Introduction
Keratocystic odontogenic tumors (KCOTs) are
classified as benign odontogenic tumors by the World Health
Organization (WHO) (1). A KCOT is
defined as a benign unicystic or multicystic intraosseous tumor of
odontogenic origin, with a characteristic lining of parakeratinized
stratified squamous epithelium that is noted for its locally
aggressive nature and capacity for recurrence (1,2). The high
rate of recurrence is due to the neoplastic nature of these tumors,
including a high rate of proliferative activity and angiogenesis,
and the presence of daughter cysts and epithelial islands (3–5). The
incomplete surgical removal of the epithelial components of KCOTs
due to their fragility is also a factor contributing to recurrence
(3,6).
Various markers of proliferation as well as
microvessel density (MVD) have previously been examined with regard
to their capacity to predict tumor recurrence in
immunohistochemical studies (3–8). One such
marker of proliferation is Ki-67, a prototypical cell
cycle-associated nuclear protein that is expressed by proliferating
cells in all phases of the cell cycle, with the exception of
G0, and is rapidly degraded following mitosis, with a
half-life of ≤1 h (7,8). The immunohistochemical detection of
Ki-67 has been used to evaluate the proliferative potential of
tumor cells, and to predict the recurrence of KCOTs (7,8).
MVD is determined by detecting the expression levels
of cluster of differentiation (CD)34, and is frequently used to
quantify angiogenesis in benign and malignant oral tumors,
including KCOTs (4,9,10). In our
previous study, it was reported that CD34 expression was associated
with tumor growth in oral squamous cell carcinoma (10).
Podoplanin is a transmembrane glycoprotein that is
specifically expressed by lymphatic endothelial cells, and its
expression is associated with lymph node metastasis in head and
neck squamous cell carcinoma (11).
Podoplanin has also been detected in a variety of neoplastic
tissues, and its expression may be associated with the
extracellular matrix signaling pathways, neoplastic nature and
proliferative capacity of KCOTs (5,12,13).
Although these markers are useful for predicting
tumor recurrence, to the best of our knowledge, no previous studies
have examined their utility in the context of surgical treatment
procedures for KCOTs. In the present study, clinicopathological and
immunohistochemical analyses were combined in order to investigate
how these factors may be associated with KCOT recurrence.
Materials and methods
Patients
The present study was approved by the independent
ethics committee of Nagasaki University Hospital (Nagasaki, Japan;
approval no. 15061127). The medical records of patients who were
diagnosed with a typical parakeratinized cyst and KCOT between 1992
and 2014 at Nagasaki University Hospital according to the WHO
classification, and who underwent a complete surgical tumor
excision, were retrospectively reviewed in the current study.
Paraffin embedded samples from these patients were obtained from
the pathology department of Nagasaki University Hospital.
Information regarding patient gender and age, the site and duration
of the lesion prior to treatment, surgical modality and the time to
follow-up and tumor recurrence was obtained from the patient
records. Patients for whom the follow-up period was <6 months,
and those who were diagnosed with nevoid basal cell carcinoma
syndrome (NBCCS), were omitted from the current study. One patient
with multiple lesions was selected for the present study as they
had not satisfied the diagnostic criteria for NBCCS (14).
Immunohistochemistry
Paraffin-embedded sections of resected KCOT tissue
specimens cut at 4 µm were deparaffinized in xylene and were soaked
in 10 mmol/l citrate buffer (pH 6.0) and placed in an autoclave at
121°C for 5 min for antigen retrieval. Endogenous peroxidase was
blocked by incubation with 0.3% H2O2 in
methanol for 30 min. Immunohistochemical staining was performed
using the Envision system (ENVISION+; Dako; Agilent Technologies,
Inc., Glostrup, Denmark). The following antibodies were used as
primary antibodies: Monoclonal antibody for Ki-67 (dilution, 1:50;
cat. no., M7240; Dako, Agilent Technologies, Inc.), CD34
(undiluted; cat. no., M716529; Dako, Agilent Technologies, Inc.)
