Introduction
Colon cancer is ranked as the third most common
malignancy of the gastrointestinal system (1–3). At
present, the comprehensive treatment based on surgery is the widely
accepted treatment option in colon cancer therapeutics.
Chemotherapy is an important part of colon cancer treatment, which
has been demonstrated to be effective for metastasis control.
However, the anticancer effects of these therapies, including
fluorouracil (5-FU), which is regarded as the first-line drug for
colon cancer chemotherapy, remains limited due to poor efficacy and
significant side effects (1–3). Therefore, it is necessary to investigate
novel bioactive molecules for the comprehensive treatment of colon
cancer.
Pulsatilla chinensis regel is a traditional
Chinese herb known to exhibit anti-inflammatory properties and may
be used in various infectious diseases, such as malaria, intestinal
amebiasis and bacterial infections (4,5). To date,
at least 15 saponin derivatives had been found in P
chinensis extracts (5), several
of which have been reported to exhibit antitumor activities. For
example, Pulsatilla saponin A (PsA), one of the P
chinensis extracts, has been reported to demonstrate an
antitumor effect by inducing DNA damage and apoptosis, and causing
G2 arrest in hepatocellular carcinoma (HCC) cells (6).
In the present study, the anti-colon cancer
activities of PsA or PsA combined with 5-FU were determined in cell
culture and xenograft mouse models. The possible mechanisms of
action were examined using western blot assays.
Materials and methods
Cell culture
The human colon cancer HT-29 cell line was purchased
from the Cellular Biological Institute of the Science Academy of
China in Shanghai (Shanghai, China), and cultured in McCoy's 5A
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10%
fetal bovine serum, 100 mg/ml streptomycin and 100 IU/ml
penicillin. All cells were cultured in a 5% CO2
humidified atmosphere at 37°C.
Cell proliferation assay
Cell proliferation was measured in 96-well plates
using the Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Kumamoto, Japan). Subsequent to incubation at 37°C in
corresponding drugs with a certain concentration (5 ng/µl PsA, 5
ng/µl 5-FU or 2.5 ng/µl 5-FU + 2.5 ng/µl PsA) for a 0, 1, 3 or 5
days all the cells were cultured with 10 ml CCK-8 solution at 37°C
for 2 h. Optical absorbance was monitored in a plate reader at a
450-nm wavelength. In these experiments, each data point was
assayed in triplicate. Each experiment was performed at least three
times as described previously (7).
Tumor xenograft study
To establish the human colon cancer mouse xenograft
models, 20 six-week-old male athymic BALB/c mice (18–20
g/each) were fed in center animals rooms of the Second Affiliated
Hospital of Soochow University (temperature, 18–22°C; humidity
50–60%) and injected subcutaneously with 5×107 HT-29
cells on the right dorsal flank to initiate tumor growth.
Subsequent to tumor volumes reaching 50–100 mm3 at ~2
weeks, the mice were randomly divided into four groups for
additional treatment. The control group mice were administered
purified saline, PsA group were injected with 10 mg/kg PsA, the
5-FU group was provided 20 mg/kg 5-FU and a combination of 5-FU and
PsA was administered to the 5-FU + PsA group. The injections were
performed intraperitoneally 3 times/week for 3 weeks. Body weight
and tumor size in the mice were recorded every 3 days. Tumor size
(in the living animal) was measured using a slide gauge. Tumor
volume was calculated using the following equation: Volume
(v)=tumor mass length × width2/2 (6). The present study was approved by the
ethic committees at the Second Affiliated Hospital of Soochow
University hospital and was conducted in accordance with the
Declaration of Helsinki. All experiments were conducted in
accordance with Animal Ethical Care (3).
Assessment of apoptosis by annexin V
staining and terminal deoxynucleotidyl transferase 2′-deoxyuridine
5′-triphosphate nick end labeling (TUNEL) assay
Subsequent to drug treatment as aforementioned, for
3 h, the cells were washed with 1X PBS, harvested and resuspended
in 100 ml staining solution containing annexin V fluorescein and
propidium iodine in a
N-2-Hydroxyethylpiperazine-N'-2-ethanesulfonic acid buffer;
Annexin-V FLUOS staining kit (Boehringer-Mannheim, China).
