Introduction
Colorectal cancer is not only an accumulation of
malignant cells but may be interpreted as an abnormal tissue
composed of cancer cells and their stroma, the tumor
microenvironment, which is composed of various cell types such as
fibroblast, endothelial cells and different cells of the immune
system (1). These cells are embedded
in, or interact with, the extracellular matrix (ECM). The ECM
contains proteins such as collagens, proteoglycans and
glycoproteins, and forms a three-dimensional protein structure
supporting the cells and tissue (2).
In addition to the ECM, multiple proteins such as secreted
intracellular proteins, various growth factors and different types
of enzymes are also part of the tumor microenvironment (1–3).
During cancer progression, the tumor
microenvironment is heavily remodeled due to altered proteolytic
activity (3). Matrix metalloproteases
(MMPs) are the main proteases responsible for the degradation of
extracellular proteins and these are typically upregulated in
cancer (4). Limited knowledge is
currently available on MMP-mediated degradation of specific
proteins of the microenvironment associated with the tumor tissue
compared with that in the corresponding non-neoplastic adjacent
tissue (NAT) in the same patient.
In a recent study of the ECM protein composition in
normal and malignant colorectal tissue, a total of 37
tumor-specific proteins were identified, including several MMPs
(5). However, measurements of altered
MMP expression/activity are not sufficient to fully understand the
effect of distinct MMP profiles between normal and malignant
colorectal tissue. Biomarkers that are capable of reflecting
altered MMP expression/activity and, specifically, the dynamic
processes of MMP-mediated degradation of signature proteins from
the colorectal tissue are required.
A biomarker specifically reflecting MMP-degraded
type III collagen (C3M) was previously developed by our group
(6). In normal and malignant
colorectal tissue, type III collagen is one of the major components
of the interstitial ECM compartment (7). The interstitial ECM is an essential
component of the fibrotic-like microenvironment identified in
numerous solid tumors, and is degraded as part of the inflammation
and ECM remodeling associated with tumor progression (8).
Another notable biomarker in this context is
citrullinated and MMP-degraded vimentin (VICM). VICM reflects
MMP-mediated degradation and citrullination of vimentin (9). Vimentin is an intermediate filament
protein shown to be associated with the pathology of inflammatory
bowel disease, a group of idiopathic gastrointestinal chronic
inflammatory conditions (10), and is
expressed in tissue from patients with colorectal cancer (11). Vimentin has been identified on the
surface of tumor cells (12) and is
released from activated macrophages (10), making it a target for proteolysis and
citrullination, a post-translational modification associated with
chronic inflammation (13).
Levels of C3M and VICM have been revealed to be
elevated in serum from patients with inflammatory bowel disease
(14). Levels of C3M have also been
revealed to be increased in serum from patients with pancreatic
(15), breast and ovarian cancer
(16), and levels of VICM have been
revealed to be elevated in lung cancer (17).
The present study hypothesized that C3M and VICM may
be used as tools to investigate specific MMP-mediated
micro-environmental changes in association with colorectal cancer,
and hereby increase the knowledge of MMP-mediated degradation of
extracellular proteins in tumor tissue compared with that in the
NAT of the same patient. The aim of the present study was to
provide support for the concept that C3M and VICM are applicable as
tools for the investigation into dynamic tissue changes in an ex
vivo culture setting of colorectal tumor tissue and
corresponding NAT.
Materials and methods
Patients/study population
Biopsies from colorectal tumor tissue and NAT from
tissue removed during bowel resection in patients with colorectal
cancer were obtained subsequent to informed consent and approval by
the Ethical Committee of the Capital Region of Denmark (Copenhagen,
Denmark; approval no. H-1-2014-048) in compliance with the Helsinki
Declaration. Biopsy samples were collected by medical staff
immediately after surgery, and stored in Dulbecco's modified
Eagle's medium (DMEM) supplemented with Ham's F12 medium (DMEM:F12;
VWR International, Søborg, Denmark), 1% penicillin and streptavidin
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Samples were
immediately placed on ice and transported directly to the
laboratory where they were processed (see below) within 1 h of
collection. Information associated with the included patients
(n=13) is shown in Table I. Tumor
staging was evaluated according to the Union for International
Cancer Control classification system. None of the patients received
any form of neo-adjuvant anticancer therapy. Tumor resections were
performed at Bispebjerg Hospital, Copenhagen, Denmark between
November 2014 and October 2015.
 | Table I.Characteristics of patients. |
Table I.
Characteristics of patients.
| Characteristic | Number of
patients |
|---|
| Age, mean ± SD
(range), years | 67.7±11.1
(41–84) |
| Gender,
female/male | 8/5 |
| BMI, mean ± SD
(range), kg/m2 | 28.5±5.4
(21.6–36.6) |
| Tumor localization,
colon/rectum | 10/3 |
| Tumor stage,
colon/rectum |
| I | 3/0 |
| II |
2/1 |
|
III |
4/1 |
| IV |
1/1 |
Cleavage of tumor tissue in vitro
A pool of tumor tissue from 2 patients was dissolved
in PBS by mechanical blending and diluted 1:20 in protease specific
buffer [MMP buffer (50 mM Tris-HCl, 200 mM NaCl, 10 mM
CaCl2, 100 µM ZnSO4 and 0.05% Brij 35 (Merck
KGaA, Darmstadt, Germany), pH 7.5) or trypsin buffer (50 mM
Tris-HCl, 150 mM NaCl, 5 mM CaCl2 and 0.05% Brij 35, pH
7.5]. For each specific cleavage, ~35 mg tissue was used. MMP-2 and
MMP-9 (BioCol GmbH, Michendorf, Germany) were activated with
freshly prepared 1 mM 4-aminophenylmercuric acetate (Sigma-Aldrich;
Merck KGaA) in dimethyl sulfoxide by incubation at 37°C for 2 h.
