Introduction
It has previously been demonstrated in human renal
cell carcinoma that the endoplasmic reticulum (ER) protein, ERp46,
a member of the protein disulfide isomerase (PDI) family of
oxidoreductases (1), is overexpressed
in renal cell carcinoma, and increased expression levels of ERp46
may promote renal cell carcinoma growth in vivo (2). ERp46, also known as thioredoxin
domain-containing 5, has three thioredoxin-like domains and is a
member of the PDI family of oxidoreductases, which includes 19
members that have been described in mammalian ER (1). PDIs are ubiquitous proteins, and their
activity facilitates the reduction of disulfides in other proteins
by cysteine-disulfide-thiol exchange (1,3). PDIs are
important in the determination of protein structure and function
and are able to augment protein stability, protect them from damage
and increase their half-lives.
The formation of disulfide bonds is reversible and
may be a key element in the regulation of protein stability
(1). Previously, ERp46 was revealed
to interact with adiponectin receptor 1, leading to decreased
activation of adenosine monophosphate-activated protein kinase in
HeLa cells (4). ERp46 has a large
number of other interacting partners as determined by previous
proteomic and interactome studies (5–7); the
majority of ERp46 interacting partners are involved in
oxidoreductive reactions (5).
Although little is known about ERp46′s biological function, it has
previously been demonstrated to be highly overexpressed in
castration-resistant prostate cancer compared with hormone naïve
prostate cancer, and was revealed to interact with the androgen
receptor in human prostate cancer LNCaP cells, which led to an
increase in androgen receptor stability and signaling (8).
Using various methodologies in vitro and
in vivo, the present study additionally investigated ERp46
as a potential therapeutic target in prostate cancer (PC) with a
focus on ERp46-mediated ER stress. The present study demonstrated a
significantly increased ERp46 expression level in PC tissue samples
with a Gleason score ≥7 compared with normal prostate tissues, and
demonstrated that an increased expression level of ERp46 promoted
PC growth, at least in part, due to an increased protective
mechanism of dealing with ER stress.
Materials and methods
Patient material and ERp46
immunohistochemistry
A tissue microarray (TMA) with duplicate cores from
a total of 57 prostate cancer and 9 normal prostate tissue
specimens was obtained from US Biomax (#PR208; Rockville, MD, USA).
Immunohistochemistry for ERp46 was performed as described
previously (2). Immunohistochemical
staining controls included a human lymph node (positive control)
and the omission of primary antibody (negative control). The
H-score for the tissue within each core of the TMA was determined
as a measure of staining intensity, as described in our previous
study (9). For each individual, the
adjusted H-score was determined by subtracting the H-score of the
negative control and averaging triplicate H-scores.
Strains, plasmids, cell lines and cell
culture
Human prostate adenocarcinoma 22Rv1 cells were
obtained from the American Type Culture Collection (Mannasas, VA,
USA) and were propagated in RPMI-1640 medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1.0 mM sodium
pyruvate and 10% (v/v) fetal bovine serum (Thermo Fisher
Scientific, Inc.). Cells were cultured in a humidified atmosphere
at 5% CO2 and 37°C. The cells were routinely verified to
be mycoplasma-free.
The short hairpin (sh)RNA vector for ERp46 (a
pGFP-V-RS plasmid with the ERp46 shRNA sequence as follows: GGT GTG
GTC ATT GTA AGA CTC TGG CTC CT), the non-effective negative
scrambled control and the pCMV6-Kan/Neo plasmid containing the
full-length cDNA encoding human ERp46 were purchased from Origene
Technologies, Inc. (Rockville, MD, USA). Transfection of 22Rv1
cells (4×106) was performed using electroporation
(Bio-Rad Laboratories, Inc., Mississauga, ON, Canada) with 1.5 µg
plasmid DNA in 400 µl ice-cold PBS, at 1,000V. Cells stably
transfected with shERp46 or scrambled control were selected in the
presence of 0.5 µg/ml puromycin, cells transfected with full-length
ERp46 were selected in the presence of 1.5 mg/ml G418.
Cell growth and survival curves
Cell growth was assessed by quantifying DNA content
using the Hoechst-DNA binding assay following 6 days of growth, and
cells were seeded at 1,000 cells/well on a 96-well plate (10). Fluorescence was evaluated using a
SpectraMax Gemini plate reader [Molecular Devices LLC, Sunnyvale,
CA, USA (λexc=360 nm; λem=460 nm)]. Doubling
times were determined from ≥2 separate experiments performed in 8
repeats (mean ± standard error of the mean).
For survival curves, subconfluent cells were
trypsinized, resuspended and seeded at a density of 5,000
cells/well in a 96-well plate in 100 µl fetal bovine serum
(FBS)-supplemented RPMI-1,640 medium. Cells were allowed to adhere
for 24 h, at which time 200 µl fresh medium containing 0.06–60 nM
thapsigargin or 0.06–20 µg/ml tunicamycin was added. Stock
solutions of tunicamycin (2 mg/ml; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) and thapsigargin (1 mM, Sigma-Aldrich; Merck
Millipore) were prepared in dimethyl sulfoxide, aliquoted and
frozen until use. After 24 h, the medium was aspirated and 200 µl
fresh FBS-supplemented medium was added. Cell survival was assessed
by quantifying DNA content using the Hoechst-DNA binding assay
after 5 days, as described above.
