Introduction
Gastric cancer (GC) is one of the most common types
of malignancy and is the leading cause of cancer-associated
mortality worldwide (1). A large
number of chemotherapy drugs have been tested for patients with
advanced gastric cancer, and considerable progress has been
achieved with platinum drug-based regimens (2). However, the overall prognosis of GC
remains poor (3). Therefore,
additional efforts are ongoing to develop safer and more effective
therapeutic strategies.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) has emerged as an attractive anticancer agent, since
it selectively induces cell death in various human cancer cells
with little effect on normal cells (4). Thus far, five TRAIL receptors have been
identified in humans. Among them, TRAIL-R1 (DR4) and TRAIL-R2 (DR5)
mediate apoptosis through characteristic interactions of their
cytoplasmic death domains (5).
Engaged TRAIL receptors recruit adaptor proteins and form the
death-inducing signaling complex, which subsequently activates
caspase cascades with or without mitochondrial amplification
(6). However, it has also been shown
that numerous TRAIL-resistant cancer cells exist (7). Therefore, a number of approaches, such
as combined administration of TRAIL with various sensitizers,
including synthetic small molecules, natural compounds and enzyme
inhibitors, are currently being tested in attempt to overcome TRAIL
resistance (8–10).
The diterpenoid lactone andrographolide is one of
the biologically active constituents of Andrographis
paniculata, a medicinal plant traditionally used for prevention
and treatment of various diseases (11–13). A
number of studies have demonstrated that andrographolide and its
analogues possess potential anti-inflammatory and antitumor effects
mediated by attenuation of nuclear factor-κB activation in various
systems (11–14). Andrographolide also induces cell cycle
arrest and apoptosis of cancer cells by inhibiting phosphoinositide
3-kinase/protein kinase B, mitogen-activated protein kinase and
other tumor growth pathways, depending on the type of treated cells
(15–18). Andrographolide triggers intrinsic and
extrinsic apoptotic pathways in different cancer cells via
mechanisms involving activation of p53, reactive oxygen species
(ROS) and topoisomerase II (17,19,20).
Furthermore, andrographolide demonstrated a potent anticancer
effect when it was applied in combination with other anticancer
agents, including cisplatin and doxorubicin (21,22). In
the present study, the effect of andrographolide was determined in
GCs, and it was reported to perform as a GC sensitizer to the
action of TRAIL.
Materials and methods
Cell culture and dosing
The human GC SNU601, SNU638 and AGS cell lines were
obtained from the Korean Cell Line Bank (Seoul, Korea) and cultured
for 2–10 weeks in the Roswell Park Memorial Institute-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% (v/v) fetal bovine serum (PAA Laboratories;
GE Healthcare, Chalfont, UK) and 1% Penicillin-Streptomycin
(Welgene, Inc., Gyeongsan, Korea) at 37°C in a 5% CO2
atmosphere. Drug treatment of the cells was performed by adding
0–50 µM andrographolide (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) alone or with 5–20 ng/ml recombinant human TRAIL (rhTRAIL;
a gift from T.H. Kim, Department of Biochemistry and Molecular
Biology, Chosun University, Korea) (23) to the culture medium at 37°C for 24–48
h. Antioxidants N-acetyl cysteine (NAC), butylated hydroxyanisole
(BHA), Trolox and catalase, and caspase inhibitors z-DEVD, z-IETD,
z-LEHD, z-LEVD and z-VAD were purchased from EMD Millipore
(Billerica, MA, USA).
MTT viability assays
For the MTT assay, cells were plated in the wells of
a 96-well plate at a density of 1×104 cells/well,
incubated at 37°C for 24 h, and then treated with 0.2% dimethyl
sulfoxide as a vehicle or 10–50 µM andrographolide at 37°C for 48
h. The MTT solution (0.5 mg/ml) was added to the wells and
incubated at 37°C in a CO2 incubator for the last 4 h.
The plates were centrifuged at 600 × g for 10 min at room
temperature and the culture medium was removed. The cells were
solubilized using 100 µl of 100% dimethyl sulfoxide and the
solubilized formazan product was quantified using an enzyme-linked
immunosorbent assay plate reader at 595 nm. The absorbance of the
untreated cells was set as 100% and cell survival was expressed as
a percentage of this value.
Hoechst 33342 (HO)/propidium iodide
(PI) double staining
Treated cells were stained with 1 µg/ml of HO and 5
µg/ml of PI for 15 min at room temperature in the dark. Floating
and attached cells were collected and centrifuged at 500 × g for 10
min at 4°C. The pooled cell pellets were washed with ice-cold PBS,
fixed in 3.7% formaldehyde on ice, washed twice again and
resuspended with PBS, and then a fraction of the suspension was
centrifuged at 500 × g for 10 min at room temperature in Shandon
Cytospin II (Thermo Fisher Scientific, Inc.). Slides were prepared,
air dried, mounted with aqueous mounting medium (Gel Mount;
Biomeda, Foster City, CA, USA) and observed under a fluorescence
microscope (magnification, ×200; DM5000; Leica Microsystems, GmbH,
Wetzlar, Germany) at respective excitation/emission wavelengths of
340/425 nm (HO) and 580/630 nm (PI). For each slide, five fields
were randomly chosen. Morphological assessments of apoptotic and
non-apoptotic death were performed. Intact blue nuclei,
condensed/fragmented blue nuclei, condensed/fragmented pink nuclei
and intact or crushed pink nuclei were considered viable, early
apoptotic, late apoptotic or non-apoptotic dead cells,
respectively. A total of 500 cells distributed across random
microscope viewing fields were counted and the number of apoptotic
or non-apoptotic cells was expressed as a percentage of the total
number of cells scored.