Activated MMPs and trypsin (Sigma-Aldrich; Merck KGaA) were added
to the Eppendorf tubes containing tissue to a final concentration
of 25 nM and incubated overnight at 37°C. Tissue dissolved in
buffers only was used as a negative control. The reaction was
terminated by adding EDTA to the MMP cleavage products or by adding
aprotinin (BioCol GmbH) to the trypsin cleavage products to a final
concentration of 1 µM. The tissue samples were centrifuged at
10,000 × g at 4°C for 15 min and the supernatant was collected. The
supernatant was stored at −80°C until analysis by relevant
ELISA.
Viability
The tumor tissue was cut into pieces of ~2
mm3 and cultured in 96-well plates with 200 µl/well
DMEM:F12, 1% penicillin and streptavidin (Thermo Fisher Scientific,
Inc.) at 37°C and 5% CO2. Cell viability was tested in
three different culture conditions: Upon addition of 2% fetal calf
serum (FCS) (Sigma-Aldrich; Merck KGaA), upon addition of 2%
Ultroser® G (Pall Life Sciences, Port Washington, NY,
USA) and without the addition of FCS or Ultroser® G. To
assess the metabolic activity of the tissue/cells, the medium was
removed after 30 min and 24 h, and alamarBlue® (Thermo
Fisher Scientific, Inc.) was added to the cultures in a 10%
dilution in PBS. alamarBlue® exhibits colorimetric
changes as a function of the metabolic activity depending on
proliferation and viability of the cells, and has previously been
validated in the ex vivo culture setting (18). After 3 h of incubation with
alamarBlue®, the supernatants were collected and the
absorbance determined at 540–590 nm. The experiment was performed
in quadruplicate for each condition and repeated twice (2
patients).
Ex vivo culture
The tissue was cut into pieces of ~2 mm3
and cultured in the highest possible number of replicates (2–6
replicas per patient per tissue type) in a 96-well plate with 200
µl/well DMEM:F12, 1% penicillin and streptavidin at 37°C and 5%
CO2. The supernatant was removed after 24 h. Any
cell/tissue debris was removed by centrifugation at 10,000 × g for
15 min at 4°C, and the supernatant was pooled and stored at −80°C
until analysis by the relevant ELISA.
Biomarker measurements
The levels of C3M (6)
and VICM (9) were assessed in the
culture supernatant by competitive ELISA. As is common for C3M and
VICM assay procedures (6,9), a biotinylated target peptide (Chinese
Peptide Company, Beijing, China) was added to a 96-well
streptavidin-coated plate in 40 mM
NaHPO4·12H2O, 7 mM KHPO4, 137 mM
NaCl, 2.7 mM KCl, 25 mM EDTA, 0.1% Tween 20, 1% BSA (Sigma-Aldrich;
Merck KGaA) and 10% sorbitol (pH 7) for C3M, or 50 mM Tris-HCl, 1%
BSA, 0.1% Tween-20 and 0.36% 5-bromo-5-nitro-1,3-dioxane (pH 7.4 at
20°C) for VICM. A total of 100 µl/well was added and plates were
then incubated for 30 min at 20°C while agitating at 300 rpm. The
plate was washed five times in 20 mM Tris-HCl and 50 mM NaCl buffer
(washing buffer, pH 7.2). Next, 20 µl target peptide calibrator
(Chinese Peptide Company) or sample and 100 µl horseradish
peroxidase-conjugated monoclonal antibody specific for the target
peptide sequence of interest (provided as part of C3M and VICM
assay kits; cat. nos. 1200 and 1800, respectively; Nordic
Bioscience A/S, Herlev, Denmark) was added. The anti-C3M antibody
was dissolved (final concentration, 23 ng/ml) in 8 mM
NaHPO4·12H2O, 1.5 mM KHPO4, 13.7
mM NaCl, 2.7 mM KCl, 0.1% Tween-20, 1% BSA and 0.003% phenol red
(pH 7.4), and the anti-VICM antibody was dissolved (final
concentration, 4 ng/ml) in 50 mM Tris-HCl, 1% BSA, 0.1% Tween 20
and 0.36% 5-bromo-5-nitro-1,3-dioxane (pH 7.4 at 20°C). The C3M and
VICM assay kits were used according to the manufacturer's protocol.
The plate was incubated for 1 h at 20°C (C3M) or overnight at 4°C
(VICM). The plate was washed five times in the aforementioned
washing buffer prior to the addition of 100 µl
3,3′,5,5′-tetramethylbenzidine (Kem-En-Tec Diagnostics A/S,
Taastrup, Denmark; cat. no. 438OH). The plate was incubated for 15
min at 20°C in the dark. The reaction was stopped by the addition
of 100 µl stopping solution (1% H2SO4) and
the optical density was measured at 450 nm with 650 nm as a
reference. Biomarker levels were determined in duplicate.
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism v6.05 (GraphPad Software, Inc., La Jolla, CA, USA). Viability
was compared using two-way analysis of variance adjusted for
multiple comparisons with the Sidak test. Biomarker levels in the
supernatant were compared using the Friedman test or the
Kruskal-Wallis test adjusted for multiple comparisons with the
Dunn's test. P<0.05 was considered to indicate a statistically
significant difference. The correlation between colorectal tumor
tissue and corresponding NAT was calculated by the Pearson's
correlation coefficient and goodness of fit. Results are presented
as mean ± standard error of the mean, Tukey plots or scatter
plots.