and podoplanin (dilution, 1:50; cat. no., M3619; Dako, Agilent
Technologies, Inc.) derived from mouse. The sections were then
washed in Dulbecco's PBS, followed by incubation with the primary
antibodies at 4°C overnight. Following the incubation of sections
with secondary antibodies (undiluted; cat. no., K4001; Dako,
Agilent Technologies, Inc.) for 30 min at room temperature, the
reaction products were visualized by immersing the sections in
diaminobenzidine solution, and the samples were counterstained with
Meyer's hematoxylin and mounted. Negative controls were created by
the replacement of the primary antibody with phosphate-buffered
saline (PBS). Ki-67 expressions were evaluated by light microscopy
in the basal to suprabasal cell layers by calculating the mean
number of positive cells in 5 randomly selected visual fields of
each tissue section. The MVD was determined from CD34 expression
levels in the stromal cells bordering the tumor parenchyma, by
evaluating the number of CD34-positive capillaries in the 200-µm
region immediately below the epithelium, as previously described
(4). The neoplastic character of the
tumor tissues was assessed using podoplanin labeling in the basal
to suprabasal cell layers by calculating the total immunoreactivity
score as the product of proportional scores, which were based on
the estimated fraction of positively labeled tumor cells for all
tumor cells (0, none; 1, <25%; 2, 25–50%; 3, >50%) (5). All immunohistochemical assessments were
performed blind by two examiners, and based on the results of these
assessments, the present study compared the responses and
characterized the tumors.
Statistical analysis
The associations between marker expression levels
and the patient clinicopathological features were analyzed using
Fisher's exact test. Continuous data are presented as the median
with interquartile range (IQR). Multiple logistic regression
analysis and univariate and multivariate logistic regression
analyses were performed in order to identify independent factors
for predicting tumor recurrence. Predictors that were not
determined to be associated with recurrence by the univariate
analysis were not included in the multivariate analysis. P<0.05
was considered to indicate a statistically significant result.
Results
Clinical characteristics of patients
with KCOTs
Medical records were reviewed for a total of 65
tumors in 63 patients who were treated during the aforementioned
22-year period, and the clinicopathological features are summarized
in Table I. Males and females
accounted for 58.7 and 41.3% of patients, respectively. The median
age of the patients was 41 years (range, 10–87 years). Regarding
the site of tumor involvement, 44 patients (69.8%) had mandibular
tumors, 18 (28.6%) had maxillary tumors, and 1 (1.6%) had tumors
involving the maxilla and the mandible. Radiographic examination
using panoramic and computed tomography revealed unilocular
radiolucency in 53 tumors (81.5%), with the remainder (18.5%) being
multilocular. The median tumor size was 35 mm (range, 5–120 mm).
Single tumors were detected in 62 patients (98.4%), whereas 1
patient (1.6%) had three tumors. Recurrence was observed for 13/65
tumors (20.0%) and the median time to recurrence was 36 months
(range, 10–137 months). Of the 65 tumors, 55 (84.6%) were treated
with surgical enucleation, and 10 (15.4%) with marsupialization and
subsequent enucleation. The median follow-up period for
marsupialization was 5.5 months (range, 5–14 months). In 24 tumors
(36.9%), enucleation was used in combination with peripheral
ostectomy with a bone bur (Table
II). The most frequently used treatment modality for cases in
which the roots of teeth were in contact with the margins of the
primary tumor was conservative (no extraction; 37 cases; 56.9%),
which included 29 cases of no treatment and 8 cases of apicoectomy;
radical treatment (extraction) was administered for 28 tumors
(43.1%), including 22 extractions and 6 cases in which there was no
contact with the root (solitary tumor; Table III). No patients underwent a partial
mandibulectomy or maxillectomy.
 | Table I.Clinicopathological characteristics of
65 keratocystic odontogenic tumors in 63 patients. |
Table I.
Clinicopathological characteristics of
65 keratocystic odontogenic tumors in 63 patients.
| Characteristic | Value |
|---|
| Gender, n (%) |
|
| Male | 37 (58.7) |
|
Female | 26 (41.3) |
| Age, years |
|
|
Range | 10–87 |
|
Median | 41 |
| Site of involvement,
n (%) |
|
|
Maxilla | 18 (28.6) |
|
Mandible | 44 (69.8) |
|
Mixed | 1 (1.6) |
| Number of tumors, n
(%) |
|
|
Single | 62 (98.4) |
|
Multiple | 1 (1.6) |
| X-ray results, n
(%) |
|
|
Unilocular | 53 (81.5) |
|
Mutilocular | 12 (15.5) |
| Tumor size, mm |
|
|
Range | 5–120 |
|
Median | 35 |
| Follow-up period,
months |
|
Range | 6–252 |
|
Median | 16 |
| Daughter
cysts/epithelial islands, n (%) |
|
|
Absent | 33 (50.7) |
|
Present | 32 (49.3) |
| Recurrence, n
(%) |
|
| No | 52 (80.0) |
| Yes | 13 (20.0) |
| Recurrence period,
months |
|
|
Range | 10–137 |
|
Median | 36 |
 | Table II.Surgical modality used in the
treatment of keratocystic odontogenic tumors (n=65). |
Table II.