Immunoblotting
Protein extracts (50 µg) were electrophoretically
separated using 10–12% SDS-PAGE and transferred to a nitrocellulose
membrane using a standard technique (24). Antibodies specific to B-cell
lymphoma-2 (Bcl-2; dilution, 1:200; catalog no., 2876S) and B-cell
lymphoma-extra-large (Bcl-xL; dilution, 1:500; catalog no., 2762S)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Anti-p53 (dilution, 1:1,000; catalog no., sc-126), anti-p21
(dilution, 1:1,000; catalog no., sc-6246) and anti-α-tubulin
(dilution, 1:500; catalog no., sc-32293) were obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Anti-DR4 (dilution,
1:200; catalog no., 1139) and anti-DR5 (dilution, 1:500; catalog
no., 2019) were purchased from ProSci, Inc. (Poway, CA, USA).
Anti-Bcl-2 associated X protein (Bax; dilution, 1:200; catalog no.,
BD 610983) was purchased from BD Biosciences (San Hose, CA, USA)
and anti-Bcl-2 homologous antagonist/killer (Bak; dilution, 1:200;
catalog no., 06-536) was purchased from EMD Millipore. Antibody
signals were detected using an Image Station 4000 MM image analyzer
(Kodak, Rochester, NY, USA).
RNA interference (RNAi)
For the RNAi experiment, the sequences of small
interfering (si)RNA were as follows: DR4 forward,
5′-CUGGAAAGUUCAUCUACUU(dtdt)-3′ and reverse,
5′-AAGUAGAUGAACUUUCCAG(dtdt)-3′; DR5 forward,
5′-CAGACUUGGUGCCCUUUG(dtdt)-3′ and reverse,
5′-UCAAAGGGCACCAAGUCUG(dtdt)-3′; and control siRNA forward,
5′-CCUACGCCACCAAUUUCGU(dtdt)-3 and reverse,
5′-ACGAAAUUGGUGGCGUAGG(dtdt)-3′ (Bioneer Corporation, Daejeon,
Korea). Cells were individually transfected with siRNA
oligonucleotides using an Amaxa Transfection System™ (Basel,
Switzerland) and grown at 37°C for 24–36 h prior to the drug
treatment.
Clonogenic assay
Clonogenic activity was measured according to
established procedures with certain modifications in cell numbers
and incubation period (25). For the
clonogenic assay, 2.5×105 cells/35-mm dishes were
pre-incubated with andrographolide or vehicle at 37°C for 18 h,
followed by incubation with rhTRAIL 37°C for another 6 h. Cells
were then trypsinized, and counted under light microscope at ×100
magnification. The mean value of the cell number from 5 counts per
sample was calculated and 2,000 cells were re-plated in 60-mm
dishes in duplicate, and maintained at 37°C/5% CO2 for
14 days in a humidified atmosphere. The grown cells were fixed with
3.7% formaldehyde, stained with 0.5% crystal violet, and colonies
(>0.7 mm diameter) were scored to determine cell proliferating
ability (26).
Detection of ROS generation
Cells were treated with andrographolide for 8, 18
and 24 h and loaded with 50 µM 2′, 7′-dichlorofluorescin diacetate
(DCFDA; Molecular Probes; Thermo Fisher Scientific, Inc.) to
measure ROS generation and 0.5 µg/ml HO to quantify cell number for
30 min. Subsequent to rinsing twice with PBS, fluorescent images
were captured with an inverted fluorescence microscope
(magnification, ×200) or fluorescence intensities were obtained
with a Fluorocount (PerkinElmer, Inc., Port Richey, NJ, USA) at
excitation/emission wavelengths of 490/530 nm (DCFDA) and 340/425
(HO), and values of ROS production were obtained by determining the
ratio of DCFDA/HO signals per well.
Statistical analysis
All numerical data are reported as the mean ±
standard error. P<0.05 was considered to indicate a
statistically significant difference. All data represent the
results of ≥3 independent experiments. Student's t-test was used to
evaluate the differences between control and treated group values,
and one-way analysis of variance was applied to analyze the
significance of differences caused by the effects of gene silencing
or caspase inhibition.
Results and Discussion
Andrographolide inhibits cell growth
and triggers apoptotic and non-apoptotic cell death in human GC
cells
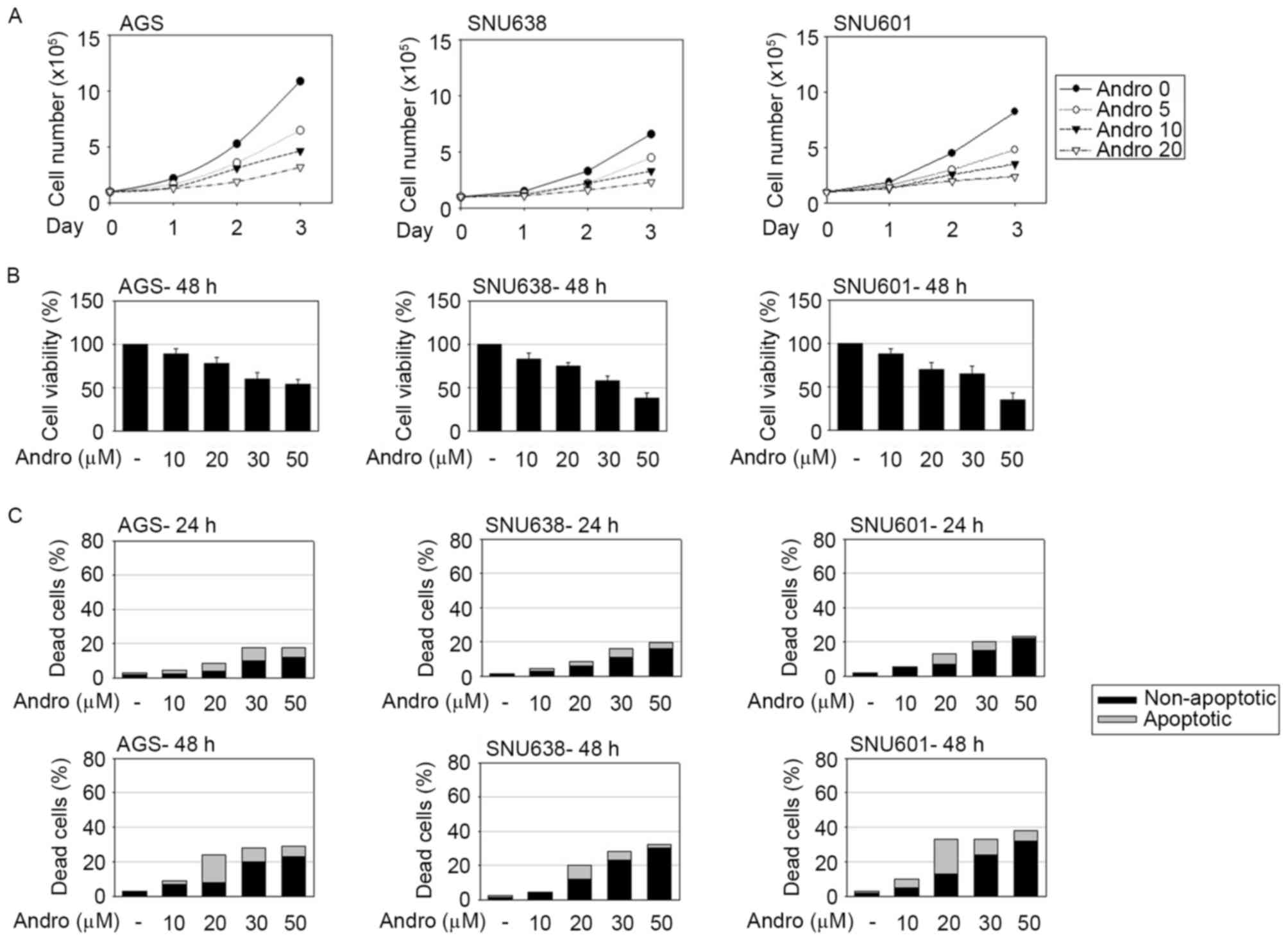