Results
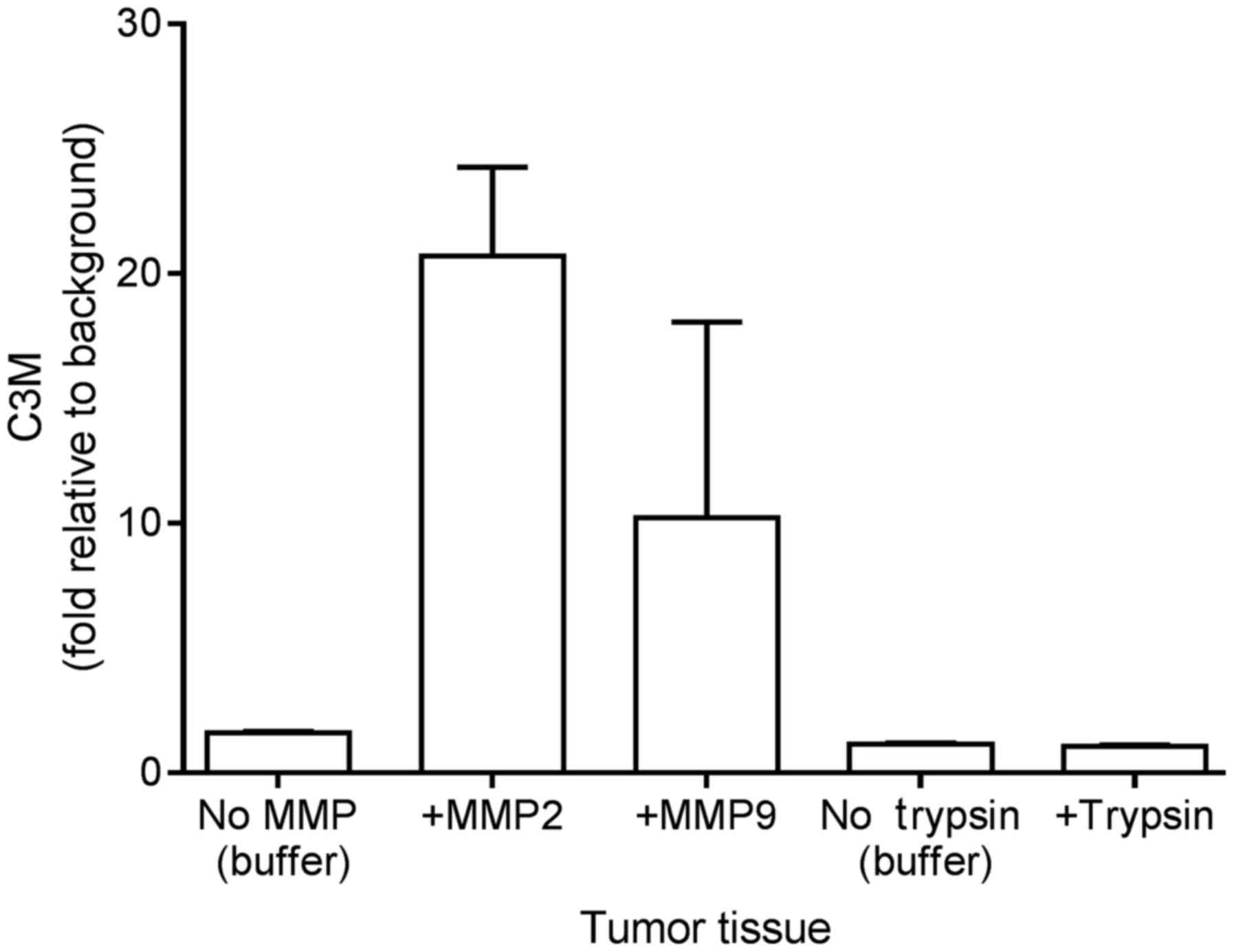
In vitro cleavage of colorectal tumor
tissue
Following resection and mechanical processing, the
tumor tissue was incubated with MMP-2, MMP-9 and trypsin to
investigate if C3M may be generated from the tissue in
vitro. As illustrated in Fig. 1,
the level of C3M increased by 20- and 10-fold upon incubating the
tumor tissue with MMP-2 and MMP-9, respectively. By contrast,
trypsin did not generate C3M. These findings suggest that C3M is
associated with colorectal tumor tissue and that C3M is generated
specifically by MMP cleavage.