Surgical modality used in the
treatment of keratocystic odontogenic tumors (n=65).
| Factor | Value |
|---|
| Marsupialization
and subsequent enucleation, n (%) | 10 (15.4) |
| Follow-up period of
marsupialization, months |
|
|
Range | 5–14 |
|
Median | 5.5 |
| Enucleation alone,
n (%) | 55 (84.6) |
| Peripheral
ostectomy, n (%) |
|
|
Absent | 41 (63.1) |
|
Present | 24 (36.9) |
 | Table III.Surgical modality used when the root
of the tooth was in contact with the margin of the primary
keratocystic odontogenic tumor (n=65). |
Table III.
Surgical modality used when the root
of the tooth was in contact with the margin of the primary
keratocystic odontogenic tumor (n=65).
| Surgical
modality | No. of cases
(%) |
|---|
| Conservative
treatment (non-extracted) | 37 (56.9) |
| No
treatment | 29 (44.6) |
|
Apicoectomy | 8 (12.3) |
| Radical treatment
(extracted) | 28 (43.1) |
| No
contact with primary tumors | 6 (9.3) |
|
Extraction | 22 (33.8) |
Histopathological and
immunohistochemical analysis
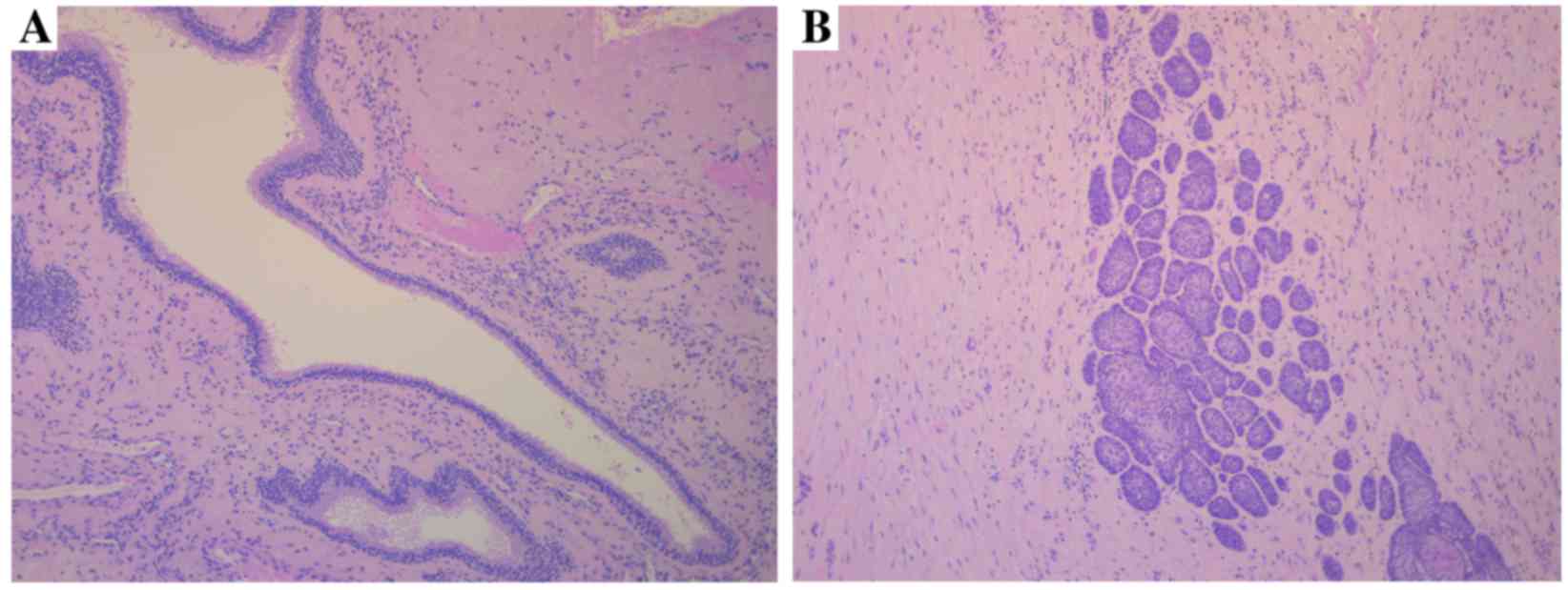
The presence of one or more daughter cyst (Fig. 1A) or epithelial island (Fig. 1B) in the cyst wall was observed in
32/65 tumors (49.3%), of which 7 recurred during the follow-up
period. No daughter cysts or epithelial islands were observed in
the cyst wall of 33 tumors (50.7%), and 6 of these cases
demonstrated recurrence during the follow-up period.