To investigate the antitumor efficacy of
andrographolide in GC, AGS, SNU601 and SNU638 human GC cells were
treated with andrographolide at several concentrations and the
effect of the compound on cell growth and cell viability was
assessed by counting the number of live cells and by performing the
MTT assay, respectively. Andrographolide evidently decreased the
cell growth rate and viability of GC cells (Fig. 1A and B). To examine whether a decrease
of cell viability was accompanied by andrographolide-induced cell
death, occurrence of apoptotic and non-apoptotic cell death was
detected using the HO/PI double staining method. Apoptotic and
non-apoptotic cell death was assessed by the percentage of
condensed or cleaved apoptotic nuclei following HO staining
(apoptotic death) and PI-stained red cells without apoptotic
features (non-apoptotic death). As shown in Fig. 1C, andrographolide induced apoptotic
and non-apoptotic cell death in GC cells. While the total number of
dead cells was linearly associated with the concentration of
andrographolide, the number of apoptotic dead cells with typical
cleaved or condensed nuclei peaked in presence of 20 µM
andrographolide. At concentrations of andrographolide exceeding 20
µM, PI-stained cells with rigid shapes of nuclei became
predominant, indicating an increase of andrographolide-induced
non-apoptotic cell death.
Andrographolide regulates expression
of intrinsic and extrinsic apoptotic proteins
To investigate the mechanisms involved in
andrographolide-induced cytotoxicity, expression levels of cell
cycle inhibitory proteins and apoptosis-inducing proteins were
analyzed by immunoblotting assay. As shown in Fig. 2, andrographolide increased expression
of cyclin inhibitor p21 in all tested GC cells and levels of the
p27 protein in SNU638 cells. Andrographolide also induced
expression of membrane death receptors DR4 and DR5 in GC cells. DR5
expression was induced by relatively low concentrations of
andrographolide (10–20 µM), whereas induction of DR4 expression was
observed following exposure to increased concentrations of
andrographolide (30–40 µM). Andrographolide also increased p53
levels in AGS and SNU638 cells, but not in SNU601 cells. The
transcription factor p53 has been reported to regulate expression
of p21, DR4 and DR5 in response to various cytotoxic stimuli
(27–29). However, the role of p53 as a
transcription regulator may not be essential in this system, since
SNU638 and SNU601 cells carry transcriptionally mutant p53 protein
(30). The effects of andrographolide
on the expression level of Bcl-2 family members (intrinsic
apoptotic regulators) was also detected. The level of
anti-apoptotic Bcl-2 was inversely proportional to the
concentration of andrographolide in all three GC cells, while
expression of another anti-apoptotic protein, Bcl-xL, was
unaffected. At high concentrations, andrographolide also affected
the levels of pro-apoptotic Bcl-2 proteins: Expression of Bak was
elevated in all three GC cells tested, while the level of Bax was
increased only in SNU638 cells. Based on these results, it was
hypothesized that at high concentrations, andrographolide triggers
various stress signaling events, including activation of the
extrinsic and intrinsic apoptotic pathways in GC cells. However,
although expression of pro-apoptotic proteins was directly
proportional to the concentration of andrographolide, apoptotic
death or total cell death was not considerably increased in the
presence of high concentrations of andrographolide (Fig. 1C). This potential discrepancy may be
explained by overload of apoptotic signaling under severe stress
conditions: Extremely harsh stress or high doses of
chemotherapeutic drugs may cause abrupt and multiple stimulation of
destructive pathways as opposed to the sequential apoptotic
cascades.