The fraction of cells affected
(fa=1-fu) for each drug dose
was averaged and analyzed using CalcuSyn software version 1.2
(Biosoft, Cambridge, UK). CalcuSyn uses the median-effect principle
to determine the potency of each drug, in addition to extrapolating
the dose for any given effect (11),
according to the following equation:
fa/fu=(D/Dm)m,
where D is the dose, Dm is the dose
required to achieve half maximal inhibitory concentration
(IC50) and fa is the fraction affected
by D. Median-effect analyses that yielded a linear
correlation coefficient >0.90 demonstrated that the dose-effect
data conformed to the median-effect principle and were included in
the analysis.
Western blot analysis
For western blot analyses, subconfluent cells were
washed twice with PBS and placed in fresh supplemented medium
containing 60 nM thapsigargin or 2.5 µg/ml tunicamycin. Protein
lysates (10–30 µg) were prepared following 6 and 24 h treatment,
resolved by 10% SDS-PAGE and transferred onto nitrocellulose
membranes. Primary antibodies used were goat anti-ERp46 (dilution,
1:1,000; sc-49660; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), mouse anti-GRP78 (dilution, 1:1,000; #610979, BD Pharmingen,
San Diego, CA, USA) and rabbit anti-PDI antibody (dilution,
1:1,000; #2446, Cell Signaling Technology, Inc., Danvers, MA, USA).
Equal protein loading was verified using mouse β-actin-specific
antibody (A00702; dilution, 1;1,000; GenScript, Piscataway, NJ,
USA). Secondary horseradish peroxidase-conjugated antibodies
(anti-goat, sc-2378; anti-rabbit, sc-2030; anti-mouse, sc-2031;
dilution, 1:1,000; Santa Cruz Biotechnology Inc.) were used.
Incubation in primary and secondary antibody was carried out for 2
h at room temperature in Tris-buffered saline/0.1% Tween 20.
Protein bands were visualized by enhanced chemiluminescence using
Pierce ECL substrate (Thermo Fisher Scientific, Inc.) and Amersham
Hyperfilm ECL film (GE Healthcare Life Sciences, Chalfont, UK).
Animal studies
The animal studies were performed in strict
accordance with the guidelines of the Canadian Council of Animal
Care and were reviewed and approved by the McMaster University
Animal Research Ethics Board (Hamilton, ON, Canada). All necessary
steps were taken to minimize suffering and distress to the mice.
Per treatment group, five-week old male inbred BALB/c nude mice
(Charles River Laboratories, St. Constant, PQ, Canada) weighing
15–20 g, were randomly divided into groups of ten mice. The mice (5
mice/microisolator cage) were kept in an ultraclean room on a
12/12-h-light/dark cycle in a temperature (21–24°C) and humidity
(35–45%) controlled environment, with food (#2918 irradiated Teklad
rodent diet; Harlan Laboratories, Mississauga, ON, Canada) and
water available ad libitum. Each cage contained Alpha-dri
bedding and a rubber tube to play/sleep for enrichment.
Parental human prostate carcinoma 22Rv1,
ERp46-overexpressing (ERp46+), shERp46 or scrambled control 22Rv1
cells were resuspended at a concentration of 1×107
cells/ml (1:1 (v/v) serum-free medium (Matrigel; BD Biosciences,
Franklin Lakes, NJ, USA). Cell viability was >95% by trypan blue
exclusion. Cells (1.5×106 cells in 150 µl) were injected
subcutaneously into the right flank of the mouse. In order to
assess differences in in vivo tumor growth, the size of the
tumors was determined every three days using plastic Vernier
calipers and tumor volume was evaluated [as 0.5x (length × width ×
height)]. The mice in all groups were assessed together by
alternating the cage order and randomly selecting the mice from
each cage. The data were used to construct tumor growth curves.
After 35 days, the animals were sacrificed, blood was collected and
the tumors dissected and weighed. A portion of the tumor was fixed
in formalin and embedded in paraffin, while the other part was
snap-frozen in liquid nitrogen. The amount of serum
prostate-specific antigen (PSA) was determined using a human PSA
ELISA-kit (Abcam, Cambridge, MA, USA).
Immunostaining of 4 µm-thick tumor xenograft
sections for cluster of differentiation 31 (CD31) and subsequent
image analysis were performed as previously described by Kleinmann
et al (12). As a measure of
microvessel density in the tumor tissue, two fields of view at
magnification, ×7 with the highest vessel density were selected in
order to determine the total cumulative linear endothelial length
using the ImageScope software version 12.2.2 (Aperio Technologies,
Inc., Vista, CA, USA). The endothelial length (in µm) was divided
by the area of the field of view in mm2.
Statistical analysis
Values are given as the mean ± 95% confidence
interval (CI) or standard error of the mean, as indicated. The
normally distributed data were analyzed using a two-tailed
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. The longitudinal tumor
volumes were analyzed using one-way analysis of variance (ANOVA)
and Tukey's post-hoc tests. Statistical analyses were performed
using MiniTab version 14 (MiniTab Inc., State College, PA,
USA).
