Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a unique
disease that is defined as a group of poorly differentiated
non-small cell lung cancers (NSCLCs) that demonstrate sarcoma-like
differentiation (1,2). At present, five histological subgroups
of PSC are defined: Pleomorphic carcinoma, spindle cell carcinoma,
giant cell carcinoma, carcinosarcoma and pulmonary blastoma.
Molecular biological analyses have suggested that the epithelial
and mesenchymal components in PSC arise from an identical
epithelial parental tumour cell (3–5).
Additionally, the mechanism underlying mesenchymal differentiation
in PSC is widely accepted to be epithelial-mesenchymal transition
(EMT) (6–8). However, the association between the
pathophysiology of PSC and the EMT process remains
uncharacterised.
EMT was originally defined as a process of cellular
remodelling that occurs during normal embryogenesis (9). EMT-accelerated epithelial cells lose
adhesion ability, change polarity, modulate cytoskeletal
constitution and switch expression from keratin-type to
vimentin-type intermediate filaments (10,11).
Additionally, EMT-accelerated tumour cells lose their epithelial
characteristics and acquire mesenchymal characteristics, which lead
to aggressive invasion and metastasis (12,13). A
reduction in E-cadherin expression and an increase in vimentin
expression are considered to be important hallmarks of EMT
(14,15). Zinc finger protein SNAIL
(Snail)-family proteins (Snail-1/Snail-2) are recognised as major
transcriptional repressors of the E-cadherin gene (16,17), and
in a number of malignant tumours, elevated expression of
Snail-1/Snail-2 is associated with poor patient prognosis and
resistance to antitumour chemotherapy (18–20).
Another key transcriptional regulator is homeobox
protein NANOG (NANOG), which was originally identified as a master
regulator for the maintenance of pluripotency and self-renewal
capacity in embryonic stem cells (21,22). The
name of this molecule is derived from ‘Tír na nÓg’: ‘Land of the
young’ in Irish mythology. Previously, augmentation of NANOG
expression was revealed to participate in the tumourigenesis and
the self-renewal of cancer stem cells (23). Furthermore, an increase in NANOG
expression has been illustrated to be associated with the EMT
process in lung cancer (24,25). Consequently, the expression of the
NANOG gene in PSC may be higher compared with that in
pulmonary epithelial tumours, as the EMT process in PSC is enhanced
compared with that in other histological subtypes of lung cancer.
However, to the best of our knowledge, no previous studies have
estimated the expression level of NANOG in PSC.
In the present study, patients with PSC were
retrospectively reviewed, and immunohistochemical (IHC) analyses
were performed to examine the expression of NANOG and
EMT-associated proteins from histological specimens obtained from
the patients. The clinicopathological characteristics of the
patients with PSC were compared with those of randomly selected,
sex-, age-, performance status- and clinical stage-matched patients
with pulmonary adenocarcinoma (PA) as the comparator group.
Notably, the results revealed that NANOG expression in the patients
with PSC was significantly lower compared with that in patients
with PA.
Patients and methods
Data collection
The medical records of all patients in whom NSCLC
had been histologically diagnosed between December 2006 and
December 2010 at Kansai Medical University Takii Hospital
(Moriguchi, Japan) and Hirakata Hospital (Hirakata, Japan) were
retrospectively reviewed. All patients provided informed consent
prior to acquisition of the histological samples. Histological
diagnoses were made according to the 2004 World Health
Organization/International Association for the Study of Lung Cancer
histological Classification of Lung and Pleural Tumors (2). Patients were included in the present
study if the diagnosis made was PSC. The clinical disease stage was
assigned according to the seventh edition of the
Tumor-Node-Metastasis Classification for Lung Cancer (26). Data on sex, age, smoking history,
clinical stage, histological typing of cancer, Eastern Cooperative
Oncology Group (ECOG) performance status (PS) and overall survival
(OS) were obtained retrospectively from the medical records of the
patients (27). Patients whose
histological samples were not adequate for additional analyses were
excluded. The age-, sex-, ECOG PS- and clinical stage-matched
comparator group comprised of randomly selected patients for whom
PA had been diagnosed at the Takii and Hirakata Hospitals of Kansai
Medical University (Moriguchi, Japan) during the aforementioned
period. The case-control ratio was 1:1. Patients from whom
inadequate amounts of histological samples were obtained were
excluded from the comparator group. The present study was performed
in accordance with the Declaration of Helsinki and was approved by
the Ethics Review Board of Kansai Medical University (institutional
ID no. 1203; University Hospital Medical Information
Network-Clinical Trials Registry no. UMIN000008737).
IHC
IHC was used to estimate the expression level of a
number of EMT-associated molecules, including NANOG, in each
tumour. The 7-µm-thick sections obtained from formalin-fixed and
paraffin-embedded tissues were deparaffinised in xylene and
rehydrated in a graded series of alcohol to water. Antigen
retrieval was performed using 10 mM citrate buffer (pH 6.0) at
121°C for 15 min. Sections were washed in Tris-buffered saline.
Antigen retrieval was not performed when examining the expression
of E-cadherin and vimentin. Sections were blocked with 3%
H2O2 at room temperature for 10 min and then
incubated for 1 h at room temperature with the antibodies against
E-cadherin or vimentin, and at 4°C overnight for antibodies against
other proteins. The primary antibodies and experimental conditions
used are detailed in Table I. The
sections were subsequently incubated with the ChemMate
EnVision™/horseradish peroxidase kit (K5027; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) for 45 min at room
temperature according to the manufacturer's protocol. Staining was
visualised by adding 3,3′-diaminobenzidine (K5007; Dako; Agilent
Technologies, Inc.) for 10 min at room temperature. Sections were
counterstained with haematoxylin for 1 min and then dehydrated with
a series of alcohols and xylene. Observation was then performed
with light microscopy.
 | Table I.Primary antibodies and conditions
used for immunohistochemistry. |
Table I.