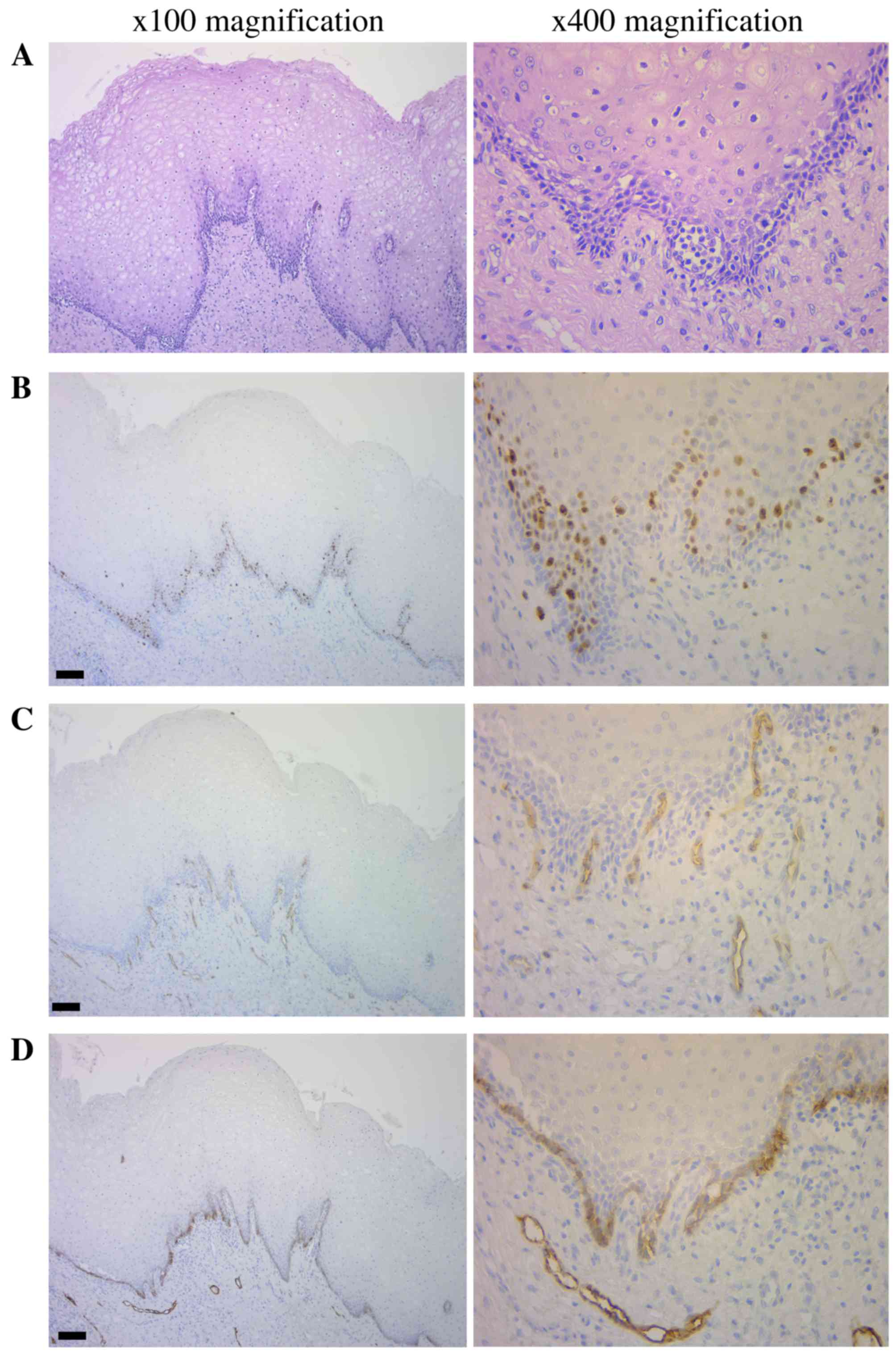
Hematoxylin-eosin staining (Fig. 2A) and immunohistochemical analysis
(Fig. 2B-D) revealed the presence of
Ki-67-positive cells in the basal and suprabasal cell layers of the
tissue samples. The median Ki-67 labeling indexes (LI) was 7.5%
(IQR=1.8–17.26) in all tumors, and 5.0% (IQR=0–17.3) and 12.5%
(IQR=7.5–17.1) in non-recurrent and recurrent tumors, respectively.