Results
ERp46 expression level is increased in
clinical specimens of human PC tissues of Gleason score ≥7
Using ERp46-immunohistochemistry on tissue
microarrays containing human PC tissue samples of various stages
and normal prostate specimens, the present study identified the
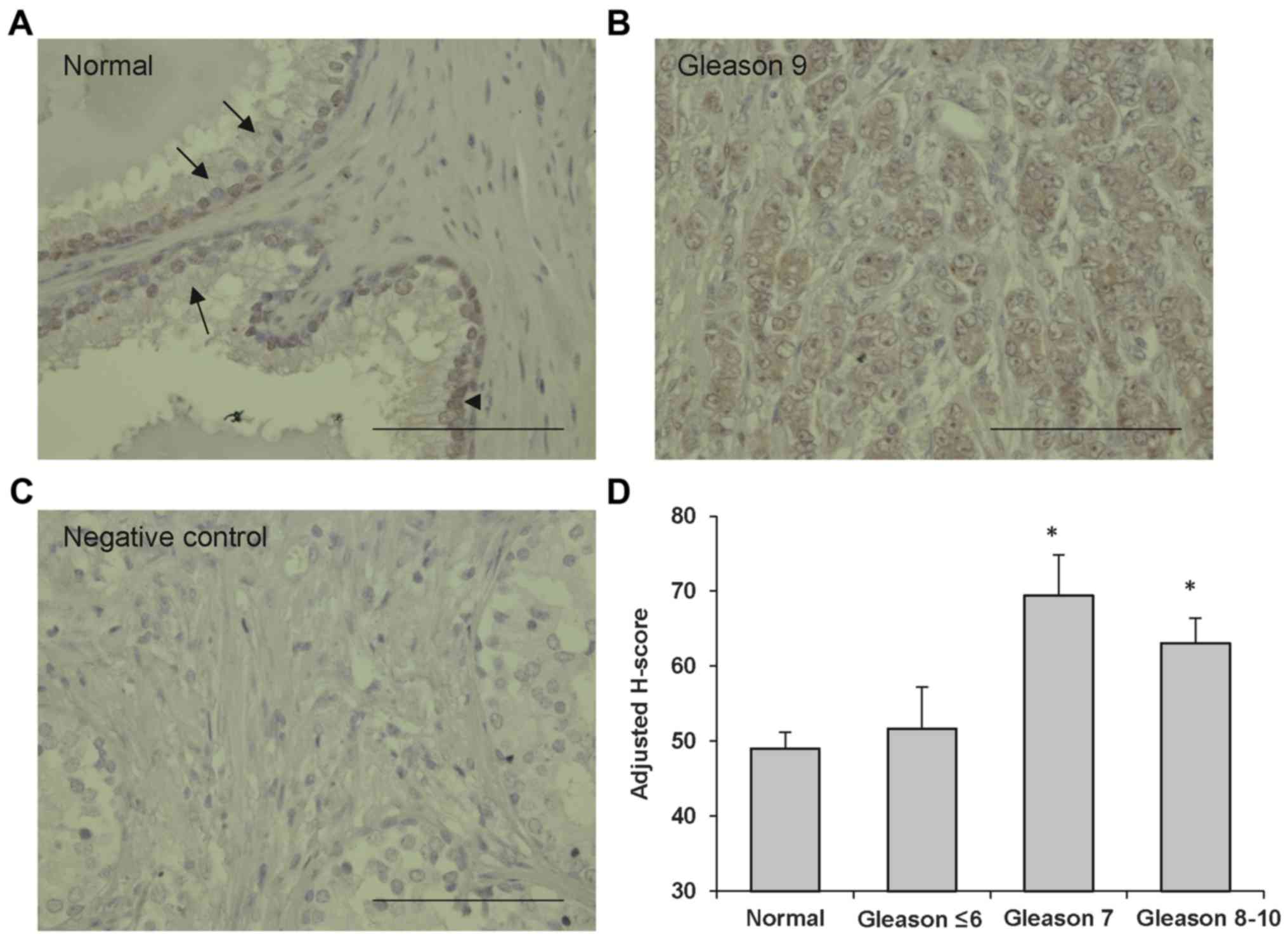
presence of ERp46 protein in specimens from normal prostate tissue
(Fig. 1A) and from prostate carcinoma
tissue samples (Fig. 1B). ERp46
staining was prominent in the cytoplasm with a granular pattern
indicative of ER staining. Staining was also observed in the
nucleus. The negative control, consisting of the omission of
primary antibody, did not reveal any background staining (Fig. 1C). The amount of ERp46 protein was
quantified by image analysis (H-score; Fig. 1D). Prostate carcinoma tissue samples
of Gleason scores of ≤6 did not exhibit increased ERp46 staining
compared with normal prostate tissue samples, whereas prostatic
tumors of Gleason scores 7 or ≥8 demonstrated significantly
stronger ERp46 staining compared with normal tissue samples
(P<0.005 and P=0.02, respectively).