Primary antibodies and conditions
used for immunohistochemistry.
| Antibody | Cat. no. | Clone | Company |
Species/clonality | Dilution,
incubation duration and temperature |
|---|
| E-cadherin | 999 | 42E | Cell
Signalinga | Rabbit/MC | 1:200, 1 h at
RT |
| Vimentin | 413541 | SP20 |
Nichireib | Rabbit/MC | 1:200, 1 h at
RT |
|
Snail-1/Snail-2 | ab63371 | N/A | Abcamc | Rabbit/PC | 1:200, O/N at
4°C |
| p-P38 MAPK | 4631 | 12F8 | Cell
Signalinga | Rabbit/MC | 1:50, O/N at
4°C |
| NANOG | 79923 | D73G4 | Cell
Signalinga | Rabbit/MC | 1:800 O/N at
4°C |
Evaluation of IHC results
The results of the IHC analysis were assessed using
a semi-quantitative scoring method in which the immunoreactive
score (IRS) was obtained, as described previously (28). Briefly, the proportions of positively
stained and intensely stained cells were determined and then
staining intensity was graded as: 0, negative; 1, weak; 2, moderate
or 3 strong. The percentage of positively stained cells was scored
as: 0, negative; 1, ≤25%; 2, >25-≤50%; 3, >50-≤75% or 4,
>75%. These two scores were multiplied to calculate the IRS,
which ranged from 0–12 as follows: 0–3, low expression or 4–12,
high expression. The levels of membrane staining for E-cadherin,
nuclear staining for Snail-1/Snail-2 and the staining for
phosphorylated p38 mitogen-activated protein kinase (p-p38 MAPK)
were estimated. To estimate the expression of vimentin and NANOG,
their cytoplasmic staining was evaluated.
Statistical analysis
Non-normally distributed data are presented as the
median and interquartile range. The Shapiro-Wilk test was used to
assess the distribution conformity of the examined parameters
featuring a normal distribution. The statistical significance of
differences between groups were determined using the χ2
or Mann-Whitney U test. OS was defined as the time from initial
diagnosis to the time of mortality from any cause or the last date
of follow-up. Univariate analyses of OS were performed using the
Kaplan-Meier estimator with the log-rank test. The 95% confidence
interval (CI) for the survival rate was calculated using
Greenwood's method (29). To
calculate the 95% CI of the median survival time (MST), the
Brookmeyer and Crowley method (30)
was used. All statistical analyses were conducted using JMP
software for Windows (version 9.0.2; SAS Institute Inc., Cary, NC,
USA). All statistical tests were two-tailed, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient clinicopathological
characteristics
Between December 2006 and December 2010,
histological diagnoses were performed on 996 patients, and PSC and
PA were diagnosed in 14 patients (1.4%) and 612 patients (61.4%),
respectively. In the present study, 12 patients with PSC for whom
adequate amounts of histological specimens were available were
enrolled. The clinicopathological characteristics of these 12
patients are summarised in Table II.
All patients were Asian, the median age was 65 years (range, 36–79
years), and included 4 females and 8 males. A total of 7 patients
had a history of smoking, while the remaining 5 had never smoked.
The numbers of patients with pleomorphic carcinoma, spindle cell
carcinoma and pulmonary blastoma were 10, 1 and 1, respectively.
The present study did not include patients with other histological
subtypes of PSC, including giant cell carcinoma and carcinosarcoma.
The ECOG PS was 0–1 in 8 patients and 2–4 in 4 patients. The
numbers of patients with stage IB, IIB, IIIA, IIIB and IV disease
were 1, 1, 1, 1 and 8, respectively. A total of 8 patients had
received initial systemic chemotherapy, whereas 3 patients had
undergone a surgical resection with curative intention. Only 1
patient had received supportive care alone.
 | Table II.Patient clinicopathological
characteristics. |
Table II.
Patient clinicopathological
characteristics.
| Clinicopathological
characteristic | PSC (n=12) | PA (n=112) | P-value |
|---|
| Median age, years
(range) | 65 (36–79) | 69.5 (60–83) | 0.1559 |
| Sex
(male/female) | 8/4 | 8/4 | 1.000 |
| ECOG PS
(0–1/2-4) | 8/4 | 8/4 | 1.000 |
| Smoking
history |
|
| 1.000 |
|
Never | 5 | 5 |
|
|
Past/Current | 7 | 7 |
|
| Clinical stage |
|
| 1.000 |
|
IA-IIIA | 3 | 3 |
|
|
IIIB-IV | 9 | 9 |
|
| Initial
treatment |
|
| 0.5890 |
|
Surgery | 3 | 3 |
|
|
Chemotherapy | 8 | 9 |
|
|
Radiotherapy | 0 | 0 |
|
|
Supportive care | 1 | 0 |
|
| EGFR mutation |
|
| 0.0061 |
| Exon 19
deletion | 0/3 | 5/9 |
|
To evaluate the clinicopathological characteristics
of the patients with PSC, a comparator group of 12 age-, sex-, ECOG
PS- and disease stage-matched patients were randomly selected from
among the patients with PA. Table II
summarises the data obtained for the two groups. Age, sex and
smoking history did not differ significantly between the two
groups. 9 of the 12 patients with PA and 3 of the 12 patients with
PSC were examined for epidermal growth factor receptor
(EGFR)-activating mutations. EGFR-activating mutations were
detected in 5 of the 9 tested patients with PA, whereas no
activating mutation was found in the 3 patients with PSC that were
tested. Consequently, EGFR-tyrosine kinase inhibitors were used to
treat the 5 detected patients with PA, and none of the patients
with PSC.