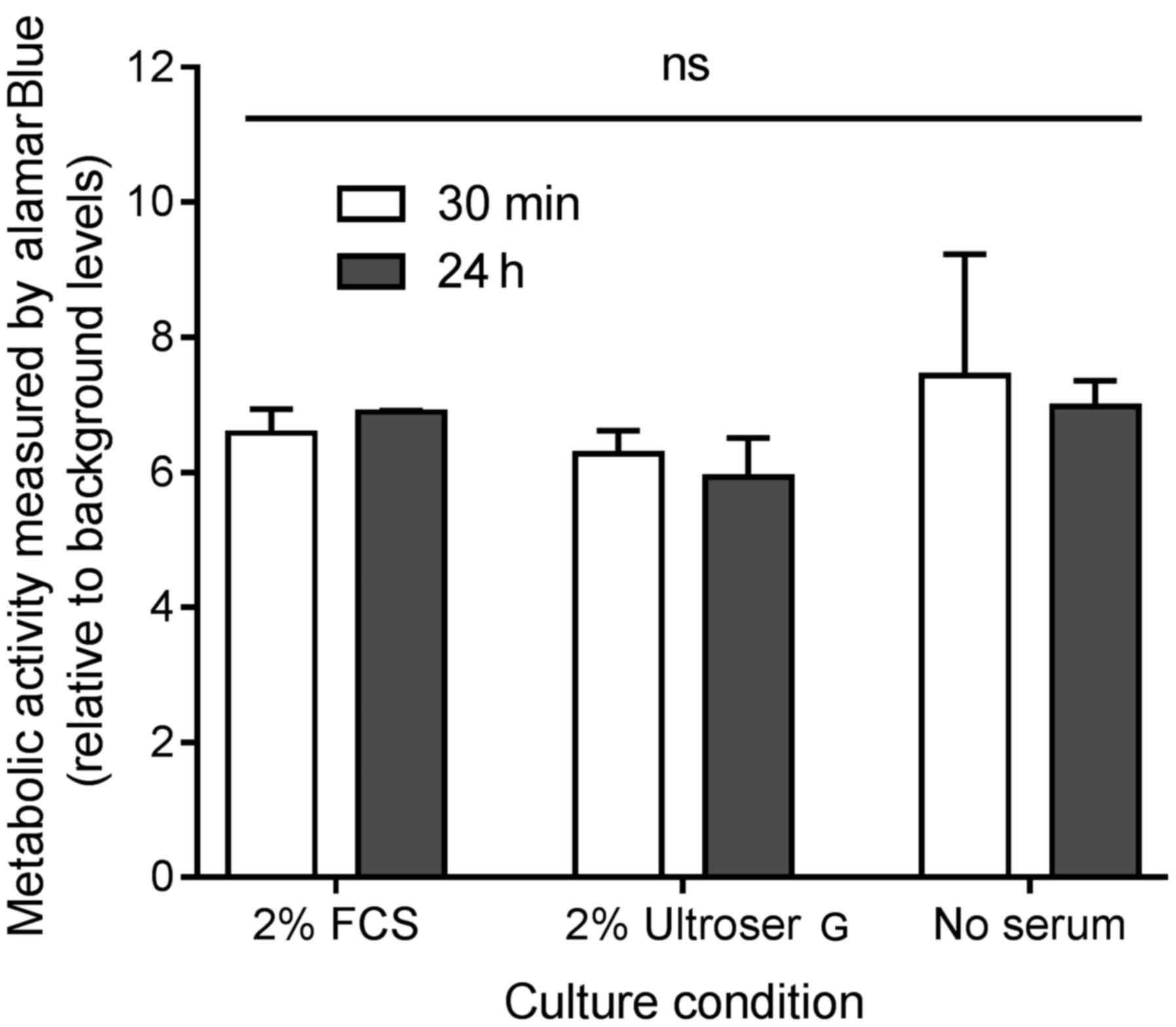
Viability of ex vivo colorectal tumor
tissue cultures under different culture conditions
The metabolic activity, as a measurement of
viability, was assessed by addition of alamarBlue® for 3
h to the ex vivo cultures after 30 min and 24 h of primary
culture. A total of three different culture conditions were
investigated: Growth medium with 2% FCS, growth medium with 2%
Ultroser® G and growth without FCS or
Ultroser® G. The results are shown in Fig. 2. No significant difference was
detected in the viability after 30 min or 24 h, indicating that the
ex vivo cultures stay viable for ≤24 h of culture. In
addition, no difference was detected when comparing the three
culture conditions, suggesting that the effect of either 2% FCS or
2% Ultroser® G on cell viability in the ex vivo
cultures is of no importance for the conditions of the present
study.
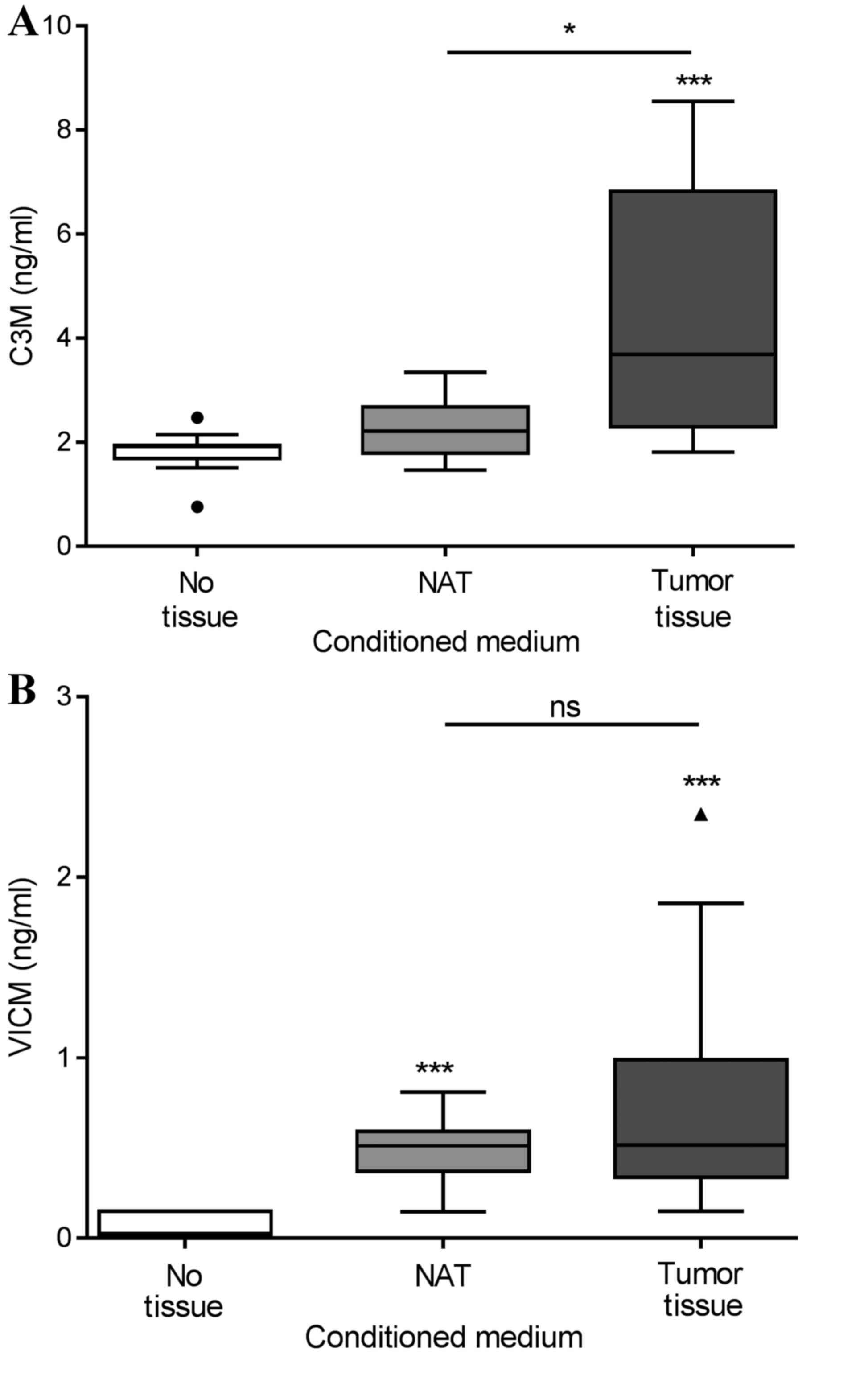
Biomarker release from ex vivo