ERp46 is associated with tumor growth
in vitro and in vivo
To investigate the significance of ERp46 expression
on PC, stably transfected human prostate adenocarcinoma 22Rv1
subclones were generated. Human PC 22Rv1 cells are
androgen-responsive and produce PSA. The 22Rv1 cells stably
overexpressing ERp46 were created by transfection with an
expression plasmid containing the full-length human ERp46, as was
the case for stable ERp46-knockdown (using shRNA specific for
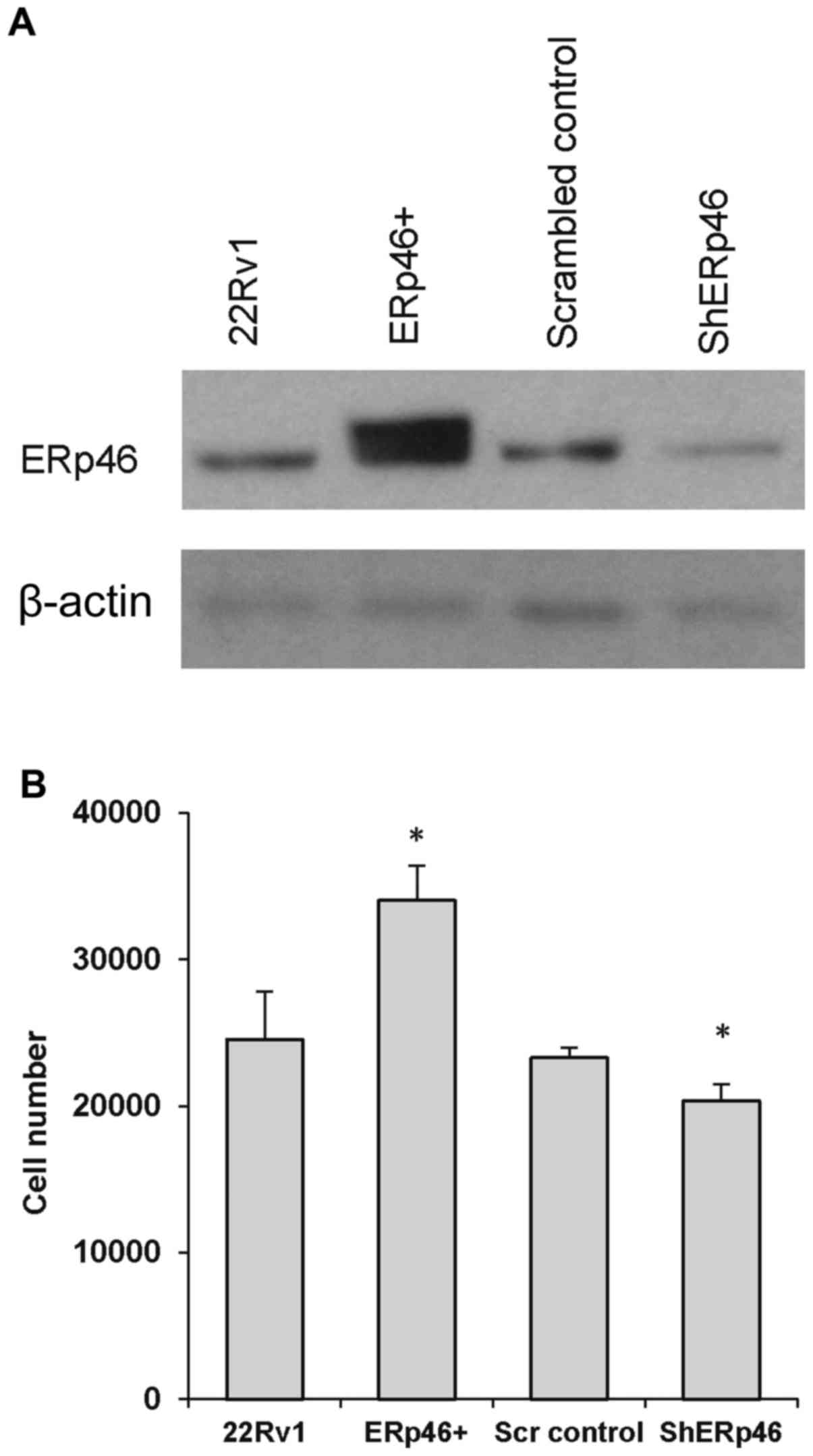
ERp46). Overall, the transfected cells expressed 89% knockdown of
ERp46 protein expression (shERp46) or a 4-fold increase in ERp46
protein expression level (ERp46+) compared with the respective
control cells (Fig. 2A). The growth
rates of the ERp46-manipulated cell lines differed from their
corresponding controls. Following 6 days of growth, the number of
ERp46+ cells was significantly increased compared with parental
22Rv1 cells (Fig. 2B). The 22Rv1
cells had a determined doubling time of 30.81±2.71 h, whereas the
doubling time of the ERp46+ cells was 29.20±2.07 h. Conversely,
shERp46 cells grew more slowly compared with the scrambled control
cells; after 6 days, the number of shERp46 cells was significantly
reduced compared with the scrambled control cells (Fig. 2B), with a determined doubling time of
32.20±1.18 h vs. 32.75±1.52 in shERp46 cells.