Comparison of the expression of
EMT-associated proteins between patients with PSC and patients with
PA
IHC analyses were performed to examine the
expression of various EMT-associated proteins and NANOG in the
histological specimens acquired from the patients in the two
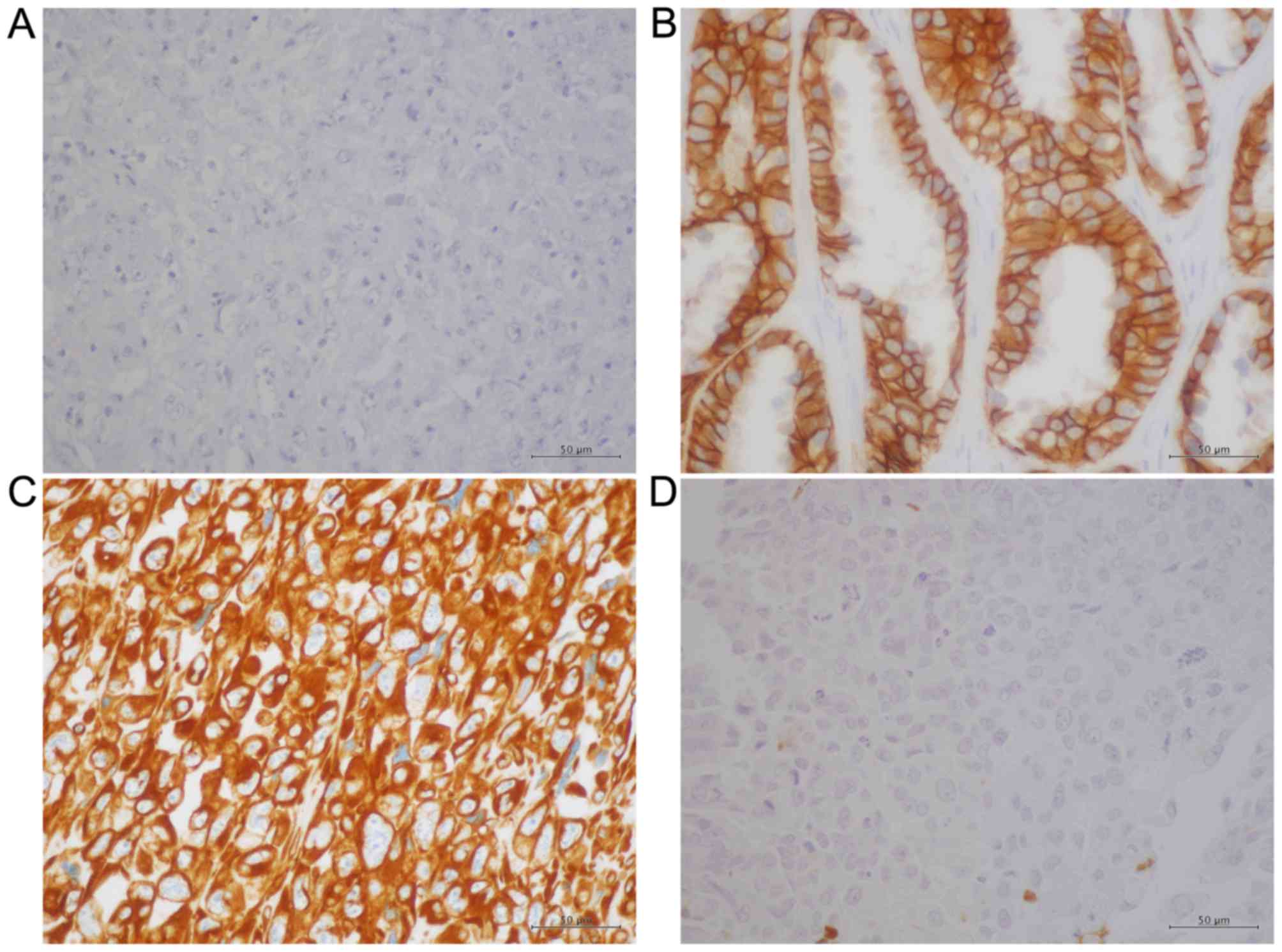
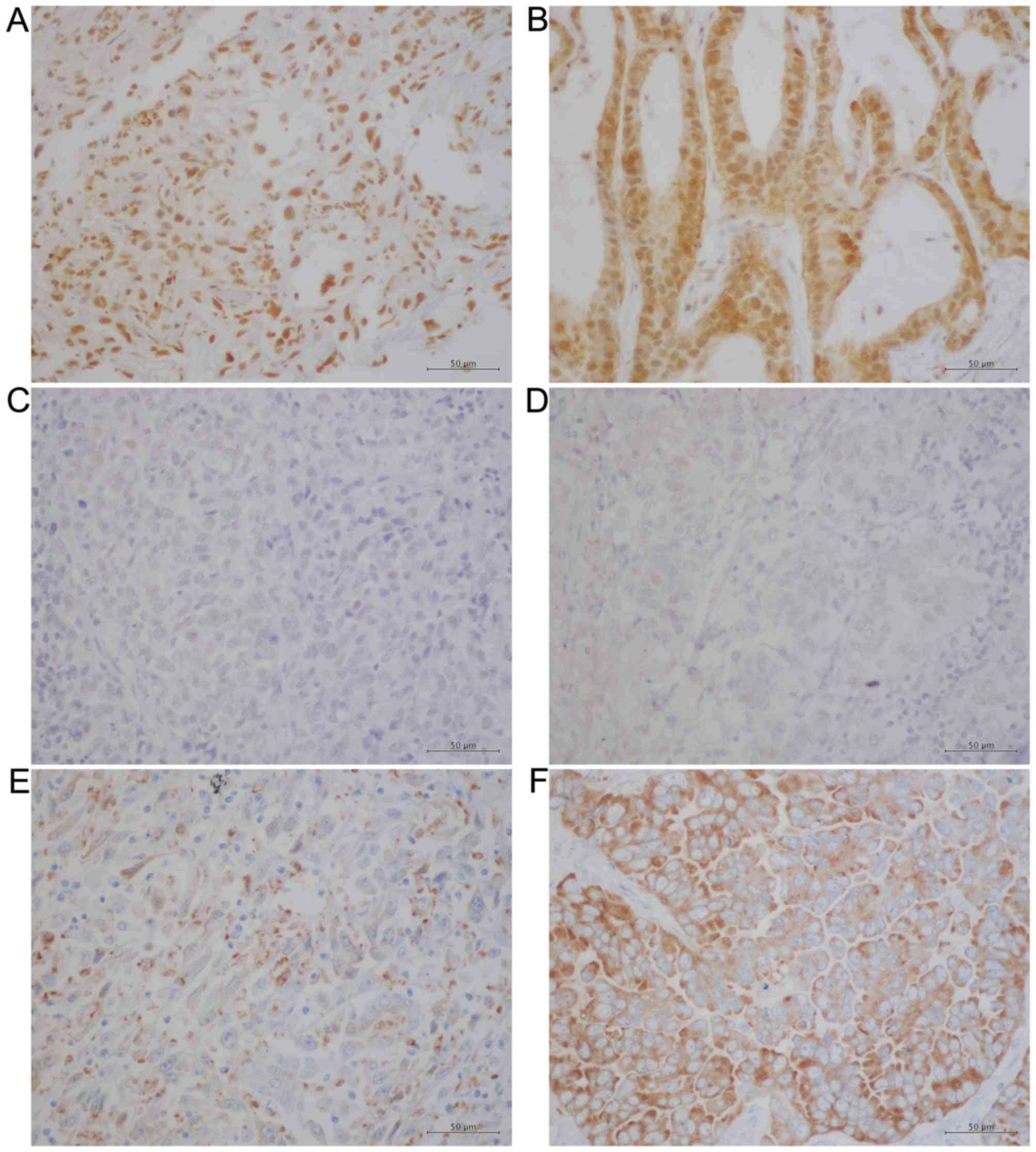
groups. Figs. 1 and 2 present representative IHC data obtained
for these proteins. Whereas E-cadherin exhibited a membranous
pattern of distribution (Fig. 1A and
1B), vimentin was detected in the cytoplasm (Fig. 1C and D). Conversely, Snail-1/Snail-2
(Fig. 2A and B) and p-p38 MAPK
(Fig. 2C and D) were observed
primarily in nucleus, which is in agreement with previous reports
(31,32), whereas NANOG was detected primarily in
the cytoplasm in PSC and PA samples (Fig.
2E and F). The IHC results were evaluated using a
semi-quantitative method using the calculation of IRS and Table III summarises these results. The IRS
of E-cadherin was significantly lower in the PSC group compared
with the PA group (P<0.0001), whereas the IRS of vimentin was
significantly higher in the PSC group compared with the PA group
(P<0.0001). These results suggest that the EMT process is
accelerated in PSC compared with PA. However, the IRS of
Snail-1/Snail-2 did not differ significantly between the two groups
(P=0.0715) and nor did that of p-p38 MAPK (P=0.3434). Finally, the