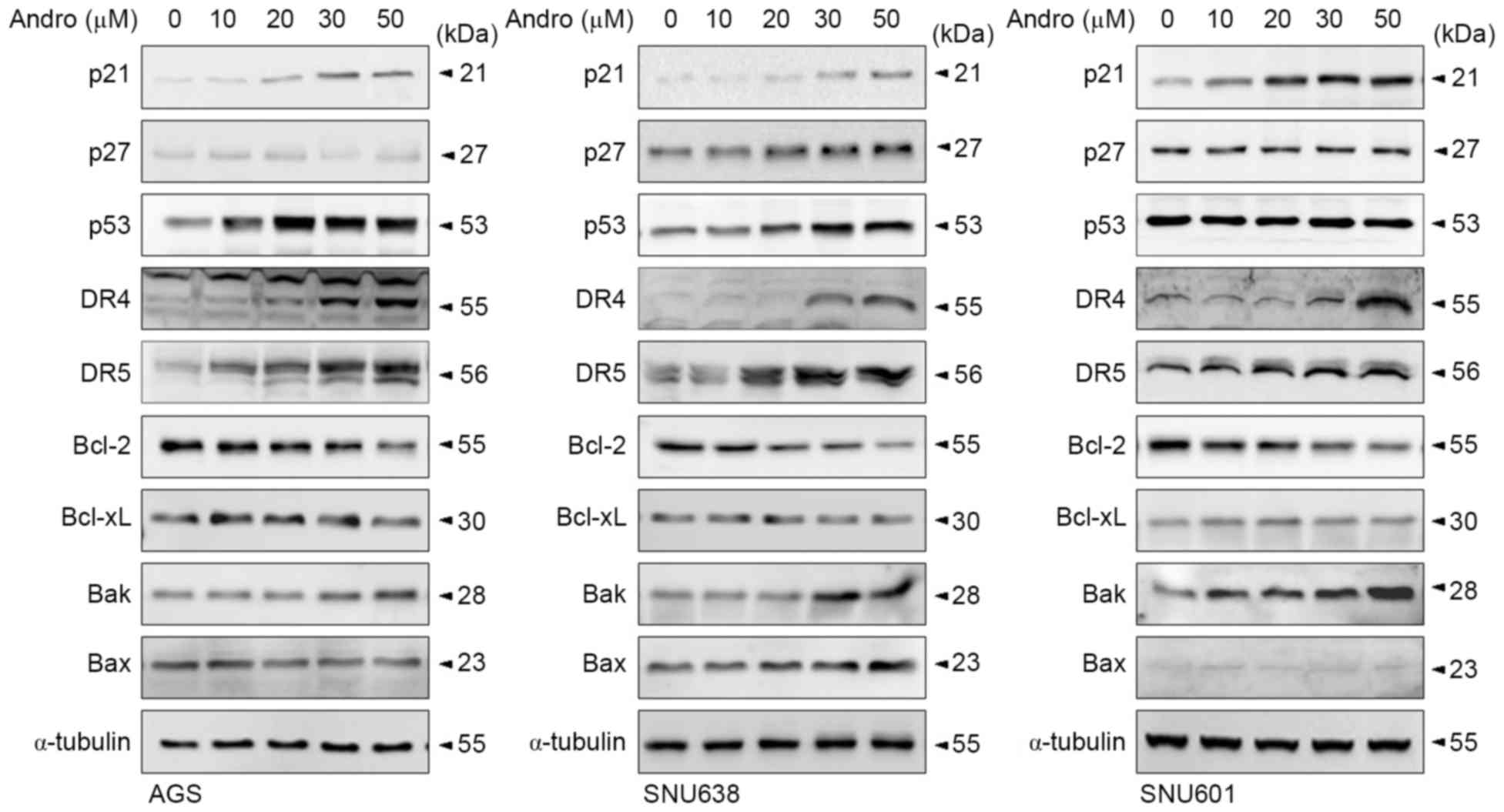 | Figure 2.Andrographolide affected expression
levels of various cell death-regulating proteins in human GC cells.
AGS, SNU638 and SNU601 cells were incubated with andrographolide at
indicated concentrations for 48 h and then harvested to prepare
total protein lysates, in which levels of p21, p27, p53, DR4, DR5,
Bcl-2, Bcl-xL, Bak and Bax proteins were determined by
immunoblotting. α-tubulin was used as a loading control. Bcl-2,
B-cell lymphoma-2; Bcl-xL, B-cell lymphoma-extra-large; DR4, tumor
necrosis factor-related apoptosis-inducing ligand-receptor 1; DR5,
tumor necrosis factor-related apoptosis-inducing ligand-receptor 2;
Bax, Bcl-2-associated X protein; Bak, Bcl-2 homologous
antagonist/killer; Andro, andrographolide. |
Andrographolide enhances
rhTRAIL-induced apoptotic cell death
Although andrographolide triggered cell death of GCs
and induced expression of several death-inducing proteins in the
present study, the use of this drug at high doses may be unsafe due
to the toxic side effects. Recently, the utility of combinations of
drugs with different mechanisms of action for cancer treatment has
been gaining increasing attention (31–34).