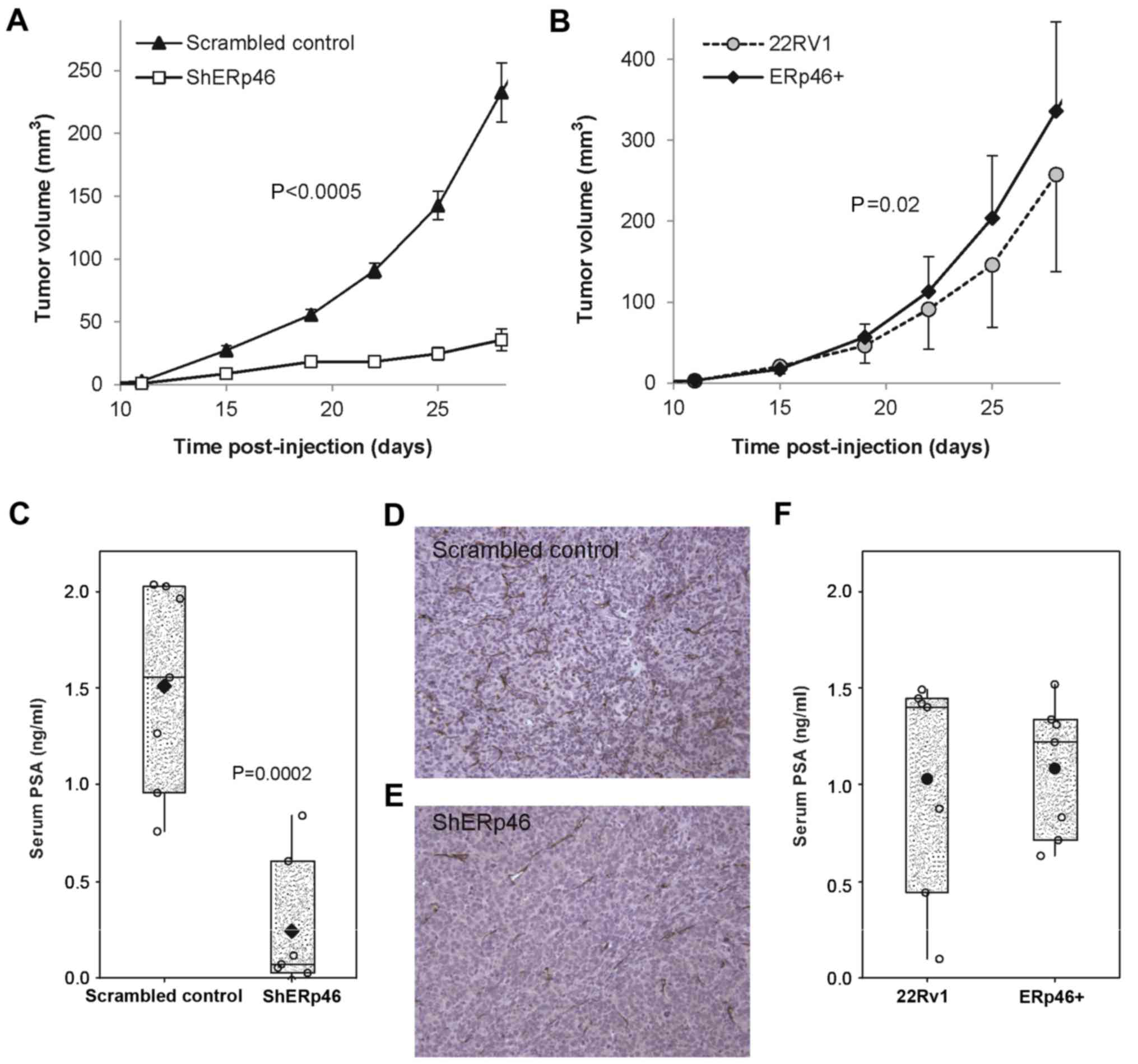
The in vivo growth rate of the various
ERp46-manipulated subclones of PC 22Rv1 cells was similarly
affected following subcutaneous injection into nude mice
(n=10/group; with a tumor take rate of 100%). Subcutaneously
growing shERp46 cells demonstrated significantly slower tumor
growth [P<0.0005 (ANOVA); Fig.
3A]. In addition, ERp46+ tumors had a significantly
increased tumor volume compared with those from 22Rv1-cell injected
mice [P=0.02 (ANOVA); Fig. 3B]. Upon
sacrifice, serum PSA values were determined. Serum PSA was
significantly reduced in mice injected with shERp46 cells compared
with mice injected with scrambled control shRNA-transfected cells
[P=0.0002 (Student's t-test); Fig.
3C]. Microarray analysis of RNA isolated from the tumors
demonstrated that ERp46 remained overexpressed in the
ERp46+-injected mice and reduced in the shERp46-mice at sacrifice,
5 weeks following tumor cell injection (data not shown). Using
immunohistochemical staining of the subcutaneous tumors for CD31,
an endothelial marker, the present study also demonstrated a
decreased amount of microvessels in the shERp46-treated mice
(Fig. 3D and E), with a linear
microvessel length decrease of 37.1% compared with scrambled
control xenografts (0.84±0.23 vs. 1.33±0.34 nm/µm2;
P=0.003). There was no significant difference in serum PSA values
(Fig. 3F) or in the amount of
microvessels between the 22Rv1- and ERp46+-injected mice (data not
shown).