There were 4 tumors (13.8%) with an LI ≤7.5% and 9 (39.1%) with an
LI >7.5% that demonstrated recurrence (Fig. 2B). CD34-positive blood vessels were
observed in the connective tissues and the MVD was 6.5% (IQR=3–10)
in all tumors, and 5.0% (IQR=3–9.25) and 8.5% (IQR=6.25–14.5) in
non-recurrent and recurrent tumors, respectively. There were 3
tumors with an MVD <6.5 and 10 with an MVD >6.5 that
demonstrated recurrence (Fig. 2C;
Table IV). Podoplanin was expressed
in the cell membrane and cytoplasm of the majority of cells in the
basal and suprabasal cell layers. Recurrence was observed in 3
tumors with scores of 0 or 1, and in 10 tumors with scores of 2 or
3 (Fig. 2D; Table V).
 | Table IV.Ki-67 and CD34 immunoreactivity (MVD)
in keratocystic odontogenic tumors. |
Table IV.
Ki-67 and CD34 immunoreactivity (MVD)
in keratocystic odontogenic tumors.
| Factor | No. of cases | Ki-67 LI, %
(IQR) | MVD, % (IQR) |
|---|
| Non-recurrence | 52 | 5.0 (0–17.3) | 5.0 (3–9.25) |
| Recurrence | 13 | 12.5
(7.5–17.1) | 8.5
(6.25–14.5) |
| Total | 65 | 7.5 (1.8–17.3) | 6.5 (3–10) |
 | Table V.Podoplanin immunoreactivity in
keratocystic odontogenic tumors. |
Table V.
Podoplanin immunoreactivity in
keratocystic odontogenic tumors.
|
| Number of
patients |
|---|
|
|
|
|---|
| Factor | Total | Score 0 | Score 1 | Score 2 | Score 3 |
|---|
| Non-recurrence | 52 | 6 | 8 | 16 | 22 |
| Recurrence | 13 | 0 | 3 | 2 | 8 |
Uni- and multivariate analyses of tumor recurrence.
A univariate analysis revealed that high CD34 expression levels and
conservative treatment were significantly associated with tumor
recurrence (P=0.034 and P=0.003, respectively). The presence of
daughter cysts or epithelial islands and the expression of Ki-67
and podoplanin, were not associated with recurrence; however the
rate of each was observed to increase in tumor tissue that were
positive for these factors, as compared with tissues that were
negative (Table VI). A multivariate
analysis revealed that conservative treatment was the only
independent predictor of tumor recurrence (odds ratio=13.337;
P=0.018; Table VII).
 | Table VI.Univariate analysis of keratocystic
odontogenic tumor recurrence. |
Table VI.
Univariate analysis of keratocystic
odontogenic tumor recurrence.