Subsequent to incubation at room temperature for 15 min, cells were
analyzed by flow cytometry (7). The
cells were treated for 8–12 h, then the apoptotic tumor cells were
observed by fluorescence microscope using the TUNEL assay kits
(Shanghai XiTang Biotechnology Corp., Shanghai, China). Apoptotic
cells were stained with green fluorescence (3).
Western blot assay
Subsequent to treatment at 37°C for 24–48 h, the
cells were collected and lysed in a buffer containing 50 mmol/l pH
8.0 Tris-HCl, 150 mmol/l NaCl, 1% (v/v) Triton X-100, and a
protease inhibitor mixture [4-(2-aminoethyl) benzenesulfonyl
fluoride hydrochloride, aprotinin, bestatin, EDTA, E-64 and
leupeptin; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; dilution,
1:100]. Protein samples were analyzed by 10 or 12% SDS-PAGE and
western blotting using antibodies against B-cell lymphoma 2
(catalog no. o.3033; dilution 1:1,500; BioVision, Inc., Milpitas,
CA, USA), tumor protein p53 (catalog no., 2524S; dilution, 1:1,000)
or cleaved caspase 9 (grant no. 9505T; dilution, 1:1,500; Cell
Signaling Technology, Inc., Danvers, MA, USA). Nitrocellulose
membranes were developed using electrochemiluminescent reagents
(Denville Scientific, South Plainfield, NJ, USA) and exposed to
X-ray films as described previously (8).
Statistical analysis
All data were presented as the mean ± standard
deviation, and all experiments were repeated at least three times.
Statistical analysis was conducted with an unpaired Student t-test
using SPSS 15 software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