ERp46 overexpression induces less ER
stress
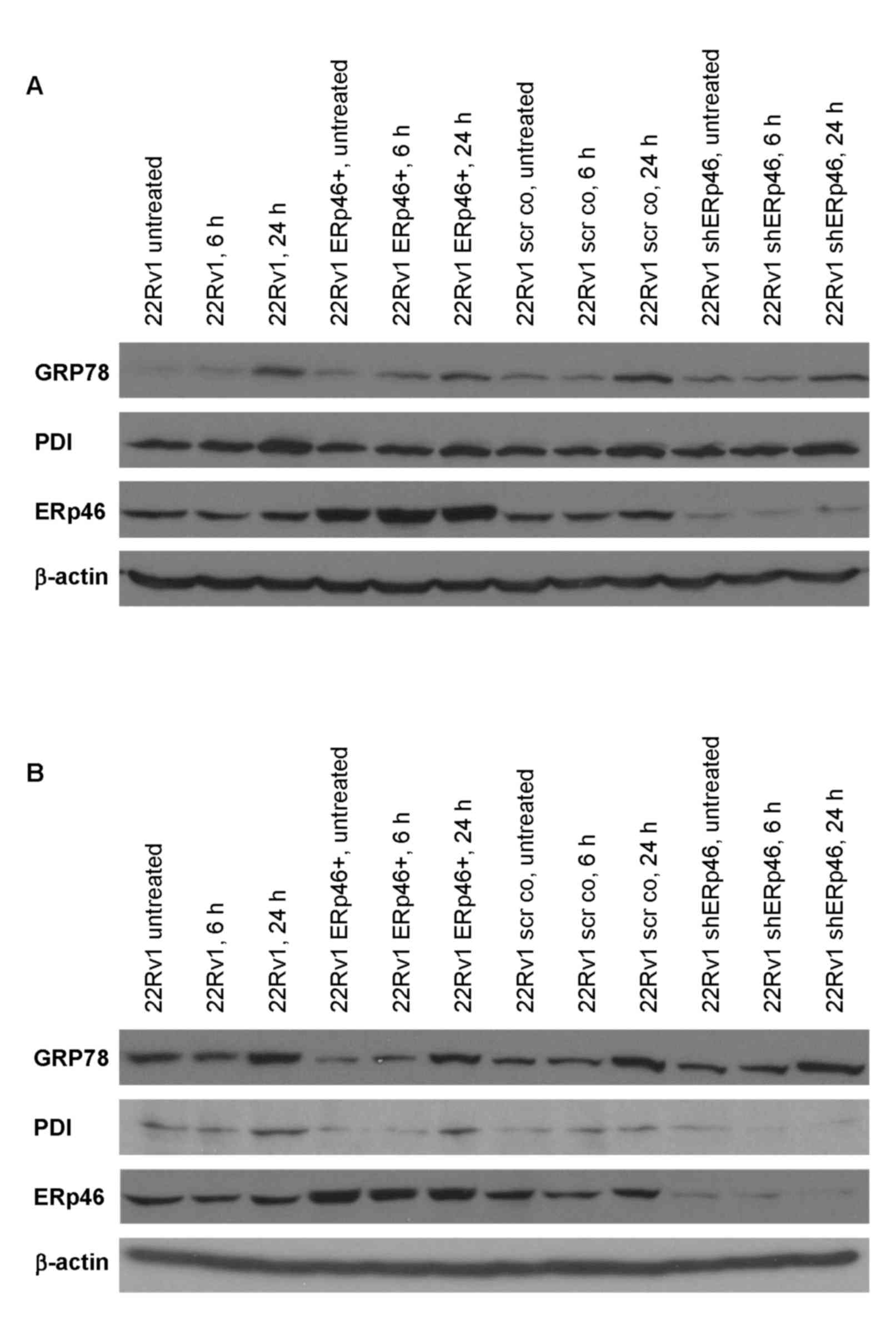
Since ERp46 belongs to the family of protein
disulfide isomerases, which are involved in protein folding in the
ER, the effects of ER-stress-inducing compounds, tunicamycin and
thapsigargin, on GRP78, an ER stress-associated protein, in the
various ERp46-expressing subclones were determined (Fig. 4). GRP78 protein expression increased
in all subclones 3–8-fold following 24 h of treatment with
thapsigargin or tunicamycin (Fig. 4).
PDI, another member of the protein disulfide isomerase family, was
also increased, except in the shERp46 cells treated with
tunicamycin where a decrease in PDI protein expression was
revealed. The dose-effect relationships for tunicamycin and
thapsigargin were also determined via cell survival following a
24-h exposure. The data were subjected to median-effect analysis in
order to determine potency (IC50) and the linear
correlation coefficient (Table I).
Overall, ERp46-overexpressing 22Rv1 cells were better able to deal
with ER stress. For tunicamyin and thapsigargin, the
IC50 was significantly increased in ERp46+ cells
compared with the parental 22Rv1 cells. For thapsigargin, the
IC50 amounted to 31.54 nM (95% CI, 14.78–67.33) in
ERp46+ cells vs. 6.98 nM (95% CI, 5.96–8.16) in 22Rv1 cells, which
was a 4.5-fold increase. For tunicamycin, the IC50
amounted to 0.75 µg/ml (95% CI, 0.57–0.98) in ERp46+ cells vs. 0.18
µg/ml (95% CI, 0.07–0.46) in 22Rv1 cells, which was a 4-fold
increase.
 | Table I.Dose-effect analysis following
treatment of the ERp46-manipulated human prostate cancer 22Rv1
cells with ER stress-inducing agents for 24 h. |
Table I.
