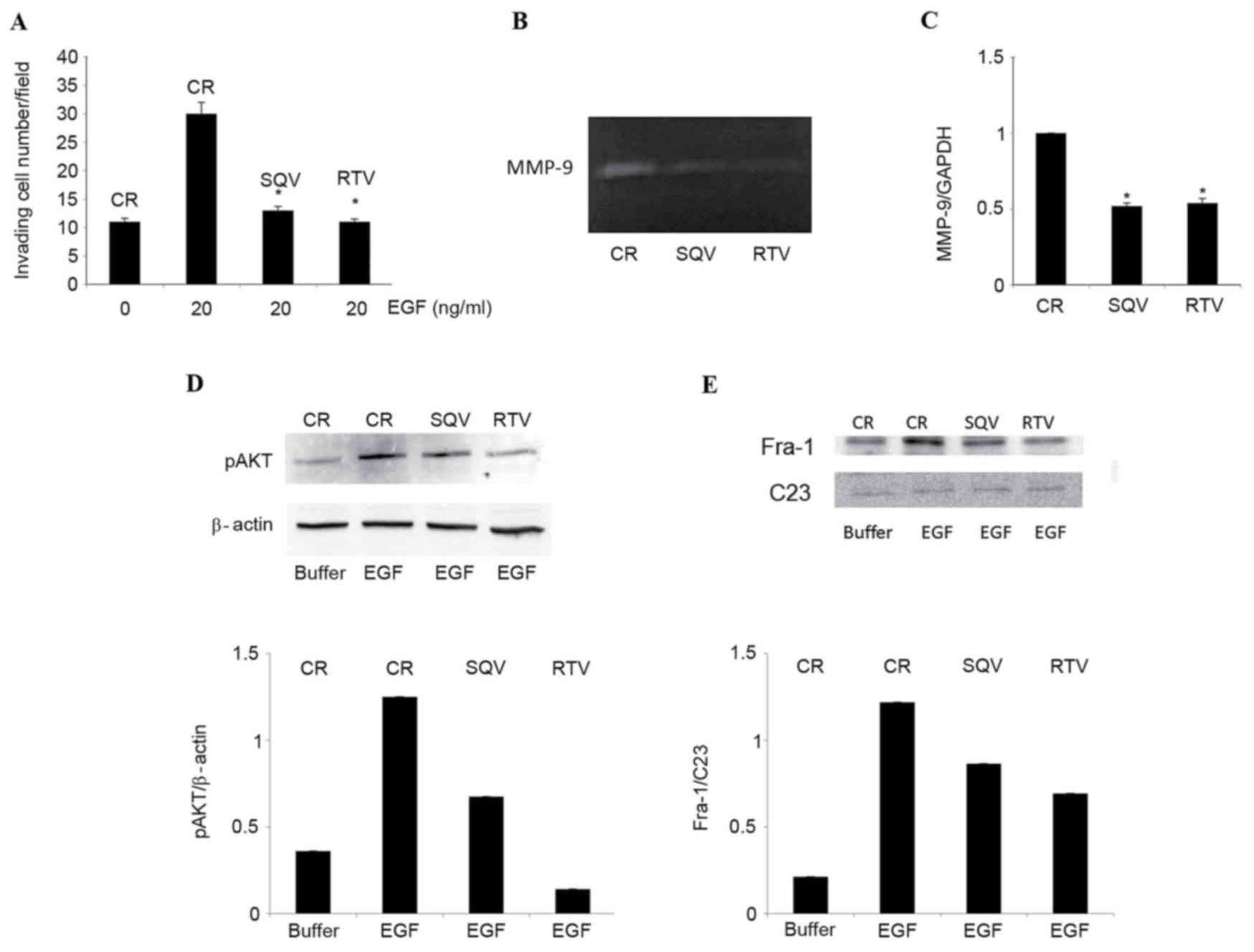 | Figure 2.SQV and RTV counteract EGF-induced
cell invasion, MMP-9 expression, AKT phosphorylation and nuclear
Fra-1 protein expression in W12 cells. W12 cells were cultured for
96 h in the presence of 10 µM SQV or RTV, or in their absence
(control). (A) Cells were stimulated to invade a reconstituted
basement membrane in response to 20 ng/ml human recombinant EGF, or
to its suspension buffer (0.1% bovine serum albumin in
phosphate-buffered saline, indicated here as EGF 0 ng/ml). Results
are expressed as the mean ± standard deviation from 3 experiments,
each performed in duplicate chambers. (B) Representative zymography
of EGF-supplemented, serum-free supernatants. The de-stained areas
indicate gelatinolytic activity corresponding to MMP-9 (92 kDa)
released by the cells. (C) Reverse transcription-quantitative
polymerase chain reaction analysis of MMP-9 messenger RNA levels
(relative to GADPH) in cells cultured in EGF-supplemented growth
medium, in the absence or presence of 10 µM SQV/RTV. Results are
expressed as the mean ± standard deviation from 3 experiments. (D)
Representative western blot analysis and quantification by
densitometry of pAKT protein levels (relative to β-actin) in W12
cells lysed following a 30-min exposure to EGF. (E) Representative
western blot analysis and quantification by densitometry of nuclear
Fra-1 protein levels (relative to C23) in W12 cells lysed following
a 6-h exposure to EGF. *P<0.05 vs. the control group. SQV,