cultures of colorectal tumor tissue and NAT
Tumor tissue and corresponding NAT were removed
during the resection of colorectal cancer in 13 patients. The
tissue was cut into equally sized pieces and cultured for 24 h in
growth medium without serum. As shown in Fig. 3A, only the tumor tissue generated
significantly elevated levels of C3M in the conditioned medium,
with median levels of 3.7 ng/ml compared with those in the NAT and
growth medium alone groups, which displayed median levels of C3M of
2.2 and 1.9 ng/ml, respectively. As shown in Fig. 3B, VICM from the tumor tissue, median
level of 0.51 ng/ml, and the NAT, median level of 0.52 ng/ml, was
significantly increased compared with that from the growth medium
alone, 0.03 ng/ml. No significant differences were detected between
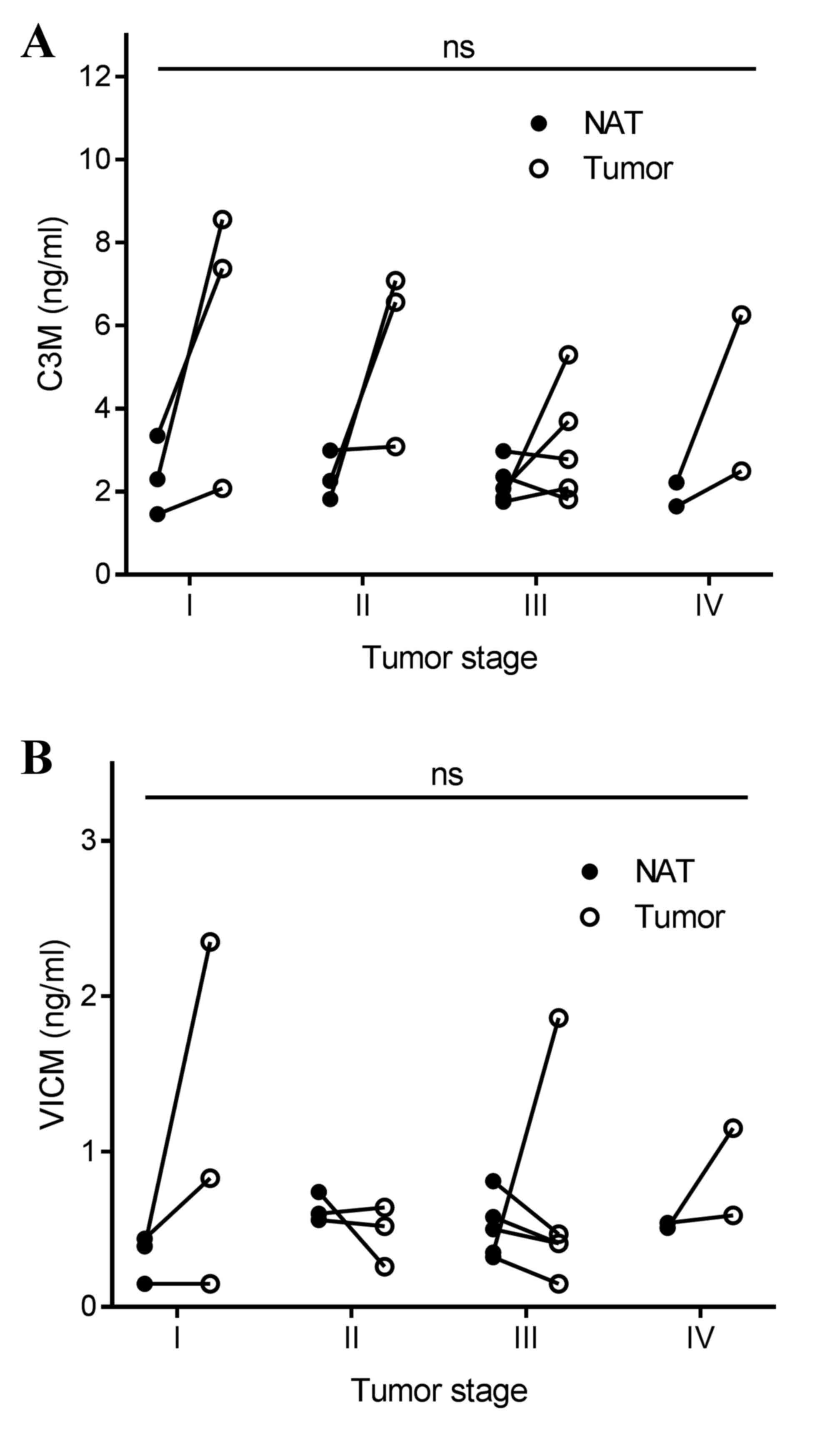
the tumor tissue and NAT. Subsequently, the present study addressed
the difference in biomarker levels according to tumor stage, and
detected no significant difference (Fig.