Effective combinations of anticancer drugs enhance therapeutic
efficacy, as well as reduce toxicity, since each constituent may be
used at a lower, non-toxic dose. Thus, it was investigated whether
a combination of a low concentration of andrographolide with
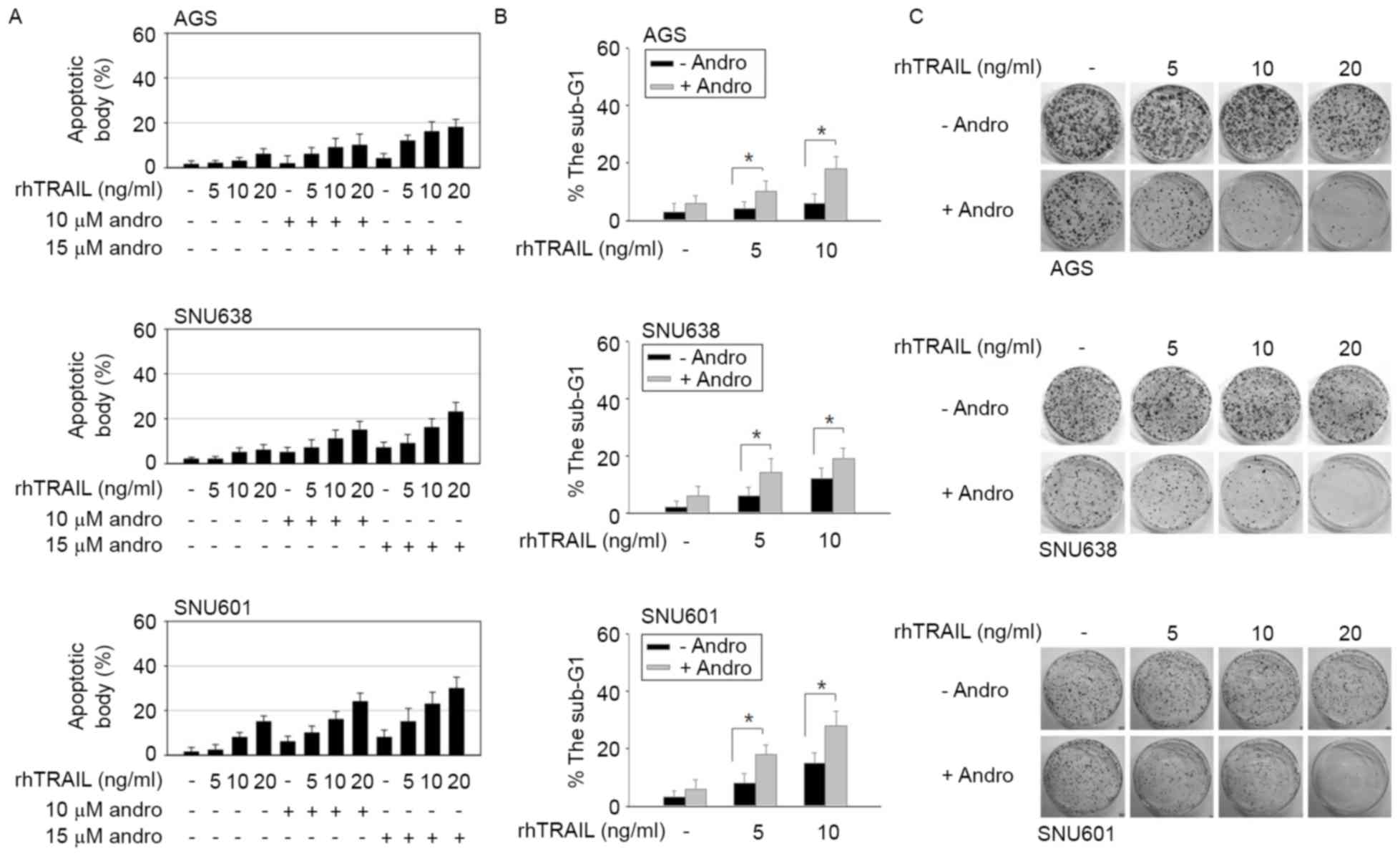
rhTRAIL may enhance rhTRAIL-induced apoptosis of GC cells. Combined
treatment was performed by a pretreatment of cell cultures with 10
or 15 µM andrographolide for 24 h, and a subsequent incubation with
rhTRAIL for another 24 h. As demonstrated in Fig. 3A, the number of apoptotic bodies was
significantly increased following the combined treatment with
andrographolide and rhTRAIL compared with following incubation with
rhTRAIL alone. The effect of the combined treatment with
andrographolide was particularly evident in AGS cells, which
demonstrated resistance to rhTRAIL action. The
andrographolide-mediated enhancement of the apoptotic rate was
confirmed again by flow cytometric analysis, in which the increased
proportion of cells in the sub-G1 area of the cell cycle
was regarded as a sign of apoptosis. As shown in Fig. 3B, the combined treatment with rhTRAIL
and andrographolide caused an increased number of cells in the
sub-G1 phase. Subsequently, to determine whether the
combined treatment with andrographolide reduced colony-forming
ability of GC cells, the clonogenic assay was performed, which is
based on the capacity of a single cell to proliferate into a clone.
It was revealed that the combined treatment with rhTRAIL and
andrographolide caused a more substantial decrease in clonogenic
activity compared with the effect of rhTRAIL alone (Fig. 3C). Together, these results indicated
that andrographolide promotes tumor-suppressing activity of rhTRAIL
in GC cells.
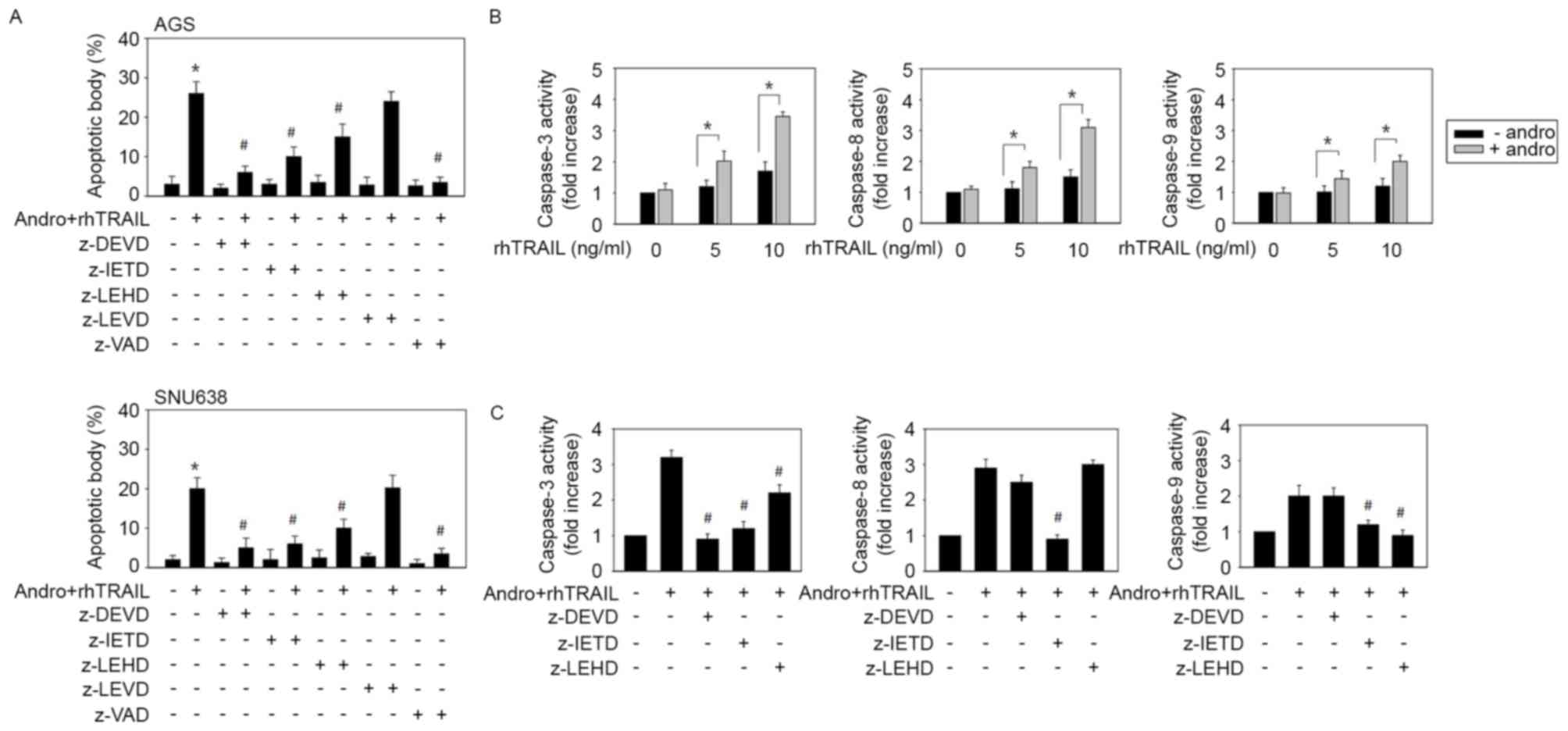
The effect of various caspase inhibitors on
apoptosis triggered by the combination of andrographolide and
rhTRAIL was then examined to estimate the signal pathways involved.
As shown in Fig. 4A, the pan-caspase
inhibitor z-vad-fmk and the caspase-3 inhibitor z-DEVD-fmk almost
completely prevented apoptosis. The caspase-8 inhibitor z-IETD-fmk
also significantly inhibited apoptosis, while the caspase-9
inhibitor z-LEHD-fmk caused weaker but statistically significant
inhibition. At the same time, the caspase-4 inhibitor z-LEVD-fmk
had little effect on apoptosis. These results indicated that the
combined treatment with andrographolide and rhTRAIL causes