saquinavir; RTV, ritonavir; Fra-1, Fos-related antigen 1; CR,
control; p, phosphorylated; MMP, matrix metalloproteinase; EGF,
epidermal growth factor. |
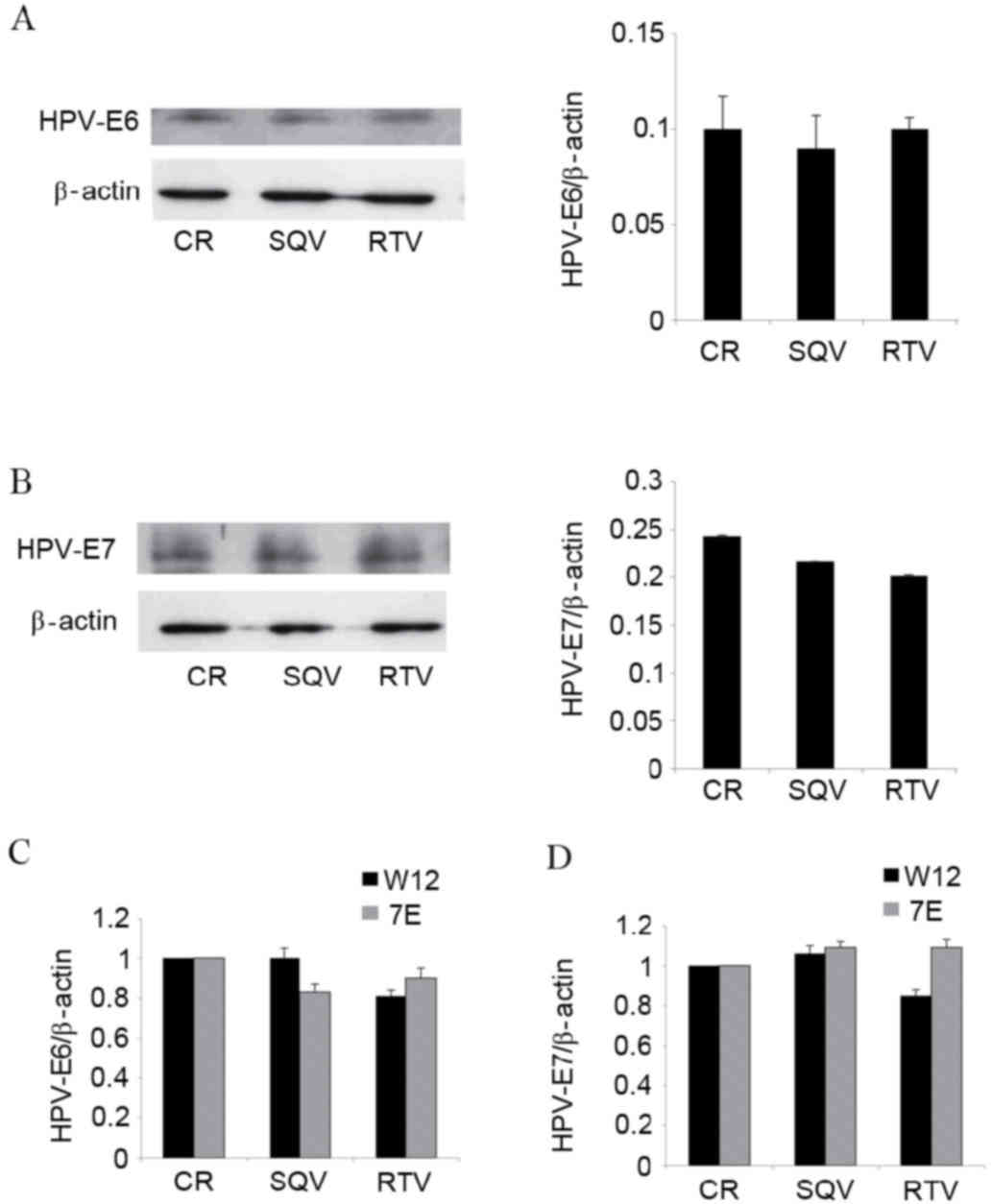
Assays using antibodies raised against the E6 and E7
proteins of HPV16 demonstrated that SQV/RTV did not modify the
content of these proteins in W12 cells (Fig. 3A and B). In addition, neither of the
HIV-PIs tested altered the messenger RNA (mRNA) levels of HPV16 E6
or E7 in W12 cells (Fig. 3C).
Similarly, SQV/RTV did not affect the mRNA expression of HPV31 E6
(Fig. 3C) or E7 (Fig. 3D) in CIN612-7E cells.
Discussion
It has previously been demonstrated that SQV/RTV, at
concentrations present in the plasma of treated patients,
efficiently inhibit EGF-induced invasion of HR-HPV31+
CIN cells via reducing MMP-9 expression (5). This explains the results of clinical
studies that observed HIV-PI efficacy against uterine CIN (2–4). The
present study confirmed the anti-invasive activities of SQV/RTV in
HPV16+ CIN cells. In addition, to the best of our
knowledge, the present study demonstrated for the first time that,
in HPV16+ and HPV31+ CIN cells, inhibition of
EGF-induced MMP-9 expression by SQV/RTV is preceded by a reduction
in AKT phosphorylation. This is consistent with previous results
obtained in other models, which indicated that HIV-PIs can
counteract AKT phosphorylation (12,13), and
that compounds abrogating EGF-induced AKT phosphorylation inhibit
MMP-9 expression and cell invasion (20).