IRS of NANOG was significantly decreased in the PSC group compared
with the PA group (P<0.0001). These results indicate that NANOG
does not serve an essential role in the EMT process in PSC.
 | Table III.Results of immunohistochemical
analysis. |
Table III.
Results of immunohistochemical
analysis.
| Protein | PSC (n=12) | PA (n=12) | P-value |
|---|
| E-cadherin | 1.0 (0–3.3) | 12.0
(9.0–12.0) | <0.0001 |
| Vimentin | 12.0
(12.0–12.0) | 1.0 (1.0–1.0) | <0.0001 |
|
Snail-1/Snail-2 | 6.0 (2.0–8.0) | 10.5
(6.0–12.0) | 0.0715 |
| p-p38 MAPK | 1.5 (1.0–2.0) | 2.0 (1.0–5.0) | 0.3434 |
| NANOG | 3.4 (2.7–4.7) | 11.5
(8.0–12.0) | 0.0001 |
Univariate analyses of OS
A series of survival analyses were conducted on the
24 enrolled patients on April 30th 2015, by which 21 patients had
succumbed due to PSC or other causes, 2 had been lost to follow-up
and 1 remained alive. Consequently, the censoring rate was
estimated to be 12.5%. The MSTs were 7.0 months (95% CI, 3.2–13.3)
and 35.6 months (95% CI, 4.9–48.5) for patients with PSC and PA,
respectively (Fig. 3A). The 1-year
survival rates were 41.7% (95% CI, 13.8–69.6) and 75.0% (95% CI,
50.5–99.5) for patients with PSC and PA, respectively (Fig. 3A). Univariate analyses revealed that
OS was significantly decreased in patients with PSC (P=0.0256 vs.
PA), in patients with a poor ECOG PS (P=0.0063; 0–1 vs. 2–4) and in
patients with clinical stage IIIB or IV disease (P=0.0080 vs.