apoptosis primarily by stimulating the extrinsic apoptotic pathway
(caspase-8/caspase-3) and, to a lesser extent, via activation of
mitochondria-linked caspase-9. Pro-apoptotic effects of the
combined treatment with andrographolide and rhTRAIL did not appear
to involve the endoplasmic reticulum stress-associated apoptotic
cascade in this system. The TRAIL-sensitizing effect of
andrographolide was examined in TRAIL-resistant AGS cells by
assessing actual activation of caspases during induction of
apoptosis by the combined treatment. In response to the treatment
with 5 and 10 ng/ml rhTRAIL, activity levels of caspase-3,
caspase-8 and caspase-9 slightly increased. Treatment of AGS cells
with 10 µM andrographolide alone did not induce any increase in
activity of these enzymes. However, the combined treatment with
andrographolide and rhTRAIL enhanced activity of these three
caspases (Fig. 4B). To determine the
identity of the caspase cascades involved, several selective
inhibitors of corresponding enzymes in AGS cells were used. It was
revealed that z-IETD-fmk significantly blocked activity levels of
caspase-9 (P=0.035) and caspase-3 (P=0.012), whereas z-DEVD-fmk
partially inhibited activation of caspase-8. At the same time,
z-LEHD-fmk had little effect on activation of caspase-8 and only
partially decreased caspase-3 activity (Fig. 4C). Collectively, these results
indicated that the combined administration of andrographolide with
rhTRAIL induces caspase-8 activation upstream of caspase-9 and
caspase-3.
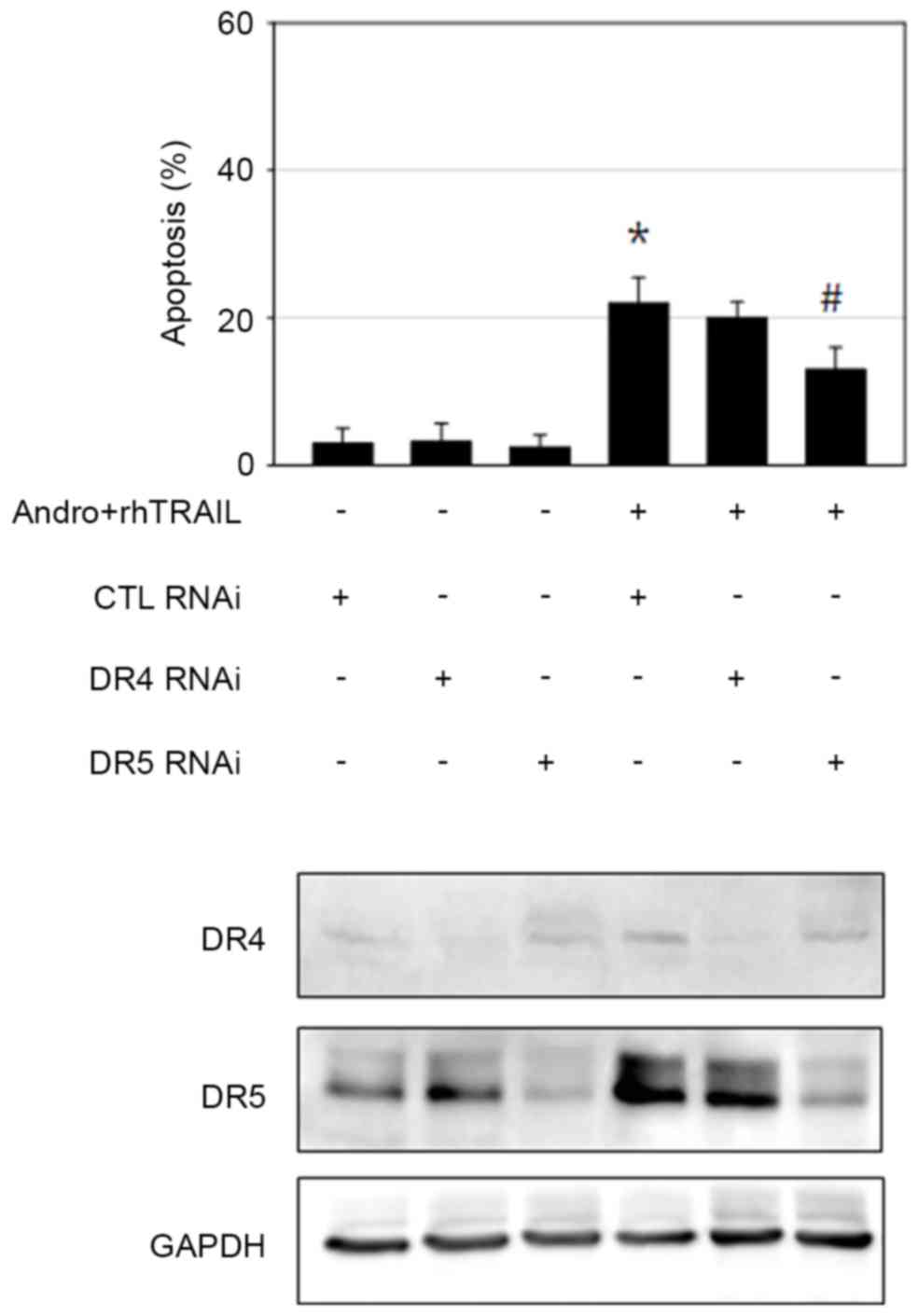
DR5 signaling is essential for
andrographolide-mediated sensitization to the action of
rhTRAIL
The extrinsic apoptotic pathway appeared to be
important in mediating apoptosis caused by the combined treatment
with andrographolide and rhTRAIL, as demonstrated by the major role
of the caspase-8/caspase-3 axis in this process. It was observed
that incubation with andrographolide led to increased expression
levels of DR4 and DR5, the receptors mediating TRAIL-induced
apoptosis in GC cells. Thus, the RNA interference approach was
performed to confirm the role of DR4 and DR5 in sensitization to
the effects of rhTRAIL caused by andrographolide. AGS cells
transfected with scrambled control RNA, DR4 siRNA or DR5 siRNA were
incubated with andrographolide and rhTRAIL, and apoptosis was then
assessed by evaluating apoptotic body formation. Knockdown of DR5
significantly reduced the extent of apoptosis (P=0.028). By
contrast, DR4 knockdown failed to prevent apoptosis induced by the