|
| Recurrence, n |
|
|
|---|
|
|
|
|
|
|---|
| Variable | − | + | Recurrence rate
(%) | P-value |
|---|
| Gender |
|
|
| 0.127 |
|
Male | 27 | 10 | 27.0 |
|
|
Female | 25 | 3 | 10.7 |
|
| Age, years |
|
|
| 0.764 |
|
≤41 | 25 | 7 | 21.9 |
|
|
>41 | 27 | 6 | 18.2 |
|
| Tumor size, mm |
|
|
| 0.356 |
|
≤35 | 29 | 5 | 17.2 |
|
|
>35 | 23 | 8 | 25.8 |
|
| Tumor site |
|
|
| 0.485 |
|
Maxilla | 15 | 3 | 16.7 |
|
Mandible | 37 | 10 | 21.3 |
| Radiographic
findings |
|
|
| 0.447 |
|
Unilocular | 43 | 10 | 18.9 |
|
Multilocular | 9 | 3 | 25.0 |
| Daughter cyst +
epithelial islands |
|
|
| 0.764 |
|
Absent | 27 | 6 | 18.1 |
|
Present | 25 | 7 | 21.9 |
| Ki-67, % |
|
|
| 0.096 |
|
≤7.5 | 29 | 4 | 12.1 |
|
>7.5 | 23 | 9 | 28.1 |
| CD34, MVD |
|
|
| 0.034 |
|
≤6.5 | 29 | 3 |
9.4 |
|
>6.5 | 23 | 10 | 30.3 |
| Podoplanin
score |
|
|
| 0.542 |
|
0–1 | 14 | 3 | 17.6 |
|
2–3 | 38 | 10 | 20.8 |
| Surgical
modality |
|
|
| 0.317 |
|
Marsupialization +
enucleation | 7 | 3 | 30.0 |
|
Enucleation alone | 45 | 10 | 18.1 |
| Surgical
modality |
|
|
| 0.003 |
|
Conservative | 25 | 12 | 32.4 |
|
Radical | 27 | 1 |
3.6 |
| Peripheral
osteotomy |
|
|
| 0.431 |
|
Absent | 32 | 9 | 21.9 |
|
Present | 20 | 4 | 16.7 |
 | Table VII.Multivariate analysis of regional
keratocystic odontogenic tumor recurrence. |
Table VII.
Multivariate analysis of regional
keratocystic odontogenic tumor recurrence.
| Parameter | Odds ratio | 95% CI | P-value |
|---|
| CD34 (≤6.5 vs.
>6.5) |
4.366 | 0.992–19.206 | 0.051 |
| Surgical modality
(conservative vs. radical) | 13.337 |
1.565–113.647 | 0.018 |
Discussion
Investigation of the pathological and neoplastic
characteristics and the proliferative and angiogenic activities of
KCOTs may provide a means of predicting tumor recurrence and reveal
novel treatment approaches. Therefore, the present study aimed to
identify the most useful markers associated with KCOT
recurrence.
The association between the tumor histopathological
features and KCOT recurrence was examined, and the presence of
daughter cysts or epithelial islands showed a high recurrence rate
compared with the absence of them, but the result was not
significant. Previous studies have reported that the presence of
daughter cysts is significantly associated with a high rate of
tumor recurrence (6), as well as a
high frequency of allelic loss in tumor suppressor genes,
suggesting a neoplastic nature (15).
However, another study refuted these results (8). The disparity may be due to the number of
cases examined in each of these studies.
The median LI for Ki-67 for basal and suprabasal
cell layers was previously demonstrated to be 4.5–13.8% in
recurrent tumors, representing a significant association (4,7,8). Ki-67 is a marker that is often used to
assess cell proliferation in aggressive tumors such as oral cancer
or ameloblastoma (16,17). In the present study, the median LI for
Ki-67 was ~12.5% in the recurrent group, as compared with ~5.0% in
the non-recurrent group; however, univariate analysis determined
that there was no significant difference between the two groups.
The discrepancy between the previously reported values and the
results of the present study may be due to variations in the
evaluations. In the present study, the median positive cell rate
was used as cut off value for univariate analysis, but different
statistical analysis was used in other studies (4,7) or 10% for
Ki-67 positive cells were used as cut off value for high expression
(8).
Angiogenesis is essential for the proliferation of
tumor cells (4) and is evaluated by
MVD, of which has previously been implicated in oral squamous cell
carcinoma (10). When nutrient
consumption in the tumor parenchyma exceeds the local supply of
nutrients, the tumor cells enter a hypoxic state and produce
vascular endothelial growth factor to ensure the provision of the
necessary nutrients and oxygen by promoting angiogenesis (10,18).
Previous studies have reported that the typical diffusion range for
nutrients and oxygen is 70–200 µm (4); therefore, the tumor cells that are
located >100 µm from blood vessels often become hypoxic
(19). From these studies,
angiogenesis in the 200 µm region immediately below the epithelium
was investigated in the present study, and it was identified that
the number of blood vessels positive for CD34 expression was
associated with the rate of tumor recurrence. Previous studies have
reported that CD34 expression is a histopathological marker of
tumor aggressiveness (4,9,20);
however, to the best of our knowledge, the present study is the
first to demonstrate that angiogenesis directly beneath the
epithelium is a significant factor in predicting the recurrence of
KCOTs.
The expression of podoplanin in KCOT tissues
reflects the neoplastic activity of the tumor, including cell
proliferation and local invasiveness (5,12,13,21).