IA-IIIA) (Table IV). However, sex
(P=0.4637; male vs. female), patient age (P=0.6989; <75 vs. ≥75
years) and smoking history (P=0.1319; never vs. past/current) were
not significantly associated with OS (Table IV). The contribution of the IRS of
NANOG to OS was also analysed. The MSTs were 12.6 months (95% CI:
3.2–14.5) and 26.6 months (95% CI: 6.3- 38.7) for patients who
expressed NANOG protein at low and high levels, respectively
(Fig. 3B). Univariate analysis
revealed no significant difference between the two groups
(P=0.4416; Table IV). In addition,
the contribution to OS of the IRS of the four other proteins
examined was investigated. This identified that only a low-level
expression of vimentin was a favourable factor for OS (P=0.0348 vs.
high expression); the expression levels of E-cadherin (P=0.2166;
low vs. high expression), Snail-1/Snail-2 (P=0.7065; low vs. high
expression) and p-p38 MAPK (P=0.7400; low vs. high expression) did
not demonstrate a statistically significant effect on OS (Table IV).
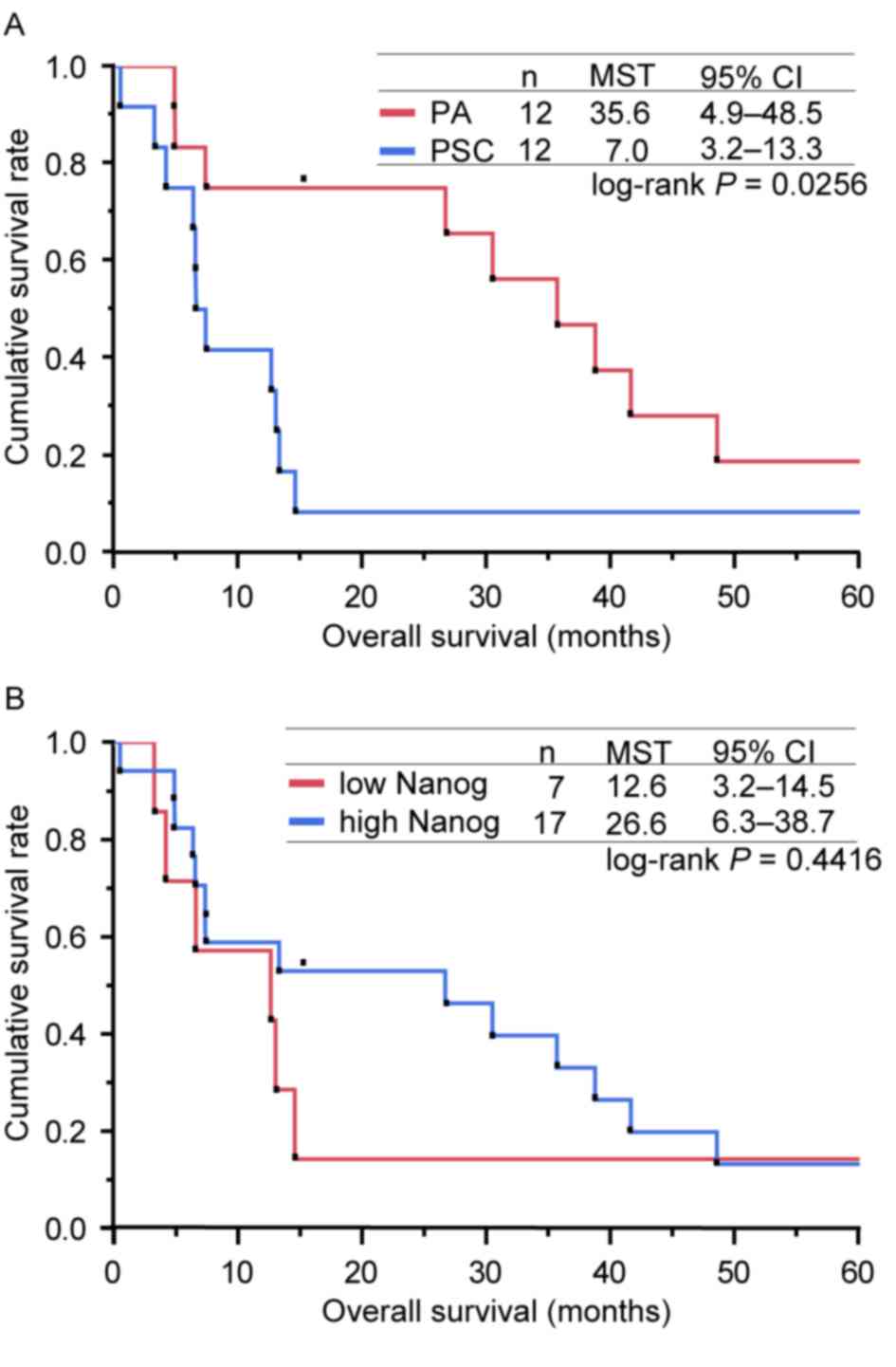 | Figure 3.Kaplan-Meier curves for the overall
survival of patients in the present study. (A) Kaplan-Meier curves
for the OS of patients according to the type of lung cancer (PA or
PSC). The MSTs determined for the patients with PSC and PA were 7.0
and 35.6 months, respectively (log-rank test, P=0.0256). (B)
Kaplan-Meier curves for OS of patients, presented according to the
expression level of NANOG (low or high). The MSTs calculated for
the patients who expressed NANOG at low and high levels were 12.6
and 26.6 months, respectively (log-rank test, P=0.4416). OS,
overall survival; PSC, pulmonary sarcomatoid carcinoma; PA,
pulmonary adenocarcinoma; MSTs, median survival times; CI,
confidence interval; NANOG, homeobox protein NANOG. |
 | Table IV.Results of univariate analysis of
overall survival. |
Table IV.