PsA inhibited cancer cell
proliferation
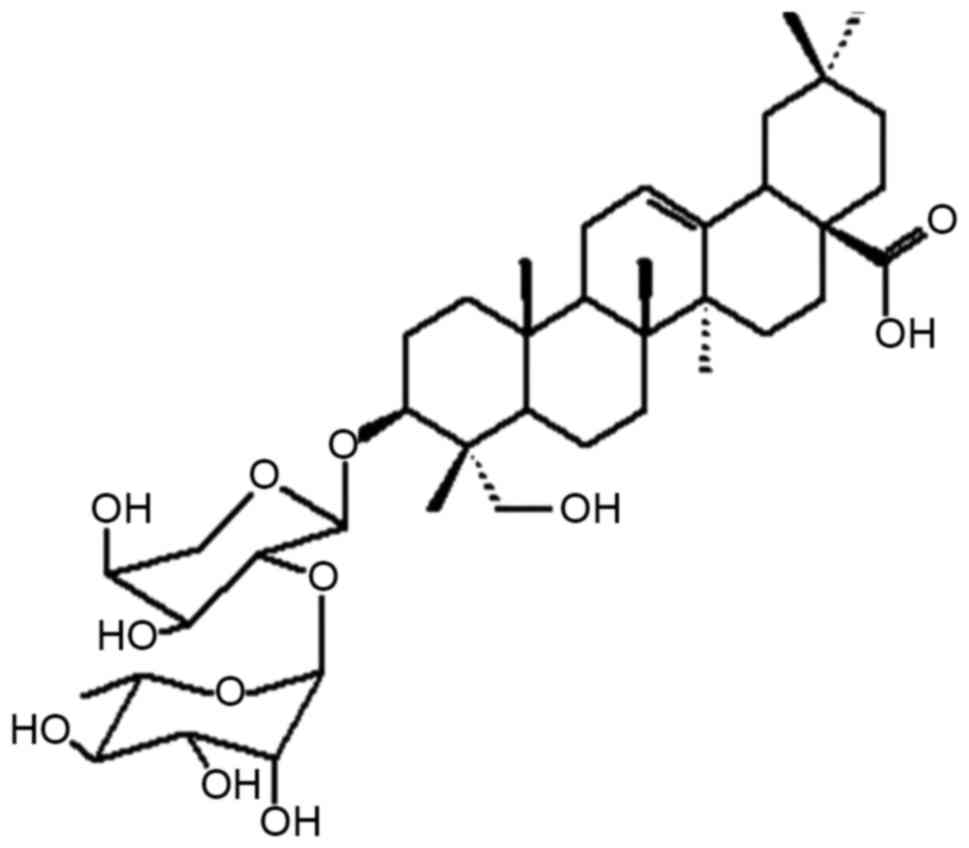
The molecule structure of PsA is illustrated in
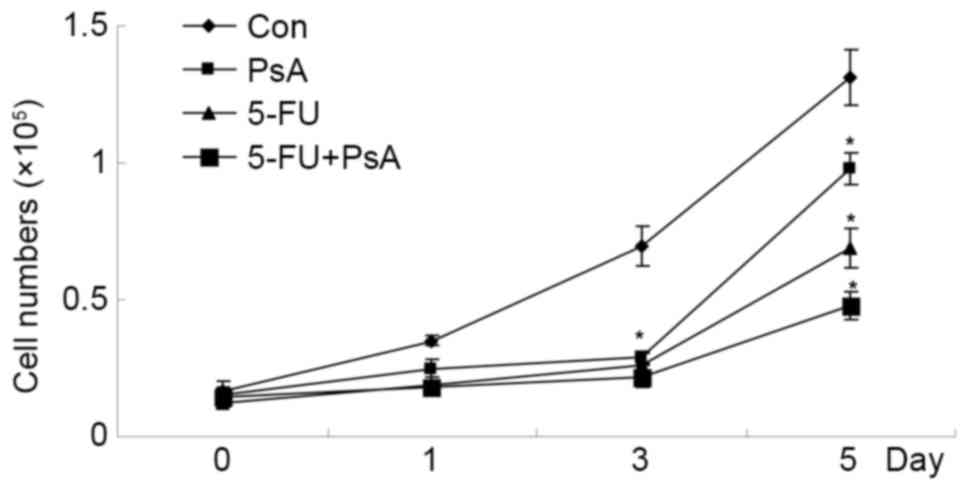
Fig. 1. Subsequent to incubation with
5 ng/µl PsA, 5 ng/µl 5-FU or 2.5 ng/µl 5-FU + 2.5 ng/µl PsA, the
viability of the HT-29 cells was measured using a CCK-8 assay. In
the presence of PsA, 5-FU, or PsA + 5-FU, the proliferation rates
were inhibited significantly compared with the PBS-treated control
cells (P<0.05). The combination of PsA and 5-FU demonstrated a
synergic inhibition on HT-29 cells growth (P<0.05). The results
indicate that PsA exhibited an antiproliferative effect on cultured
colon cancer cells, in isolation or combined with 5-FU (Fig. 2).
PsA inhibited tumor growth in mouse
xenograft models
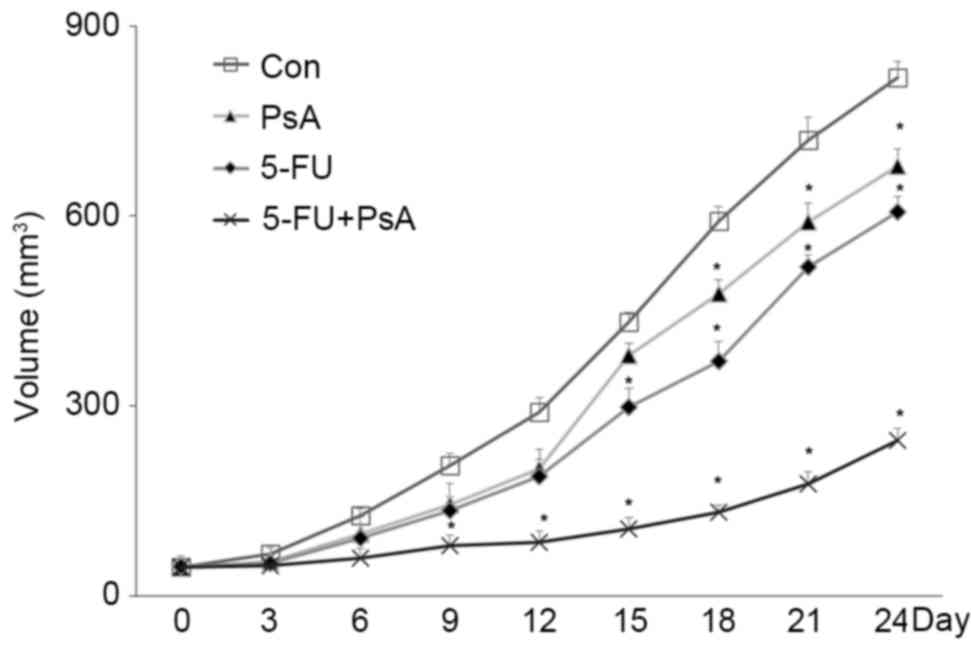
To examine whether PsA exhibited antitumor growth
activity in vivo, xenograft tumor models were established in
athymic BALB/c mice using HT-29 cells. The mice were then treated
with PsA, 5-FU, PsA + 5-FU or a vehicle. As demonstrated in
Fig. 3, PsA and 5-FU markedly
inhibited HT-29 derived tumor growth in mice compared with the
vehicle treated control, as indicated by smaller tumor volumes
(P<0.05). Additionally, the tumors grew more slowly subsequent
to the combined application of PsA and 5-FU, compared with each
treatment alone (P<0.05). These data indicate that PsA exhibited
inhibitory activities against human colon tumor in vivo, and
that PsA and 5-FU possessed synergic antitumor effects in
vivo.