Dose-effect analysis following
treatment of the ERp46-manipulated human prostate cancer 22Rv1
cells with ER stress-inducing agents for 24 h.
|
| Thapsigargin | Tunicamycin |
|---|
|
|
|
|
|---|
| Cell line | IC50 (95%
CI), nM | r | IC50 (95%
CI), µg/ml | r |
|---|
| 22Rv1 | 6.98 (5.96–8.16) | 0.99 | 0.18 (0.07–0.46) | 0.91 |
| ERp46+ | 31.54
(14.78–67.33) | 0.98 | 0.75 (0.57–0.98) | 0.99 |
| Scrambled
control | 2.62 (1.43–4.80) | 0.99 | 0.29 (0.16–0.51) | 0.94 |
| shERp46 | 5.46
(1.58–18.84) | 0.95 | 0.36 (0.14–0.92) | 0.91 |
Discussion
An increased protein expression level of ERp46 in
human metastatic renal cell carcinoma has previously been
demonstrated (2), while other studies
have demonstrated increased ERp46 protein expression in non-small
cell lung carcinoma (13), colorectal
adenoma and carcinoma specimens (14), as well as in castration-resistant
prostate tumor samples compared with specimens of hormone-naive
prostate cancer (8). Similarly, the
results of the present study support an association between ERp46
overexpression and prostate cancer aggressiveness. The present
study revealed that ERp46 is overexpressed in prostate cancer
samples with a Gleason score ≥7, compared with normal prostate
tissue samples, and that increased expression of ERp46 promotes PC
growth in vitro and in vivo. While the differences in
doubling times of the subclones in vitro may appear small
(1.61 h for ERp46+ vs. 22Rv1 and 0.55 h for shERp46 vs. scrambled
control cells), significant differences in cell numbers were
observed following several doubling times. Knockdown of ERp46
demonstrated more significant results on tumor growth in
vivo compared with overexpression, which may be due to an
already robust expression level of ERp46 in the parental cells.
Similar to the results of the present study, ERp46 overexpression
also induced an increased tumor cell growth rate in human prostate
adenocarcinoma LNCaP cells, in vitro and in vivo
(8). This was revealed to be at least
in part due to increased androgen receptor stability and signaling
in the presence of increased ERp46 (8). Accordingly, in the in vivo
experiments of the present study, a significant reduction of PSA
secretion, a potential indication of tumor volume, was identified
following knockdown of ERp46 (Fig.
3C); however, no significant alterations in PSA secretion by
tumors overexpressing ERp46 were observed, which may be due to a
sufficient innate ERp46 expression level of the parental cells.
In the present study, a further potential
pro-tumorigenic mechanism underlying ERp46 in tumor cells and its
involvement in ER stress was investigated. Tumor cells adapt to ER
stress, defined by the accumulation of unfolded or misfolded
proteins within the ER, by intracellular signaling pathways, which
are collectively known as the unfolded protein response (15). The unfolded protein response is
initiated by GRP78, an ER chaperone; transcriptional activation of
GRP78 results in an upregulation of genes encoding proteins that
assist in protein folding, maturation and degradation (15). Human prostate cancer cells express
significantly more GRP78 compared with normal prostate epithelial
cells, and GRP78 overexpression correlates with recurrence and poor
survival (16). The ability to
activate the unfolded protein response has been linked to prostate
tumorigenesis and cancer progression, as prostate-specific deletion
of GRP78 in phosphatase and tensin homolog-deficient mice has been
demonstrated to inhibit prostate tumorigenesis (17).
The present study demonstrated that the
IC50 for tunicamycin and thapsigargin was increased by
~4-fold in ERp46-overexpressing cells compared with the
IC50 in the other cell lines, indicating that the ERp46+
cells were less sensitive to ER stress. However, GRP78 protein
expression increased to a similar extent in all cells following
induction of ER stress. Similar to the results of the present
study, 50 nM thapsigargin also induced an increase in GRP78 in
human prostate cancer PC-3 cells after just 6 h of treatment, in a
previous study (18). The results of
the present study also demonstrated that PDI, a member of the
protein disulfide isomerase family as ERp46 is, does not appear to
compensate for ERp46, as all different subclones expressed similar
basal PDI levels. PDI protein expression levels also increased with
ER stress in all four subclones, except in the shERp46 cells
treated with tunicamycin, where a decrease in PDI protein
expression level was observed. In neuroblastoma cells, PDI
expression is upregulated by tunicamycin and hypoxia (19), and inhibiting PDI activity sensitizes
cells to stress-induced apoptosis (20). The data of the present study may
indicate that shERp46 cells have lost the ability to upregulate PDI
upon ER stress, which may explain the decreased cell growth in
vitro and in vivo.
In vivo, knockdown of ERp46 induced a
significant overall decrease in tumor volume, with a mean reduction
of 74% between 15 and 28 days following cell injection, an effect
comparable to chemotherapeutic drugs, including docetaxol (7.5
mg/kg) or cisplatin (5 mg/kg) in 22Rv1-bearing mice (21). While targeting ERp46 alone may not be
effective at slowing tumor growth in the long-term, combining
inhibition of ERp46 with docetaxol or cisplatin treatment may be a
potential strategy to maximally inhibit numerous growth pathways
and yield synergistic effects (21).
Indeed, in vitro sensitization to docetaxol has been
demonstrated for another ER stress inducer, methylseleninic acid
(18). Targeting ERp46 may therefore
potentiate the effect of chemotherapy in prostate cancer.