Results of univariate analysis of
overall survival.
| Variable | Median survival
time (months) | P-value |
|---|
| Histology: PSC vs.
PA | 7.0 vs. 35.6 | 0.0256 |
| ECOG PS: 0–1 vs.
2–4 | 26.6 vs. 6.1 | 0.0063 |
| Sex: male vs.
female | 13.1 vs. 13.6 | 0.4637 |
| Age: <75 vs.
≥75 | 13.1 vs. 21.5 | 0.6989 |
| Smoking status:
never vs. past/current | 10.0 vs. 20.0 | 0.1319 |
| Clinical stage:
IA-IIIA vs. IIIB-IV | 59.8 vs. 10.0 | 0.0080 |
| E-cadherin: low vs.
high | 12.6 vs. 30.4 | 0.2166 |
| Vimentin: low vs.
high | 38.7 vs. 7.3 | 0.0348 |
| Snail-1/Snail-2:
low vs. high | 6.6 vs. 13.3 | 0.7065 |
| p-p38 MAPK: low vs.
high | 13.0 vs. 13.3 | 0.7400 |
| NANOG: low vs.
high | 12.6 vs. 26.6 | 0.4416 |
Discussion
NANOG expression has been widely examined in tumour
tissues obtained from patients with lung cancer (33,34), and
the results have revealed that NANOG expression does not differ
substantially between the histological subtypes of lung cancer,
including adenocarcinoma, squamous cell carcinoma, large cell
carcinoma, bronchoalveolar carcinoma and small cell carcinoma.
However, to the best of our knowledge, this is the first report
describing the expression of NANOG in PSC.
The correlation between NANOG expression and the EMT
process has been discussed extensively. Meng et al (28) revealed that NANOG protein expression
was induced in response to tumour growth factor (TGF)-β stimulation
of a colorectal cancer cell line in a Snail-dependent manner. Chiou
et al (23) demonstrated that
the co-expression of NANOG and octamer-binding transcription factor
4 promoted EMT, indicated by a reduction in E-cadherin expression
and an increase in vimentin expression, in the pulmonary
adenocarcinoma A549 cell line. Additionally, Luo et al
(35) demonstrated that the
expression of NANOG was positively correlated with that of Snail,
but negatively correlated with that of E-cadherin in nasopharyngeal
carcinoma. The results of the present study indicate that the EMT
process in PSC is accelerated compared with that in PA. However,
the current results also revealed that NANOG expression in PSC was
significantly lower compared with that in PA. In addition, no
statistically significant difference was observed between the two
groups in terms of the expression of Snail-1/Snail-2 and p-p38
MAPK, which are major signal transducing molecules in EMT. A
previous study provides one possible explanation for the reduced
expression of NANOG observed in PSC: Chen et al (36) assessed the expression of NANOG in
primary tumours at the frontline between tumour and normal tissue,
and in malignant pleural effusions of PA, demonstrating that a
situation-dependent up-regulation of the NANOG gene occurred
at the frontline of tumour progression compared with quiescent
areas. According to the hypothesis of Chen et al (36), quiescent tumour cells obtained from
PSC may express only small amounts of NANOG. Thus, in the present
study, transient NANOG expression in PSC may not have been detected
with the IHC method. However, quiescent and NANOG-negative tumour
cells constitutively demonstrated mesenchymal characteristics,
indicating an acceleration of the EMT process. These discrepancies
suggest that NANOG does not serve an essential role in PSC EMT.
Numerous signalling pathways are known to promote EMT, including
the mothers against decapentaplegic homolog-dependent/independent
TGF-β, phosphoinositide 3-kinase/protein kinase B, Wnt/glycogen
synthase kinase 3β and the extracellular signal-regulated
kinase/p38 MAPK signalling pathways (37–41). One
or more of these signalling pathways may be involved in the EMT
process in PSC, rather than NANOG.
NANOG contains conserved homeodomain motifs, which
indicates that the protein functions as a transcription factor
(21). To facilitate the
transcription of a target gene, a transcription factor must
localise in the nucleus and bind to the promoter region of the
target gene in vivo. The NANOG gene product contains
a nuclear localisation signal and a nuclear export signal (42,43),
suggesting that a molecular shuttling of NANOG between the
intra-nuclear space and the cytoplasmic space occurs. The
subcellular localisation of NANOG various types of human malignant
tumours has been demonstrated, and, notably, the following three
independent types of NANOG localisation have been described: In the
nucleus, as illustrated in germ cell tumours (44); in the nucleus and the cytoplasm, as
identified in prostate and breast cancer (44,45) and
primarily in the cytoplasm, as demonstrated in colorectal and
ovarian cancer (28,46). However, the subcellular localisation
of NANOG in lung cancer remains debatable (23,25,33,34).
The results of the present study demonstrated that NANOG was
localised primarily in the cytoplasm in PSC and PA, with a small
proportion of nuclear staining. Gu et al (47) suggested a possible role for
cytoplasmic NANOG by demonstrating that cytoplasmic NANOG-positive
tumour stromal cells promoted the proliferation and tumourigenesis
of human cervical cancer cells. Cytoplasmic NANOG was previously
identified to be an independent unfavourable prognostic factor for
OS in patients with colorectal cancer (28). A previous study also revealed the
unfavourable prognostic effects of the overexpression of
cytoplasmic NANOG in patients with lung cancer (33). However, the results of the present
study did not reveal a statistically significant unfavourable
effect of cytoplasmic NANOG on the survival of the patients
examined. One possible explanation for this discrepancy is that an
unfavourable survival effect of mesenchymal differentiation through
EMT overcomes the effect of NANOG overexpression in lung
cancer.