Podoplanin-positive cells are also involved in extracellular matrix
remodeling, which is associated with cell growth (13,21). In
the present study, ~91.8% of all tumors examined were positive for
podoplanin expression; however, univariate analysis revealed that
there was no significant difference in podoplanin expression levels
between the recurrent and non-recurrent tumor tissues. Previous
studies have indicated that podoplanin expression levels are also
indicative of tumor aggressiveness (5,12,13,21), and
that they are decreased following marsupialization or decompression
(13). In the present study, no
significant difference in the rate of recurrence was identified
between those tumors treated with marsupialization and subsequent
enucleation and those treated by enucleation alone, which may also
suggest that podoplanin expression levels are not useful as a
marker for KCOT recurrence.
Although there have been numerous retrospective
studies that have investigated the association between the type of
surgical procedure administered and the rate of tumor recurrence
(6,22–24), to
the best of our knowledge, no previous studies specifically
examined the correlation between tumor recurrence and the surgical
modality used when the tooth root was in contact with the margins
of the primary tumor. The results of the current study demonstrated
that conservative treatment was significantly associated with tumor
recurrence; therefore, teeth that remain in contact with primary
tumors may present a risk for recurrence, as previously suggested
(23). The majority of primary KCOTs
occur in the mandibular molar region; an apicoectomy against
mandibular molars is often inaccurate because it is anatomically
difficult to access compared with anterior region (23). Although peripheral osteotomy with a
bone bur has previously been used to prevent recurrence (23,24), it
was identified in the present study that the use of peripheral
osteotomy was not associated with the rate of tumor recurrence.
Multivariate analysis identified conservative
treatment to be the only independent factor for predicting KCOT
recurrence. Therefore, it was hypothesized that the surgical
modality may be more useful for predicting KCOT recurrence,
compared with histopathological factors or molecular markers.
Although NBCCS was omitted and solitary KCOT cases were selected to
reduce biases associated with tumor recurrence, residual
confounding effects may remain due to the retrospective study
design. Additionally, a potential limitation of the current study
was the relatively small number of patient cases that were
examined, due to the low incidence of KCOT amongst oral lesions.
Therefore, further prospective and intergroup studies are
required.
In conclusion, overexpression of CD34 may be a
potent marker of tumor recurrence and the radical treatment
(extraction) of teeth that are in contact with tumors is a
promising approach for preventing the recurrence of KCOTs. However,
as KCOT is more prevalent in younger patients, this may not be a
widely acceptable treatment due to cosmetic and occlusional
complications; therefore, a more elaborate peripheral osteotomy
with a bone bur may be required when apicoectomy is selected.
Glossary
Abbreviations
Abbreviations:
|
KCOT
|
keratocystic odontogenic tumor
|
|
CD
|
cluster of differentiation
|
|
MVD
|
microvesssel density
|
|
NBCCS
|
nevoid basal cell carcinoma
syndrome
|
|
WHO
|
World Health Organization
|
|
LI
|
labeling index
|
References
|
1
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: Pathology and genetics of head and neck tumours. WHO
Classif Tumour. 9:2912005.
|
|
2
|
Neville BW, Damm DD, Allen CM and Bouquot
JE: Oral and Maxillofacial Pathology. 3rd (eds). Saunders/Elsevier.
St. Louis, MO: 683–687. 2009.
|
|
3
|
Mendes RA, Carvalho JFC and van der Waal
I: Characterization and management of the keratocystic odontogenic
tumor in relation to its histopathological and biological features.
Oral Oncol. 46:219–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suemitsu M: A pathomorphological study of
fractal analysis in parenchymal-stromal border on keratocystic
odontogenic tumor-with special reference to proliferative activity
and vascular distribution. Int J Oral-Medical Sci. 10:372–383.
2012. View Article : Google Scholar
|
|
5
|
Okamoto E, Kikuchi K, Miyazaki Y,
González-Alva P, Oku Y, Tanaka A, Yoshida N, Fujinami M, Ide F,
Sakashita H and Kusama K: Significance of podoplanin expression in
keratocystic odontogenic tumor. J Oral Pathol Med. 39:110–114.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Myoung H, Hong SP, Hong SD, Lee JI, Lim
CY, Choung PH, Lee JH, Choi JY, Seo BM and Kim MJ: Odontogenic
keratocyst: Review of 256 cases for recurrence and
clinicopathologic parameters. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 91:328–333. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Selvi F, Tekkesin MS, Cakarer S, Isler SC
and Keskin C: Keratocystic odontogenic tumors: Predictive factors
of recurrence by Ki67 and AgNOR labelling. Int J Med Sci.