Acknowledgements
The authors thank Ms. Stephanie Fedorov (McMaster
University, Hamilton, ON, Canada) for the technical assistance
provided. The present study was supported by a McMaster Surgical
Associates basic research grant and Prostate Cancer Canada (pilot
grant #2011-709).
References
|
1
|
Hatahet F and Ruddock LW: Protein
disulfide isomerase: A critical evaluation of its function in
disulfide bond formation. Antioxid Redox Signal. 11:2807–2850.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duivenvoorden WC, Paschos A, Hopmans SN,
Austin RC and Pinthus JH: Endoplasmic reticulum protein ERp46 in
renal cell carcinoma. PLoS One. 9:e903892014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benham AM: The protein disulfide isomerase
family: Key players in health and disease. Antioxid Redox Signal.
16:781–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Charlton HK, Webster J, Kruger S, Simpson
F, Richards AA and Whitehead JP: ERp46 binds to AdipoR1, but not
AdipoR2, and modulates adiponectin signalling. Biochem Biophys Res
Commun. 392:234–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jessop CE, Watkins RH, Simmons JJ, Tasab M
and Bulleid NJ: Protein disulphide isomerase family members show
distinct substrate specificity: P5 is targeted to BiP client
proteins. J Cell Sci. 122:4287–4295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Havugimana PC, Hart GT, Nepusz T, Yang H,
Turinsky AL, Li Z, Wang PI, Boutz DR, Fong V, Phanse S, et al: A
census of human soluble protein complexes. Cell. 150:1068–1081.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kristensen AR, Gsponer J and Foster LJ: A
high-throughput approach for measuring temporal changes in the
interactome. Nat Methods. 9:907–909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Song G, Chang X, Tan W, Pan J, Zhu
X, Liu Z, Qi M, Yu J and Han B: The role of TXNDC5 in
castration-resistant prostate cancer-involvement of androgen
receptor signaling pathway. Oncogene. 34:4735–4745. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duivenvoorden WC, Beatty LK, Lhotak S,
Hill B, Mak I, Paulin G, Gallino D, Popovic S, Austin RC and
Pinthus JH: Underexpression of the tumor suppressor LKB1 in clear
cell renal cell carcinoma is common and confers growth advantage in
vitro and in vivo. Br J Cancer. 108:327–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rago R, Mitchen J and Wilding G: DNA
fluorometric assay in 96-well tissue culture plates using Hoechst
33258 after cell lysis by freezing in distilled water. Anal
Biochem. 191:31–34. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chou TC: Theoretical basis, experimental
design and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kleinmann N, Duivenvoorden WC, Hopmans SN,
Beatty LK, Qiao S, Gallino D, Lhotak S, Daya D, Paschos A, Austin
RC and Pinthus JH: Underactivation of the adiponectin-adiponectin
receptor 1 axis in clear cell renal cell carcinoma: Implications
for progression. Clin Exp Metastasis. 31:169–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vincent EE, Elder DJ, Phillips L, Heesom
KJ, Pawade J, Luckett M, Sohail M, May MT, Hetzel MR and Tavaré JM:
Overexpression of the TXNDC5 protein in non-small cell lung
carcinoma. Anticancer Res. 31:1577–1582. 2011.PubMed/NCBI
|
|
14
|
Wang Y, Ma Y, Lü B, Xu E, Huang Q and Lai
M: Differential expression of mimecan and thioredoxin
domain-containing protein 5 in colorectal adenoma and cancer: A
proteomic study. Exp Biol Med (Maywood). 232:1152–1159. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harding HP, Calfon M, Urano F, Novoa I and
Ron D: Transcriptional and translational control in the mammalian
unfolded protein response. Annu Rev Cell Dev Biol. 18:575–599.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daneshmand S, Quek ML, Lin E, Lee C, Cote
RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, et al:
Glucose-regulated protein GRP78 is up-regulated in prostate cancer
and correlates with recurrence and survival. Hum Pathol.
38:1547–1552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu Y, Wey S, Wang M, Ye R, Liao CP,
Roy-Burman P and Lee AS: Pten null prostate tumorigenesis and AKT
activation are blocked by targeted knockout of ER chaperone
GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA.
105:19444–19449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Fabritius M and Ip C:
Chemotherapeutic sensitization by endoplasmic reticulum stress:
Increasing the efficacy of taxane against prostate cancer. Cancer
Biol Ther. 8:146–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka S, Uehara T and Nomura Y:
Up-regulation of protein-disulfide isomerase in response to
hypoxia/brain ischemia and its protective effect against apoptotic
cell death. J Biol Chem. 275:10388–10393. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko HS, Uehara T and Nomura Y: Role of
ubiquilin associated with protein-disulfide isomerase in the
endoplasmic reticulum in stress-induced apoptotic cell death. J
Biol Chem. 277:35386–35392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Festuccia C, Gravina GL, D'Alessandro AM,
Muzi P, Millimaggi D, Dolo V, Ricevuto E, Vicentini C and Bologna
M: Azacitidine improves antitumor effects of docetaxel and
cisplatin in aggressive prostate cancer models. Endocr Relat
Cancer. 16:401–413. 2009. View Article : Google Scholar : PubMed/NCBI
|