In conclusion, despite the small sample size used in
the present study, the data clearly demonstrated that cytoplasmic
NANOG expression was significantly lower in PSC compared with PA,
and that the EMT process in PSC was accelerated compared with that
in PA. Further investigations are warranted to clarify the
biological role of NANOG in lung cancer.
Acknowledgements
The authors would like to thank Editage (www.editage.jp) for their English language
editing.
Glossary
Abbreviations
Abbreviations:
|
PSC
|
pulmonary sarcomatoid carcinoma
|
|
PA
|
pulmonary adenocarcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
OS
|
overall survival
|
|
IRS
|
immunoreactive score
|
References
|
1
|
Pelosi G, Sonzogni A, De Pas T, Galetta D,
Veronesi G, Spaggiari L, Manzotti M, Fumagalli C, Bresaola E and
Nappi O: Review article: Pulmonary sarcomatoid carcinomas: A
practical overview. Int J Surg Pathol. 18:103–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: World Health Organization Classification of
TumoursPathology and genetics of tumours of the lung, pleura,
thymus and heart. IARC Press; Lyon: pp. 53–58. 2004
|
|
3
|
Dacic S, Finkelstein SD, Sasatomi E,
Swalsky PA and Yousem SA: Molecular pathogenesis of pulmonary
carcinosarcoma as determined by microdissection-based allelotyping.
Am J Surg Pathol. 26:510–516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holst VA, Finkelstein S, Colby TV, Myers
JL and Yousem SA: p53 and K-ras mutational genotyping in pulmonary
carcinosarcoma, spindle cell carcinoma, and pulmonary blastoma:
Implications for histogenesis. Am J Surg Pathol. 21:801–811. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sekine S, Shibata T, Matsuno Y, Maeshima
A, Ishii G, Sakamoto M and Hirohashi S: Beta-Catenin mutations in
pulmonary blastomas: Association with morule formation. J Pathol.
200:214–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blaukovitsch M, Halbwedl I, Kothmaier H,
Gogg-Kammerer M and Popper HH: Sarcomatoid carcinomas of the
lung-are these histogenetically heterogeneous tumors? Virchows
Arch. 449:455–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakatani Y, Miyagi Y, Takemura T, Oka T,
Yokoi T, Takagi M, Yokoyama S, Kashima K, Hara K, Yamada T, et al:
Aberrant nuclear/cytoplasmic localization and gene mutation of
beta-catenin in classic pulmonary blastoma: Beta-catenin
immunostaining is useful for distinguishing between classic
pulmonary blastoma and a blastomatoid variant of carcinosarcoma. Am
J Surg Pathol. 28:921–927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haraguchi S, Fukuda Y, Sugisaki Y and
Yamanaka N: Pulmonary carcinosarcoma: Immunohistochemical and
ultrastructural studies. Pathol Int. 49:903–908. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hay ED: Organization and fine structure of
epithelium and mesenchyme in the developing chick
embryoEpithelial-mesenchymal interactions. Fleischmajer R and
Billingham RE: Williams & Wilkins; Baltimore, Maryland, USA:
pp. 31–55. 1968
|
|
10
|
Greenburg G and Hay ED: Epithelia
suspended in collagen gels can lose polarity and express
characteristics of migrating mesenchymal cells. J Cell Biol.
95:333–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miettinen PJ, Ebner R, Lopez AR and
Derynck R: TGF-beta induced transdifferentiation of mammary
epithelial cells to mesenchymal cells: Involvement of type I
receptors. J Cell Biol. 127:2021–2036. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sommers CL, Thompson EW, Torri JA, Kemler
R, Gelmann EP and Byers SW: Cell adhesion molecule uvomorulin
expression in human breast cancer cell lines: Relationship to
morphology and invasive capacities. Cell Growth Differ. 2:365–372.
1991.PubMed/NCBI
|
|
13
|
Oka H, Shiozaki H, Kobayashi K, Inoue M,
Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S,
Takeichi M, et al: Expression of E-cadherin cell adhesion molecules
in human breast cancer tissues and its relationship to metastasis.
Cancer Res. 53:1696–1701. 1993.PubMed/NCBI
|
|
14
|
Burdsal CA, Damsky CH and Pedersen RA: The
role of E-cadherin and integrins in mesoderm differentiation and
migration at the mammalian primitive streak. Development.
118:829–844. 1993.PubMed/NCBI
|
|
15
|
Thompson EW, Torri J, Sabol M, Sommers CL,
Byers S, Valverius EM, Martin GR, Lippman ME, Stampfer MR and
Dickson RB: Oncogene-induced basement membrane invasiveness in
human mammary epithelial cells. Clin Exp Metastasis. 12:181–194.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blechschmidt K, Sassen S, Schmalfeldt B,
Schuster T, Höfler H and Becker KF: The E-cadherin repressor Snail
is associated with lower overall survival of ovarian cancer
patients. Br J Cancer. 98:489–495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shih JY, Tsai MF, Chang TH, Chang YL, Yuan
A, Yu CJ, Lin SB, Liou GY, Lee ML, Chen JJ, et al: Transcription
repressor slug promotes carcinoma invasion and predicts outcome of
patients with lung adenocarcinoma. Clin Cancer Res. 11:8070–8078.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haslehurst AM, Koti M, Dharsee M, Nuin P,
Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, et al: EMT
transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chambers I, Colby D, Robertson M, Nichols
J, Lee S, Tweedie S and Smith A: Functional expression cloning of
Nanog, a pluripotency sustaining factor in embryonic stem cells.
Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mitsui K, Tokuzawa Y, Itoh H, Segawa K,
Murakami M, Takahashi K, Maruyama M, Maeda M and Yamanaka S: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS and Wu CW:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelial-mesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XQ, Yang XL, Zhang G, Wu SP, Deng XB,
Xiao SJ, Liu QZ, Yao KT and Xiao GH: Nuclear β-catenin accumulation
is associated with increased expression of Nanog protein and
predicts poor prognosis of non-small cell lung cancer. J Transl
Med. 11:1142013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pirozzi G, Tirino V, Camerlingo R, Franco
R, La Rocca A, Liguori E, Martucci N, Paino F, Normanno N and Rocco
G: Epithelial to mesenchymal transition by TGFβ-1 induction
increases stemness characteristics in primary non small cell lung
cancer cell line. PLoS One. 6:e215482011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC lung cancer staging project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meng HM, Zheng P, Wang XY, Liu C, Sui HM,
Wu SJ, Zhou J, Ding YQ and Li J: Over-expression of Nanog predicts
tumour progression and poor prognosis in colorectal cancer. Cancer
Biol Ther. 9:295–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Greenwood M: The natural duration of
cancerReports on Public Health and Medical Subjects. No. 33. Her
Majesty's Stationery Office; London: pp. 1–26. 1926
|
|
30
|
Brookmeyer R and Crowley J: A confidence
interval for the median survival time. Biometrics. 38:29–41. 1982.
View Article : Google Scholar
|
|
31
|
Kim SH, Kim JM, Shin MH, Kim CW, Huang SM,
Kang DW, Suh KS, Yi ES and Kim KH: Correlation of
epithelial-mesenchymal transition markers with clinicopathologic
parameters in adenocarcinomas and squamous cell carcinoma of the
lung. Histol Histopathol. 27:581–591. 2012.PubMed/NCBI
|
|
32
|
Vicent S, Garayoa M, López-Picazo JM,
Lozano MD, Toledo G, Thunnissen FB, Manzano RG and Montuenga LM:
Mitogen-activated protein kinase phosphatase-1 is overexpressed in
non-small cell lung cancer and is an independent predictor of
outcome in patients. Clin Cancer Res. 10:3639–3649. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Du Y, Ma C, Wang Z, Liu Z, Liu H and Wang
T: Nanog, a novel prognostic marker for lung cancer. Surg Oncol.
22:224–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gialmanidis IP, Bravou V, Petrou I, Kourea
H, Mathioudakis A, Lilis I and Papadaki H: Expression of Bmi1,
FoxF1, Nanog, and γ-catenin in relation to hedgehog signaling
pathway in human non-small-cell lung cancer. Lung. 191:511–521.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo W, Li S, Peng B, Ye Y, Deng X and Yao
K: Embryonic stem cells markers SOX2, OCT4 and Nanog expression and
their correlations with epithelial-mesenchymal transition in
nasopharyngeal carcinoma. PLoS One. 8:e563242013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen SF, Lin YS, Jao SW, Chang YC, Liu CL,
Lin YJ and Nieh S: Pulmonary adenocarcinoma in malignant pleural
effusion enriches cancer stem cell properties during metastatic
cascade. PLoS One. 8:e546592013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Piek E, Moustakas A, Kurisaki A, Heldin CH
and ten Dijke P: TGF-(beta) type I receptor/ALK-5 and Smad proteins
mediate epithelial to mesenchymal transdifferentiation in NMuMG
breast epithelial cells. J Cell Sci. 112:4557–4568. 1999.PubMed/NCBI
|
|
38
|
Bhowmick NA, Ghiassi M, Bakin A, Aakre M,
Lundquist CA, Engel ME, Arteaga CL and Moses HL: Transforming
growth factor-beta1 mediates epithelial to mesenchymal
transdifferentiation through a RhoA-dependent mechanism. Mol Biol
Cell. 12:27–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim K, Lu Z and Hay ED: Direct evidence
for a role of beta-catenin/LEF-1 signaling pathway in induction of
EMT. Cell Biol Int. 26:463–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bhowmick NA, Zent R, Ghiassi M, McDonnell
M and Moses HL: Integrin beta 1 signaling is necessary for
transforming growth factor-beta activation of p38MAPK and
epithelial plasticity. J Biol Chem. 276:46707–46713. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Do HJ, Lim HY and Kim JH, Song H, Chung HM
and Kim JH: An intact homeobox domain is required for complete
nuclear localization of human Nanog. Biochem Biophys Res Commun.
353:770–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang DF, Tsai SC, Wang XC, Xia P,
Senadheera D and Lutzko C: Molecular characterization of the human
NANOG protein. Stem Cells. 27:812–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ezeh UI, Turek PJ, Reijo RA and Clark AT:
Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are
expressed in both seminoma and breast carcinoma. Cancer.
104:2255–2265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miyazawa K, Tanaka T, Nakai D, Morita N
and Suzuki K: Immunohistochemical expression of four different stem
cell markers in prostate cancer: High expression of NANOG in
conjunction with hypoxia-inducible factor-1α expression is involved
in prostate epithelial malignancy. Oncol Lett. 8:985–992.
2014.PubMed/NCBI
|
|
46
|
Pan Y, Jiao J, Zhou C, Cheng Q, Hu Y and
Chen H: Nanog is highly expressed in ovarian serous
cystadenocarcinoma and correlated with clinical stage and
pathological grade. Pathobiology. 77:283–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gu TT, Liu SY and Zheng PS: Cytoplasmic
NANOG-positive stromal cells promote human cervical cancer
progression. Am J Pathol. 181:652–661. 2012. View Article : Google Scholar : PubMed/NCBI
|