PsA and/or 5-FU induced apoptosis in
colon cancer cells
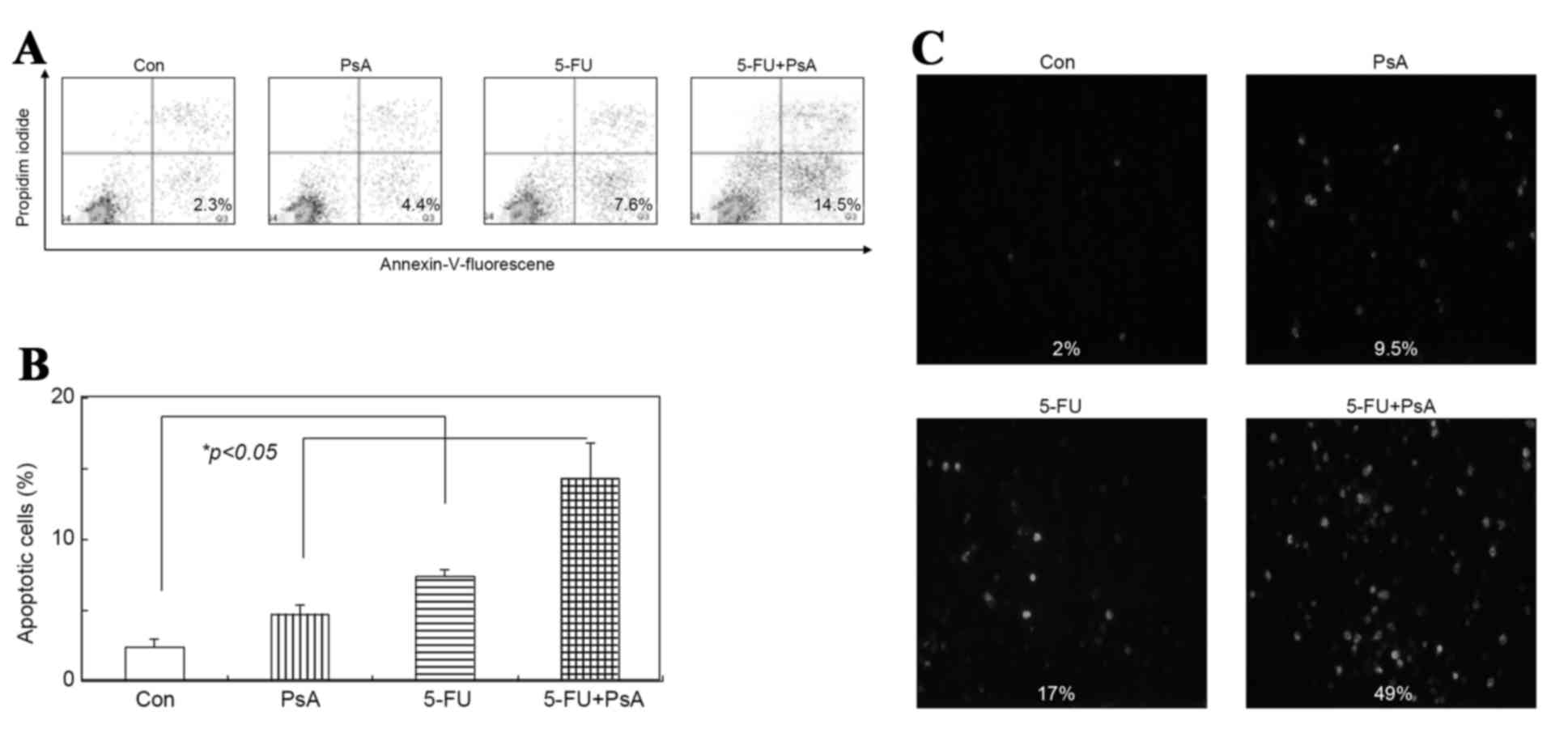
To examine whether PsA induces apoptosis in cancer
cells, the annexin V expression in HT-29 cells was analyzed. The
cells were incubated with 5 ng/µl PsA, 5 ng/µl 5-FU or 2.5 ng/µl
5-FU + 2.5 ng/µl PsA for 3 h and analyzed by flow cytometry using
an anti-annexin V antibody. As illustrated in Fig. 4A and B, 4.4, 7.6 and 14.5% of the
cells were positive for annexin V subsequent to treatment with PsA,
5-FU and 5-FU+PsA, respectively, however only 2.3% of the cells
were annexin V positive in the vehicle-treated group
(P<0.05).
The TUNEL assays demonstrated that the number of
apoptotic cells markedly increased in the treated cells compared
with the control group; the apoptotic rate was 9.5, 17 and 49% in
the PsA group, 5-FU group and 5-FU+PsA group, respectively, while
only 2% in the control group (P<0.05), as demonstrated in
Fig. 4C. These data suggest that PsA
may induce apoptosis in HT-29 cells, and the combined treatment
with PsA and 5-FU increased the rate of apoptosis in HT-29 cells
compared with these drugs in isolation.
PsA affected the p53, Bcl-2, and