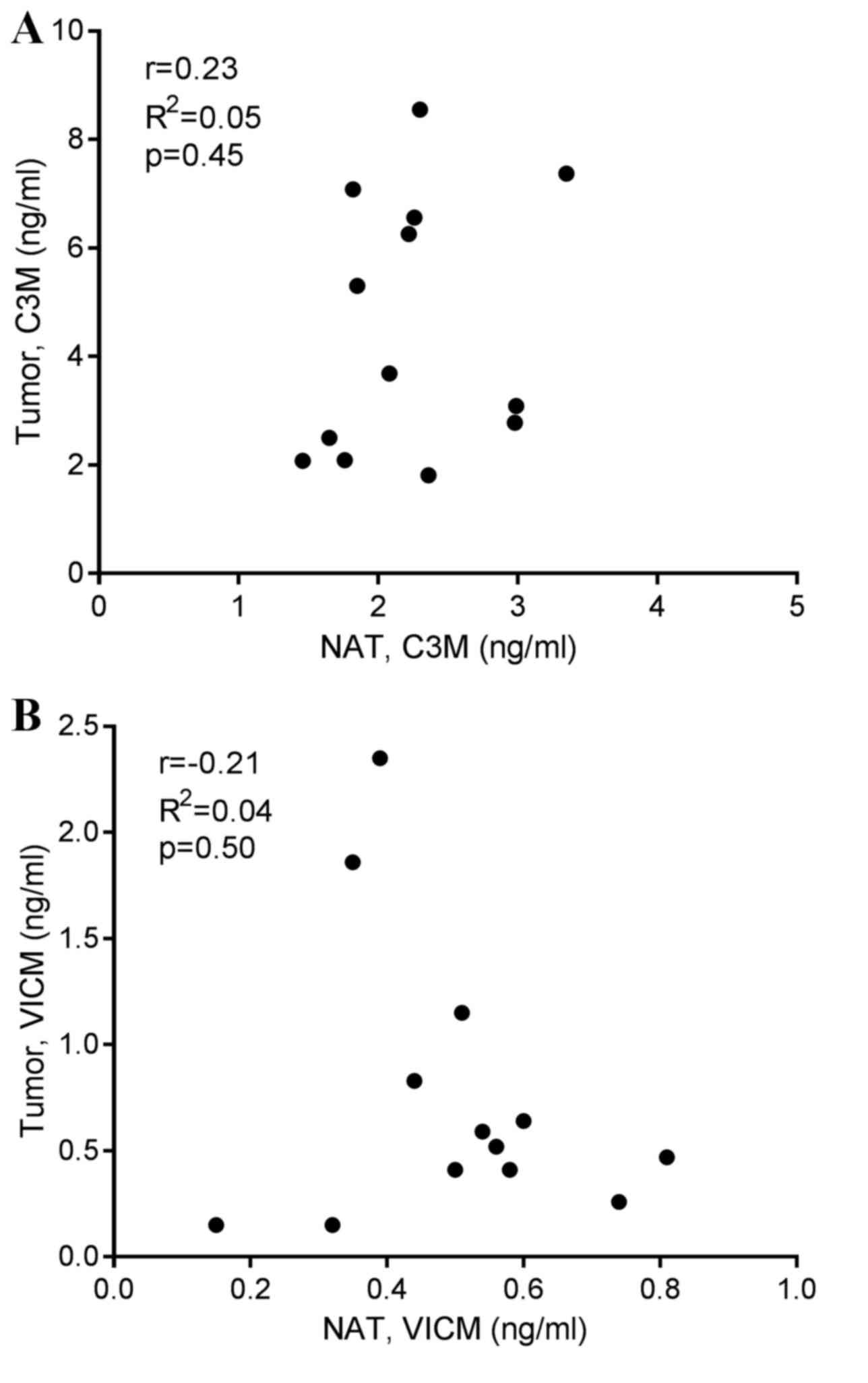
4). Finally, no correlation was observed between biomarker
levels released from the tumor tissue and corresponding NAT
(Fig. 5).
Discussion
The present study revealed that specific
MMP-mediated molecular changes, particularly the degradation of C3M
and VICM, may be assessed in conditioned medium from ex vivo
culture of biopsy tissue from patients with colorectal cancer. It
is well established that such ex vivo culture of primary
human tissues is a model that maintains tissue architecture and
reflects ongoing dynamic changes in the tumor (18,19),
thereby retaining the majority of the complexity and heterogeneity
of human tumors in a laboratory setting.
Higher levels of C3M were released from tumor tissue
compared with the corresponding levels of NAT; C3M levels of NAT
did not differ from those detected in the growth medium alone. This
suggests that C3M is specifically associated with tissue remodeling
of the tumor tissue, not NAT. The elevated levels of C3M in tumor
tissue vs. NAT are hypothesized to be a result of the pathological
turnover of the tissue. Therefore, the present findings indicate
that C3M may reflect disease activity at the time of resection.
Whether the elevated C3M levels are a result of specific ongoing
inflammatory processes or altered cancer or stromal cell activities
in the tumor remains to be established. However, C3M has been
associated with inflammatory diseases of the gut (14), suggesting that C3M may reflect the
extent of ongoing inflammation in the tumor tissue.