Indeed, the capability of SQV/RTV to impair AKT
phosphorylation in CIN cells may have clinical relevance. Levels of
phosphorylated AKT in epithelial cells change during the different
stages of cervical carcinogenesis. Specifically, phosphorylated AKT
is absent in the normal uterine cervix, while it is present in CIN
biopsies and further increased in cervical carcinoma (34,35). In
agreement with previous studies that observed that Fra-1 silencing
reduces MMP-9 expression and cell invasion (22), the present study demonstrated that
inhibition of MMP-9 expression by RTV/SQV is associated with a
reduction in nuclear, transcriptionally active Fra-1 protein in CIN
cells.
The current study identified that the inhibitory
effect of RTV/SQV on MMP-9 expression is not accompanied with the
downregulation of E6 or E7, two HPV oncogenes that trigger AKT
phosphorylation and MMP-9 expression in infected cells (25–28).
Nevertheless, the present and previous results (5) suggest that the effectiveness of RTV/SQV
against uterine CIN is influenced by HPV, in particular by its
integration into the host cell genome. Specifically, the present
study demonstrated that SQV and RTV downregulate MMP-9 expression
in low-passage, but not in high-passage, W12 cells. In this regard,
it must be highlighted that W12 cells retain HPV16 in an episomic
(not integrated) form during early passages, while long-term in
vitro cultivation of the cells leads to spontaneous loss of
episomes and selection of cells containing only integrated HPV16
(29).