combined treatment with andrographolide and rhTRAIL (Fig. 5). This may be explained by the
evidence that the concentration of andrographolide used for the
sensitizing activity was reduced compared with the concentration
required for efficient DR4 induction. Thus, DR5 appears to perform
a more important role compared with DR4 in mediating
andrographolide-induced sensitization of AGS cells to the action of
rhTRAIL. However, activation of multiple signaling apoptotic
pathways cannot be ruled out, since apoptosis was not completely
rescued by DR5 knockdown.
ROS is involved in
andrographolide-induced sensitization to effects of rhTRAIL through
potentiation of DR5 expression
Oxidative stress by chemopreventive agents,
including curcumin and casticin, has been implicated in DR5
upregulation and apoptosis (35–37).
Furthermore, numerous natural antitumor compounds have been
reported to induce ROS production (35,37). The
present study explored whether ROS is involved in apoptosis
triggered by the combined action of andrographolide and rhTRAIL by
examining the effects of various antioxidants. The general ROS
scavenger N-acetyl cysteine (NAC) profoundly suppressed apoptosis
and activation of caspases following the combination treatment,
whereas application of catalase had a weaker, but statistically
significant, inhibitory effect (Fig. 6A
and B). However, treatments with the superoxide anion scavenger
butylated hydroxyanisole (BHA) or the lipid peroxidation inhibitor
trolox had no effect (Fig. 6A and B).
Based on these results, ROS, including hydrogen peroxide but not
superoxide anion or superoxide radicals, appear to possess a
critical role in apoptosis induced by the combined treatment with
andrographolide and rhTRAIL. As determined by the
dichloro-dihydro-fluorescein diacetate assay, exposure of AGS cells
to andrographolide significantly increased ROS production, which
may be reduced by application of NAC (Fig. 6C). The effect of antioxidants on the
increase of DR5 expression induced by andrographolide was also
examined. Similar to its inhibitory effect on apoptosis induction,
NAC almost completely blocked induction of DR5 expression (Fig. 6D). BHA and trolox had no effect on DR5
induction, while catalase caused only a partial decrease in DR5
expression (Fig. 6D). Therefore,
induction of oxidative stress by andrographolide may be the
essential mechanism for DR5 induction and sensitization to the
pro-apoptotic effects of rhTRAIL in GCs.