caspase 9 protein expression in HT-29 cells
The incidence and mechanisms of action of PsA on the
expression of proteins involved in apoptosis were examined. HT-29
cells were treated with 5 ng/µl PsA, 5 ng/µl 5-FU or 2.5 ng/µl 5-FU
+ 2.5 ng/µl PsA at 37°C for between 24 and 48 h. Subsequent to cell
lysis, the proteins in the cell lysate were analyzed by SDS-PAGE
and western blotting. As demonstrated in Fig. 5, BCL-2 protein expression levels were
reduced in the PsA-treated cells, whilst the cleaved caspase 9 and
p53 protein expression levels were increased in these cells.
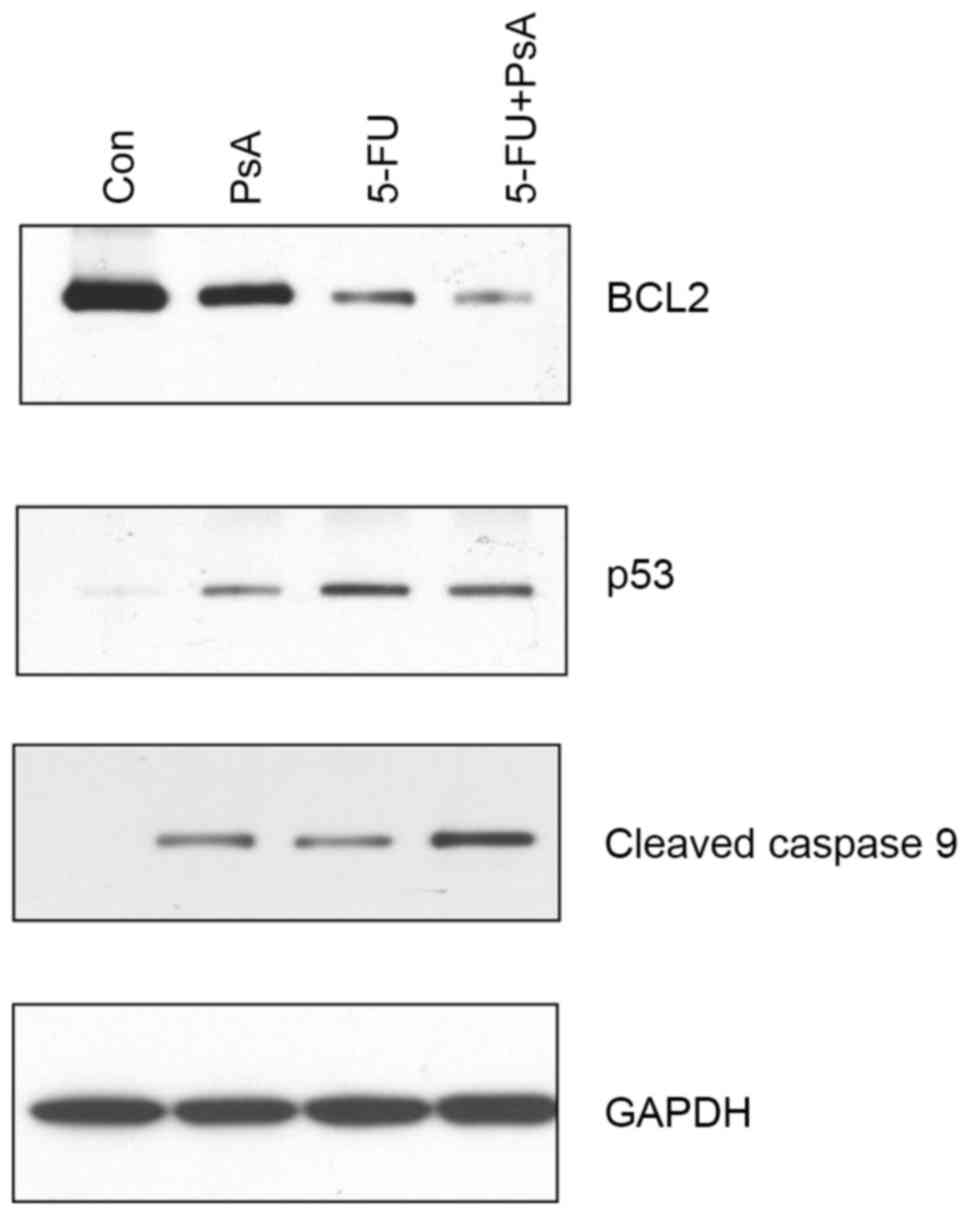 | Figure 5.PsA affected the p53, BCL-2, and
caspase 9 protein expression levels. HT-29 cells were treated with
PsA, 5-FU, 5-FU+PsA at 37°C for 24 h. The cells were lysed, and
protein samples were analyzed by western blot assay for BCL-2 (top
panel), p53 (second panel), and cleaved caspase 9 (third panel)
protein expression levels. The level of GAPDH protein expression
was used as a sample loading control (bottom panel). PsA,
Pulsatilla saponin A; 5-FU, fluorouracil; Con, control;
BCL-2, B-cell lymphoma 2, p53, tumor protein 53. |
Discussion
As one of the most common clinical chemotherapy
drugs for gastrointestinal tumors, 5-FU exerts antitumor effects
through transforming into corresponding nucleotides (3), and by inducing cancer cell apoptosis.
Hypothetically, 5-FU may cause DNA deterioration, thus accelerating
the rates of apoptosis in cancer cells (9,10).
However, the effective rate of inducing cancer cell apoptosis for
5-FU was only ~30% with respect to colon cancer clinical
chemotherapy. The severe levels of toxicity and side-effects, and
emerging drug resistance, have limited the dosage and therapeutic
applications of 5-FU (1–3). Therefore, the investigation of novel
therapeutic strategies, which may improve the effect and reduce the
possibility of adverse reactions of 5-FU, is required.
PsA is an active compound extracted from P
chinensis, similar to several saponin derivatives, which also
exhibits antitumor activities via inducing DNA damage, G2 arrest
and apoptosis among HCC and pancreatic cancer cells. However, the
effects of PsA on colon cancer cells has not been fully
characterized, particularly when administered in combination with
5-FU.