It has previously been identified that SQV and RTV
reduce MMP-9 expression in CIN612 cells independently of their
passage status, while having little or no effect on cell lines
derived from late-stage carcinoma of the uterine cervix (5). CIN612 cells permanently maintain HPV31
DNA in an episomic form (30), while
cervical carcinoma cells possess integrated HPV genomes (36). Since HPV episomes are present in
low-grade CIN, while integrated HPV is characteristic of invasive
cervical carcinomas (37), these
results indicate that SQV and RTV could be effective therapeutic
agents for the treatment of CIN, but may be less effective against
cervical carcinoma. Although this hypothesis is supported by
previous clinical observations (4,38), further
study is required to verify it.
In conclusion, in view of the high incidence of CIN
in HIV-infected women (1), the
results of the present study support the continued employment of
HIV-PIs in HIV treatment regimens. In addition, considering the key
role that MMP-9 serves in CIN progression to invasive cervical
carcinoma (15–18), the results of the current study
support the use of SQV, RTV or their derivatives for the treatment
of CIN in HIV-negative women. These recommendations are supported
by the low toxicity of SQV/RTV and the large quantity of data
available regarding their pharmacokinetics (39).
Acknowledgements
The present study was supported by the Italian
Ministry of Health (Rome, Italy; grant no. OR/70DF) and the Italian
Ministry of Education, University and Research (Rome, Italy; grant
no. RSA/0906). The authors would like to thank Professor L.A.
Laimins (Northwestern University, Chicago, IL, USA) for providing
the W12 and CIN612-7E cells, and Dr M. Falchi (National AIDS
Center, Rome, Italy) for assistance in preparing the figures.
References
|
1
|
Denslow SA, Rositch AF, Firnhaber C, Ting
J and Smith JS: Incidence and progression of cervical lesions in
women with HIV: A systematic global review. Int J STD AIDS.
25:163–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heard I, Schmitz V, Costagliola D, Orth G
and Kazatchkine MD: Early regression of cervical lesions in
HIV-seropositive women receiving highly active antiretroviral
therapy. AIDS. 12:1459–1464. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Omar T, Schwartz S, Hanrahan C,
Modisenyane T, Tshabangu N, Golub JE, McIntyre JA, Gray GE, Mohapi
L and Martinson NA: Progression and regression of premalignant
cervical lesions in HIV-infected women from Soweto: A prospective
cohort. AIDS. 25:87–94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blitz S, Baxter J, Raboud J, Walmsley S,
Rachlis A, Smaill F, Ferenczy A, Coutlée F, Hankins C and Money D:
Canadian Women's HIV Study Group: Evaluation of HIV and highly
active antiretroviral therapy on the natural history of human
papillomavirus infection and cervical cytopathologic findings in
HIV-positive and high-risk HIV-negative women. J Infect Dis.
208:454–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barillari G, Iovane A, Bacigalupo I,
Palladino C, Bellino S, Leone P, Monini P and Ensoli B: Ritonavir
or saquinavir impairs the invasion of cervical intraepithelial
neoplasia cells via a reduction of MMP expression and activity.
AIDS. 26:909–919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sgadari C, Barillari G, Toschi E, Carlei
D, Bacigalupo I, Baccarini S, Palladino C, Leone P, Bugarini R,
Malavasi L, et al: HIV protease inhibitors are potent
anti-angiogenic molecules and promote regression of Kaposi sarcoma.