Tissue remodeling is a dynamic process that is
ultimately the sum of protein formation, degradation and
post-translational modifications (2).
Normally, the ECM, including type III collagen, is maintained in a
delicate equilibrium between protein formation and degradation.
However, this balance may be altered in pathological conditions
such as cancer, leading to the altered composition and quality of
the ECM (8). The ECM and tissue
remodeling are part of the malignant changes that drive cancer
(20,21). Thus, biomarkers reflecting dynamic
changes such as ECM formation or degradation, and not merely the
total protein content of ECM (22),
are crucial for additional studies.
Proteolytic enzymes such as MMPs have been
associated with the development and progression of colorectal
cancer (23). It has also previously
been shown that MMP-2 and MMP-9 protein expression is lower in NAT
compared with that in the corresponding tumor tissue from patients
with colorectal cancer (24). As C3M
was generated by MMP-2 and MMP-9 (Fig.
1), this is in line with these previous studies. Notably,
although the MMP-2 and MMP-9 levels were lower in NAT vs. tumor
tissue in the study by Langers et al (24), the MMP levels in NAT were still
predictive of outcome (mortality), suggesting the biological
relevance of assessing the molecular composition of NAT.
Furthermore, the level of MMP-2, but not of MMP-9, was correlated
between tumor tissue and NAT. The present study did not detect a
correlation between biomarker levels in tumor tissue and
corresponding NAT. Taken together, this indicates that
investigating tumor and NAT biology in greater detail may yield
essential information.
In contrast to C3M, the tumor tissue and NAT
released VICM, and no difference was detected between the two types
of tissue. VICM is a citrullinated protein, and citrullination of
extracellular proteins is dependent on high levels of calcium
(13). Enhanced calcium levels have
been identified in tumor tissue and NAT from patients with
colorectal cancer (25). It has been
revealed that VICM is associated with activated macrophages
(Willumsen et al, unpublished data). Therefore, the present
findings may suggest ongoing macrophage activity in the tumor
tissue and NAT from patients with colorectal cancer, and suggest
that VICM may be applicable in an immuno-oncology setting by
monitoring the activity of tumor-associated macrophages.
Although no correlation was observed between the
stage of disease for C3M and VICM, the variation between individual
patients in the levels released from the tumor tissue appeared to
be more pronounced compared with the NAT findings. This may
indicate that tumor tissues are more heterogeneous than NAT.
The relatively small number of patients involved
limits the present study. Findings should be confirmed in larger
clinical settings ultimately with progression or survival as
primary endpoints. Investigating the effect of specific treatments
may also be relevant, and potentially this model may serve as a
promising preclinical tool for future drug-development studies.
Additionally, the culture condition of colorectal cancer tissues
may be a critical step that may affect the results. Although no
effect was observed on viability as a function of culture media
conditions, other factors may affect the outcomes, which require
additional investigation. In addition, although the same volume of
tissues were analyzed, a larger number of cells may be present in
cancer tissue compared with that in the normal counterparts.
Pathological analysis of cellularity and other tissue components
such as total collagen content, should be performed in future
studies to additionally interrogate the association between the
tissue remodeling biomarkers and cancer.
Several other biomarkers exist for assessing ECM and
microenvironmental alterations, and these may be relevant to
include in future studies. In line with the present findings,
biomarkers reflecting the MMP-mediated degradation of specific
proteins of the tumor microenvironment have been observed to be
significantly upregulated in serum from patients with various solid
tumors compared with those in healthy controls. Elevated levels of
different MMP-generated collagen degradation products have been
observed in pancreatic (15), breast,
ovarian (16), lung (17) and colorectal cancer (26).
In conclusion, by assessing MMP-mediated degradation
of type III collagen and vimentin in an ex vivo model, the
present study profiled and characterized colorectal tumor tissue
and NAT, thus providing novel relevant insight into the dynamic
changes occurring as part of the colorectal cancer pathogenesis.
Detailed molecular characterization, including specific protein
compositions and relevant post-translational modifications, as
determined by evaluating the levels of citrullination and
proteolysis of specific proteins, leads to an increased
understanding of tumor-associated biology, and may provide novel
biomarkers and targets for novel therapies. The increased
understanding of the microenvironmental changes associated with
colorectal cancer may ultimately help to improve the outcomes of
individual patients.
Acknowledgements
The authors would like to acknowledge Miss Tina L.
Brondum, Research Nurse, Digestive Disease Center, Bispebjerg
Hospital, University of Copenhagen, DK-2400 Copenhagen, Denmark for
her practical contribution to the present study and the Danish
Research Foundation for funding the present study. N.W., C.L.B.,
A.-C.B.-J., S.N.K., D.J.L. and M.A.K. are employed at Nordic
Bioscience A/S (Herlev, Denmark) and are involved in the
development of biomarkers.
Glossary
Abbreviations
Abbreviations:
|
MMP
|
matrix metalloprotease
|
|
NAT
|
non-neoplastic adjacent tissue
|
|
ECM
|
extracellular matrix
|
|
C3M
|
MMP-degraded type III collagen
|
|
VICM
|
citrullinated and MMP-degraded
vimentin
|
References
|
1
|
Balkwill FR, Capasso M and Hagemann T: The
tumor microenvironment at a glance. J Cell Sci. 125:5591–5596.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frantz C, Stewart KM and Weaver VM: The
extracellular matrix at a glance. J Cell Sci. 123:4195–4200. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mason SD and Joyce JA: Proteolytic
networks in cancer. Trends Cell Biol. 21:228–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naba A, Clauser KR, Whittaker CA, Carr SA,
Tanabe KK and Hynes RO: Extracellular matrix signatures of human
primary metastatic colon cancers and their metastases to liver. BMC
Cancer. 14:5182014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barascuk N, Veidal SS, Larsen L, Larsen
DV, Larsen MR, Wang J, Zheng Q, Xing R, Cao Y, Rasmussen LM and
Karsdal MA: A novel assay for extracellular matrix remodeling
associated with liver fibrosis: An enzyme-linked immunosorbent
assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of
type III collagen. Clin Biochem. 43:899–904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hilska M, Collan Y, Peltonen J, Gullichsen
R, Paajanen H and Laato M: The distribution of collagen types I,
III, and IV in normal and malignant colorectal mucosa. Eur J Surg.