9:262–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuroyanagi N, Sakuma H, Miyabe S, Machida
J, Kaetsu A, Yokoi M, Maeda H, Warnakulasuriya S, Nagao T and
Shimozato K: Prognostic factors for keratocystic odontogenic tumor
(odontogenic keratocyst): Analysis of clinico-pathologic and
immunohistochemical findings in cysts treated by enucleation. J
Oral Pathol Med. 38:386–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jamshidi S, Zargaran M, Baghaei F, Shojaei
S, Zare Mahmoodabadi R, Dehghan A and Moghimbeigi A: An
immunohistochemical survey to evaluate the expression of CD105 and
CD34 in ameloblastoma and odontogenic keratocyst. J Dent (Shiraz).
15:192–198. 2014.PubMed/NCBI
|
|
10
|
Naruse T, Kawasaki G, Yanamoto S, Mizuno A
and Umeda M: Immunohistochemical study of VEGF expression in oral
squamous cell carcinomas: Correlation with the mTOR-HIF-1α pathway.
Anticancer Res. 31:4429–4437. 2011.PubMed/NCBI
|
|
11
|
Yuan P, Temam S, El-Naggar A, Zhou X, Liu
DD, Lee JJ and Mao L: Overexpression of podoplanin in oral cancer
and its association with poor clinical outcome. Cancer.
107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Friedrich RE, Scheuer HA and Zustin J:
Expression of podoplanin in nevoid basal cell carcinoma
syndrome-associated keratocystic odontogenic tumours. Anticancer
Res. 32:2125–2128. 2012.PubMed/NCBI
|
|
13
|
Tsuneki M, Maruyama S, Yamazaki M, Cheng J
and Saku T: Podoplanin expression profiles characteristic of
odontogenic tumor-specific tissue architectures. Pathol Res Pract.
208:140–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Evans DG and Farndon PA: Nevoid basal cell
carcinoma syndrome. GeneReviews®. Pagon RA, Adam MP,
Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N,
Mefford HC, Smith RJH and Stephens K: University of Washington.
(Seattle, USA). 2015.
|
|
15
|
Agaram NP, Collins BM, Barnes L, Lomago D,
Aldeeb D, Swalsky P, Finkelstein S and Hunt JL: Molecular analysis
to demonstrate that odontogenic keratocysts are neoplastic. Arch
Pathol Lab Med. 128:313–317. 2004.PubMed/NCBI
|
|
16
|
Soluk Tekkeşın M, Mutlu S and Olgaç V:
Expressions of bax, bcl-2 and ki-67 in odontogenic keratocysts
(keratocystic odontogenic tumor) in comparison with ameloblastomas
and radicular cysts. Turk Patoloji Derg. 28:49–55. 2012.PubMed/NCBI
|
|
17
|
Yanamoto S, Kawasaki G, Yoshitomi I and
Mizuno A: Expression of p53R2, newly p53 target in oral normal
epithelium, epithelial dysplasia and squamous cell carcinoma.
Cancer Lett. 190:233–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toi M, Matsumoto T and Bando H: Vascular
endothelial growth factor: Its prognostic, predictive, and
therapeutic implications. Lancet Oncol. 2:667–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alaeddini M, Mostafaloo E,
Mirmohammadkhani O, Eshghyar N and Etemad-Moghadam S: Exploring the
concept of ‘inflammatory angiogenesis’ in keratocystic odontogenic
tumor. Med Oral Patol Oral Cir Bucal. 18:e241–e245. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Wang J, Ding X, Xing S, Zhang W
and Wang L, Wu H and Wang L: Altered expression of podoplanin in
keratocystic odontogenic tumours following decompression. Oncol
Lett. 7:627–630. 2014.PubMed/NCBI
|
|
22
|
González-Alva P, Tanaka A, Oku Y,
Yoshizawa D, Itoh S, Sakashita H, Ide F, Tajima Y and Kusama K:
Keratocystic odontogenic tumor: A retrospective study of 183 cases.
J Oral Sci. 50:205–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chirapathomsakul D, Sastravaha P and
Jansisyanont P: A review of odontogenic keratocysts and the
behavior of recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 101:5–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morgan TA, Burton CC and Qian F: A
retrospective review of treatment of the odontogenic keratocyst. J
Oral Maxillofac Surg. 63:635–639. 2005. View Article : Google Scholar : PubMed/NCBI
|