Nat Med. 8:225–232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Monini P, Sgadari C, Toschi E, Barillari G
and Ensoli B: Antitumour effects of antiretroviral therapy. Nat Rev
Cancer. 4:861–875. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brunner TB, Geiger M, Grabenbauer GG,
Lang-Welzenbach M, Mantoni TS, Cavallaro A, Sauer R, Hohenberger W
and McKenna WG: Phase I trial of the human immunodeficiency virus
protease inhibitor nelfinavir and chemoradiation for locally
advanced pancreatic cancer. J Clin Oncol. 26:2699–2706. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monini P, Sgadari C, Grosso MG, Bellino S,
Di Biagio A, Toschi E, Bacigalupo I, Sabbatucci M, Cencioni G,
Salvi E, et al: Clinical course of classic Kaposi's sarcoma in
HIV-negative patients treated with the HIV protease inhibitor
indinavir. AIDS. 23:534–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rengan R, Mick R, Pryma D, Rosen MA, Lin
LL, Maity AM, Evans TL, Stevenson JP, Langer CJ, Kucharczuk J, et
al: A phase I trial of the HIV protease inhibitor nelfinavir with
concurrent chemoradiotherapy for unresectable stage IIIA/IIIB
non-small cell lung cancer: A report of toxicities and clinical
response. J Thorac Oncol. 7:709–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barillari G, Iovane A, Bacigalupo I,
Labbaye C, Chiozzini C, Sernicola L, Quaranta MT, Falchi M, Sgadari
C and Ensoli B: The HIV protease inhibitor indinavir down-regulates
the expression of the pro-angiogenic MT1-MMP by human endothelial
cells. Angiogenesis. 7:831–838. 2014. View Article : Google Scholar
|
|
12
|
Batchu RB, Gruzdyn OV, Bryant CS, Qazi AM,
Kumar S, Chamala S, Kung ST, Sanka RS, Puttagunta US, Weaver DW and
Gruber SA: Ritonavir-mediated induction of apoptosis in pancreatic
cancer occurs via the RB/E2F-1 and AKT pathways. Pharmaceuticals
(Basel). 7:46–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kraus M, Müller-Ide H, Rückrich T, Bader
J, Overkleeft H and Driessen C: Ritonavir, nelfinavir, saquinavir
and lopinavir induce proteotoxic stress in acute myeloid leukemia
cells and sensitize them for proteasome inhibitor treatment at low
micromolar drug concentrations. Leuk Res. 38:383–392. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato A: The human immunodeficiency virus
protease inhibitor ritonavir is potentially active against
urological malignancies. Onco Targets Ther. 8:761–768. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Libra M, Scalisi A, Vella N, Clementi S,
Sorio R, Stivala F, Spandidos DA and Mazzarino C: Uterine cervical
carcinoma: Role of matrix metalloproteinases (Review). Int J Oncol.
34:897–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Talvensaari-Mattila A and
Turpeenniemi-Hujanen T: Matrix metalloproteinase 9 in the uterine
cervix during tumor progression. Int J Gynaecol Obstet. 92:83–84.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang SF, Wang PH, Lin LY, Ko JL, Chen GD,
Yang JS, Lee HS and Hsieh YS: A significant elevation of plasma
level of matrix metalloproteinase-9 in patients with high-grade
intraepithelial neoplasia and early squamous cell carcinoma of the
uterine cervix. Reprod Sci. 14:710–718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matheus ER, Zonta MA, Discacciati MG,
Paruci P, Velame F, Cardeal LB, Barros SB, Pignatari AC and
Maria-Engler SS: MMP-9 expression increases according to the grade
of squamous intraepithelial lesion in cervical smears. Diagn
Cytopathol. 42:827–833. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mathur SP, Mathur RS, Rust PF and Young
RC: Human papilloma virus (HPV)-E6/E7 and epidermal growth factor
receptor (EGF-R) protein levels in cervical cancer and cervical
intraepithelial neoplasia (CIN). Am J Reprod Immunol. 46:280–287.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsieh CY, Tsai PC, Tseng CH, Chen YL,
Chang LS and Lin SR: Inhibition of EGF/EGFR activation with
naphtho[1,2-b]furan-4,5-dione blocks migration and invasion of
MDA-MB-231 cells. Toxicol In Vitro. 27:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seitz O, Schürmann C, Pfeilschifter J,
Frank S and Sader R: Identification of the Fra-1 transcription
factor in healing skin flaps transplants: A potential role as a
negative regulator of VEGF release from keratinocytes. J
Craniomaxillofac Surg. 40:379–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Belguise K, Kersual N, Galtier F and
Chalbos D: FRA-1 expression level regulates proliferation and
invasiveness of breast cancer cells. Oncogene. 18:1434–1444. 2005.