164:457–464. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karsdal MA, Nielsen MJ, Sand JM, Henriksen
K, Genovese F, Bay-Jensen AC, Smith V, Adamkewicz JI, Christiansen
C and Leeming DJ: Extracellular matrix remodeling: The common
denominator in connective tissue diseases. Possibilities for
evaluation and current understanding of the matrix as more than a
passive architecture, but a key player in tissue failure. Assay
Drug Dev Technol. 11:70–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vassiliadis E, Oliveira CP,
Alvares-da-Silva MR, Zhang C, Carrilho FJ, Stefano JT, Rabelo F,
Pereira L, Kappel CR, Henriksen K, et al: Circulating levels of
citrullinated and MMP-degraded vimentin (VICM) in liver fibrosis
related pathology. Am J Transl Res. 4:403–414. 2012.PubMed/NCBI
|
|
10
|
Mor-Vaknin N, Punturieri A, Sitwala K and
Markovitz DM: Vimentin is secreted by activated macrophages. Nat
Cell Biol. 5:59–63. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ngan CY, Yamamoto H, Seshimo I, Tsujino T,
Man-i M, Ikeda JI, Konishi K, Takemasa I, Ikeda M, Sekimoto M, et
al: Quantitative evaluation of vimentin expression in tumour stroma
of colorectal cancer. Br J Cancer. 96:986–992. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steinmetz NF, Cho CF, Ablack A, Lewis JD
and Manchester M: Cowpea mosaic virus nanoparticles target surface
vimentin on cancer cells. Nanomedicine (Lond). 6:351–364. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gudmann NS, Hansen NU, Jensen AC, Karsdal
MA and Siebuhr AS: Biological relevance of citrullinations:
Diagnostic, prognostic and therapeutic options. Autoimmunity.
48:73–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mortensen JH, Godskesen LE, Jensen MD, Van
Haaften WT, Klinge LG, Olinga P, Dijkstra G, Kjeldsen J, Karsdal
MA, Bay-Jensen AC and Krag A: Fragments of citrullinated and
MMP-degraded Vimentin and MMP-degraded type III collagen are novel
serological biomarkers to differentiate crohn's disease from
ulcerative colitis. J Crohns Colitis. 9:863–872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Willumsen N, Bager CL, Leeming DJ, Smith
V, Karsdal MA, Dornan D and Bay-Jensen AC: Extracellular matrix
specific protein fingerprints measured in serum can seperate
pancreatic cancer patients from healthy controls. BMC Cancer.
13:5542013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bager CL, Willumsen N, Leeming DJ, Smith
V, Karsdal MA, Dornan D and Bay-Jensen AC: Collagen degradation
products measured in serum can separate ovarian and breast cancer
patients from healthy controls: A preliminary study. Cancer
Biomark. 15:783–788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Willumsen N, Bager CL, Leeming DJ, Smith
V, Christiansen C, Karsdal MA, Dornan D and Bay-Jensen AC: Serum
biomarkers reflecting specific tumor tissue remodeling processes
are valuable diagnostic tools for lung cancer. Cancer Med.
3:1136–1145. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pirnia F, Frese S, Gloor B, Hotz MA,
Luethi A, Gugger M, Betticher DC and Borner MM: Ex vivo assessment
of chemotherapy-induced apoptosis and associated molecular changes
in patient tumor samples. Anticancer Res. 26:1765–1772.
2006.PubMed/NCBI
|
|
19
|
Freeman AE and Hoffman RM: In vivo-like
growth of human tumors in vitro. Proc Natl Acad Sci USA.
83:2694–2698. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pickup MW, Mouw JK and Weaver VM: The
extracellular matrix modulates the hallmarks of cancer. EMBO Rep.
15:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karsdal MA, Delvin E and Christiansen C:
Protein fingerprints-relying on and understanding the information
of serological protein measurements. Clin Biochem. 44:1278–1279.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herszényi L, Barabás L, Hritz I, István G
and Tulassay Z: Impact of proteolytic enzymes in colorectal cancer
development and progression. World J Gastroenterol. 20:13246–13257.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Langers AM, Verspaget HW, Hawinkels LJ,
Kubben FJ, van Duijn W, van der Reijden JJ, Hardwick JC, Hommes DW
and Sier CF: MMP-2 and MMP-9 in normal mucosa are independently
associated with outcome of colorectal cancer patients. Br J Cancer.
106:1495–1498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edelstein PS, Thompson SM and Davies RJ:
Altered intracellular calcium regulation in human colorectal
cancers and in ‘normal’ adjacent mucosa. Cancer Res. 51:4492–4494.
1991.PubMed/NCBI
|
|
26
|
Kehlet SN, Sanz-Pamplona R, Brix S,
Leeming DJ, Karsdal MA and Moreno V: Excessive collagen turnover
products are released during colorectal cancer progression and
elevated in serum from metastatic colorectal cancer patients. Sci
Rep. 6:305992016. View Article : Google Scholar : PubMed/NCBI
|