In cell line experiments, PsA in isolation
significantly inhibited the proliferation of human colon cancer
HT-29 cell line, similar to 5-FU. In addition, the combined
treatment with 5-FU and PsA enhanced the inhibitory effects on
HT-29 cells growth. The antitumor activity of PsA was also
confirmed in vivo, as in a human colon cancer xenograft
mouse model, PsA elicited inhibitory effects on tumor growth in
isolation and PsA+5-FU treatment exhibited marked synergic
antitumor effects in vivo. Using a TUNEL assay and flow
cytometric analysis, apoptotic cells were detected in the PsA and
PsA+5-FU-treated HT-29 cells. Notably, a larger number of apoptotic
cells were identified the PsA+5-FU-treated group. These data
indicate one of the possible mechanisms through which PsA and 5-FU
induce the synergic anticancer effects.
To elucidate the possible mechanisms underlying
PsA-mediated tumor inhibition, western blot analyses were
performed. It was revealed that p53 and cyclin B protein expression
levels were increased in PsA-treated cells, whereas BCL-2 protein
expression levels were decreased. All these data were in agreement
with the previous findings that PsA may induce apoptosis. Unlike
PsA, which induces DNA damage and cell apoptosis, 5-FU may elicit
antitumor activities through serving as a thymidylate synthase
inhibitor, thus blocking the pyrimidine-thymidine synthesis and DNA
replication of cancer cells (2,3).
Therefore, evidence was provided for the mechanisms through which
PsA and 5-FU may exhibit synergic antitumor effects.
In summary, PsA may inhibit human colon cancer cell
growth in vitro and in vivo, in isolation or
synergistically with 5-FU, through apoptosis induction. The data
from the present study suggest that PsA and related compounds may
be a novel class of anti-colon cancer drugs.
References
|
1
|
Cassidy J, Saltz L, Twelves C, Van Cutsem
E, Hoff P, Kang Y, Saini JP, Gilberg F and Cunningham D: Efficacy
of capecitabine versus 5-fluorouracil in colorectal and gastric
cancers: A meta-analysis of individual data from 6171 patients. Ann
Oncol. 22:2604–2609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kodama Y, Fumoto S, Nishi J, Nakashima M,
Sasaki H, Nakamura J and Nishida K: Absorption and distribution
characteristics of 5-fluorouracil (5-FU) after an application to
the liver surface in rats in order to reduce systemic side effects.
Biol Pharm Bull. 31:1049–1052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang DQ, Guo Q, Zhu JH and Chen WC:
Increase of cyclooxygenase-2 inhibition with celecoxib combined
with 5-FU enhances tumor cell apoptosis and antitumor efficacy in a
subcutaneous implantation tumor model of human colon cancer. World
J Surg Oncol. 11:162013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng L, Zhang M, Zhang P, Song Z, Ma Z
and Qu H: Silver complexation and tandem mass spectrometry for
differentiation of triterpenoid saponins from the roots of
Pulsatilla chinensis (Bunge) Regel. Rapid Commun Mass
Spectrom. 22:3783–3790. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu QM, Shu Z, He WJ, Chen LY, Yang SL,
Yang G, Liu YL and Li XR: Antitumor activity of Pulsatilla
chinensis (Bunge) Regel saponins in human liver tumor 7402
cells in vitro and in vivo. Phytomedicine. 19:293–300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Q, Chen W, Jiao Y, Hou J, Wu Q, Liu Y
and Qi X: Pulsatilla saponin A, an active molecule from
Pulsatilla chinensis, induces cancer cell death and inhibits
tumor growth in mouse xenograft models. J Surg Res. 188:387–395.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi X, Chen Z, Liu D, Cen J and Gu M:
Expression of Dlk1 gene in myelodysplastic syndrome determined by
microarray, and its effects on leukemia cells. Int J Mol Med.
22:61–68. 2008.PubMed/NCBI
|
|
8
|
Li Z, Qiu HY, Jiao Y, Cen JN, Fu CM, Hu
SY, Zhu MQ, Wu DP and Qi XF: Growth and differentiation effects of
Homer3 on a leukemia cell line. Asian Pac J Cancer Prev.
14:2525–2528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Debatin K: Activation of apoptosis
pathways by anticancer treatment. Toxicol Lett. 112–113:41–48.
2000. View Article : Google Scholar
|
|
10
|
Iwaizumi M, Tseng-Rogenski S and Carethers
JM: DNA mismatch repair proficiency executing 5-fluorouracil
cytotoxicity in colorectal cancer cells. Cancer Biol Ther.
12:756–764. 2011. View Article : Google Scholar : PubMed/NCBI
|