View Article : Google Scholar
|
|
23
|
Adiseshaiah P, Vaz M, Machireddy N,
Kalvakolanu DV and Reddy SP: A Fra-1-dependent, matrix
metalloproteinase driven EGFR activation promotes human lung
epithelial cell motility and invasion. J Cell Physiol. 216:405–412.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das A, Li Q, Laws MJ, Kaya H, Bagchi MK
and Bagchi IC: Estrogen-induced expression of Fos-related antigen 1
(FRA-1) regulates uterine stromal differentiation and remodeling. J
Biol Chem. 287:19622–19630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Menges CW, Baglia LA, Lapoint R and
McCance DJ: Human papillomavirus type 16 E7 up-regulates AKT
activity through the retinoblastoma protein. Cancer Res.
66:5555–5559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spangle JM and Münger K: The human
papillomavirus type 16 E6 oncoprotein activates mTORC1 signalling
and increases protein synthesis. J Virol. 84:9398–9407. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cardeal LB, Boccardo E, Termini L,
Rabachini T, Andreoli MA, di Loreto C, Longatto Filho A, Villa LL
and Maria-Engler SS: HPV16 oncoproteins induce MMPs/RECK-TIMP-2
imbalance in primary keratinocytes: Possible implications in
cervical carcinogenesis. PLoS One. 7:e335852012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shiau MY, Fan LC, Yang SC, Tsao CH, Lee H,
Cheng YW, Lai LC and Chang YH: Human papillomavirus up-regulates
MMP-2 and MMP-9 expression and activity by inducing interleukin-8
in lung adenocarcinomas. PLoS One. 8:e544232013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stanley MA, Browne HM, Appleby M and
Minson AC: Properties of a non-tumorigenic cervical keratinocyte
cell line. Int J Cancer. 43:672–676. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pray TR and Laimins LA:
Differentiation-dependent expression of E1-E4 proteins in cell
lines maintaining episomes of human papillomavirus type 31b.
Virology. 206:679–685. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toschi E, Rota R, Antonini A, Melillo G
and Capogrossi MC: Wild-type p53 gene transfer inhibits invasion
and reduces matrix metalloproteinase-2 levels in p53-mutated human
melanoma cells. J Invest Dermatol. 114:1188–1194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barillari G, Iovane A, Bonuglia M,
Albonici L, Garofano P, Di Campli E, Falchi M, Condò I, Manzari V
and Ensoli B: Fibroblast growth factor-2 transiently activates the
p53 oncosuppressor protein in human primary vascular smooth muscle
cells: Implications for atherogenesis. Atherosclerosis.
210:400–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bertelsen BI, Steine SJ, Sandvei R, Molven
A and Laerum OD: Molecular analysis of the PI3K-AKT pathway in
uterine cervical neoplasia: Frequent PIK3CA amplification and AKT
phosphorylation. Int J Cancer. 118:1877–1883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du CX and Wang Y: Expression of P-Akt,
NFkappa B and their correlation with human papillomavirus infection
in cervical carcinoma. Eur J Gynaecol Oncol. 33:274–277.
2012.PubMed/NCBI
|
|
36
|
Xu F, Cao M, Shi Q, Chen H, Wang Y and Li
X: Integration of the full-length HPV16 genome in cervical cancer
and Caski and Siha cell lines and the possible ways of HPV
integration. Virus Genes. 50:210–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rusan M, Li YY and Hammerman PS: Genomic
landscape of human papillomavirus-associated cancers. Clin Cancer
Res. 21:2009–2019. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adler DH: The impact of HAART on
HPV-related cervical disease. Curr HIV Res. 8:493–497. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Justesen US: Protease inhibitor plasma
concentrations in HIV antiretroviral therapy. Dan Med Bull.
55:165–185. 2008.PubMed/NCBI
|