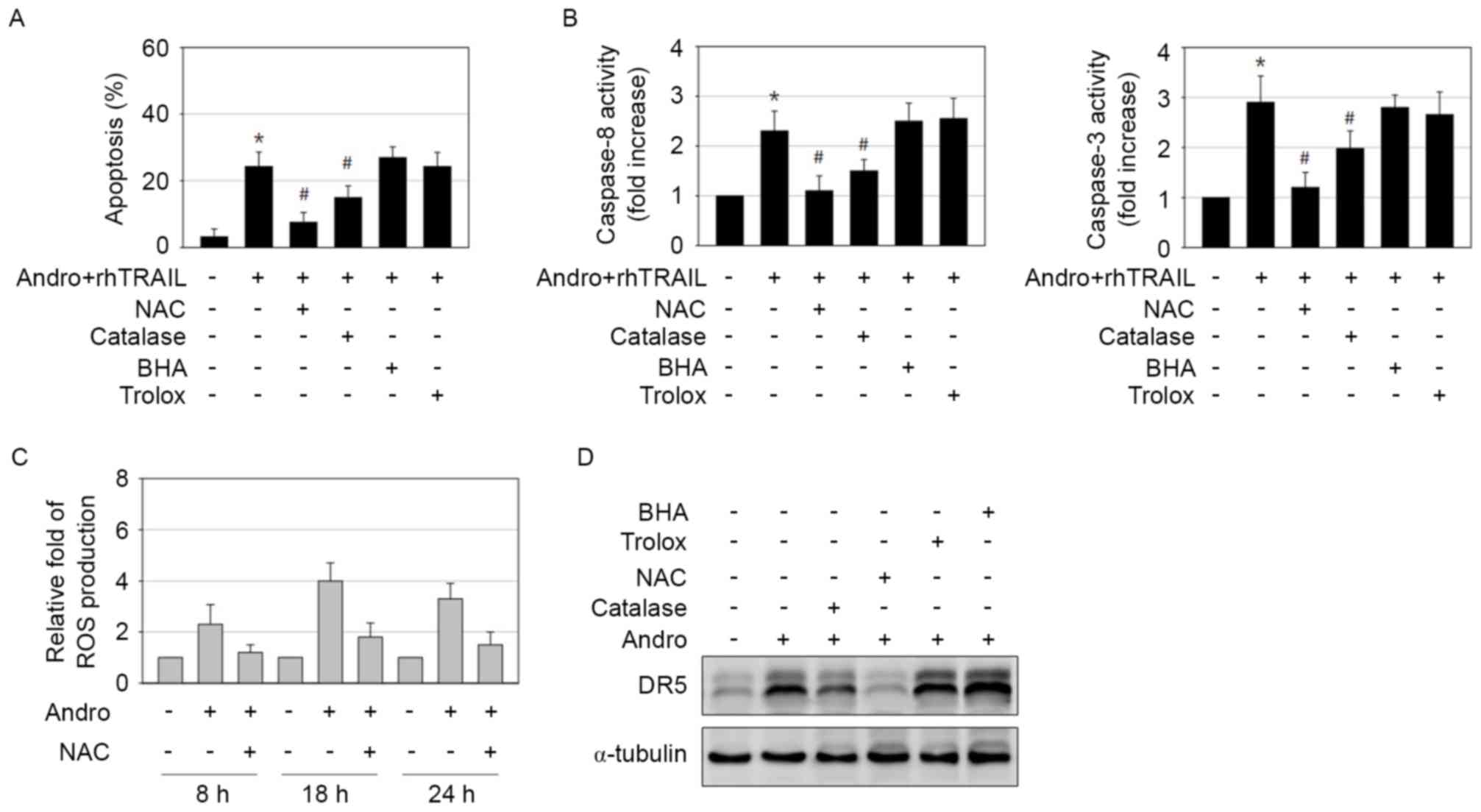 | Figure 6.Andrographolide-induced ROS generation
induces DR5 expression and is important for rhTRAIL-sensitizing
effect. (A and B) AGS cells were treated with a combination of 15
µM andrographolide and 10 ng/ml rhTRAIL in the absence or presence
of 5 mM NAC, 500 U catalase, 50 µM BHA and 50 µM trolox for 48 h.
The treated cells were (A) stained with Hoechst 33342 to allow
detection of apoptotic cells or (B) subjected to caspase-8 and −3
activity assay. *P<0.05 vs. control; #P<0.05 vs.
andrographolide and rhTRAIL-treated cells. (C) Cells treated with
15 µM andrographolide in the presence or absence of 5 mM NAC were
analyzed for ROS generation using DCFH-DA at 8, 18 and 24 h
post-incubation. (D) Cells exposed to 15 µM andrographolide in the
absence or presence of 5 mM NAC, 500 U catalase, 50 µM BHA and 50
µM trolox for 48 h were analyzed by immunoblotting. ROS, reactive
oxygen species; DR5, tumor necrosis factor-related
apoptosis-inducing ligand-receptor 2; rhTRAIL, recombinant human
tumor necrosis factor-related apoptosis-inducing ligand; NAC,
N-acetyl cysteine; BHA, butylated hydroxyanisole; Andro,
andrographolide. |
In conclusion, the present study revealed a possible
role for andrographolide as a sensitizer of GC cells to the action
of TRAIL. Based on the present results, co-application of these
drugs may improve therapeutic efficacy of GC treatment, although
additional clinical studies are required.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education, Science and Technology (grant
no. NRF-2011-0014540). The authors thank Professor Tae-Hyoung Kim
for supplying rhTRAIL and Ms. Jeong-Eun Choi for her technical
assistance.
References
|
1
|
Piazuelo MB and Correa P: Gastric cáncer:
Overview. Colomb Med (Cali). 44:192–201. 2013.PubMed/NCBI
|
|
2
|
Wagner AD, Unverzagt S, Grothe W, Kleber
G, Grothey A, Haerting J and Fleig WE: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev. CD004064:2010.
View Article : Google Scholar
|
|
3
|
Van Cutsem E, Haller D and Ohtsu A: The
role of chemotherapy in the current treatment of gastric cancer.
Gastric Cancer. 5:(Suppl 1). 17–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gura T: How TRAIL kills cancer cells, but
not normal cells. Science. 277:7681997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baker SJ and Reddy EP: Modulation of life
and death by the TNF receptor superfamily. Oncogene. 17:3261–3270.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashkenazi A: Targeting death and decoy
receptors of the tumour-necrosis factor superfamily. Nat Rev
Cancer. 2:420–430. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Srivastava RK: TRAIL/Apo-2L: Mechanisms
and clinical applications in cancer. Neoplasia. 3:535–546. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prasad S, Yadav VR, Kannappan R and
Aggarwal BB: Ursolic acid, a pentacyclin triterpene, potentiates
TRAIL-induced apoptosis through p53-independent up-regulation of
death receptors: Evidence for the role of reactive oxygen species
and JNK. J Biol Chem. 286:5546–5557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siddiqui IA, Malik A, Adhami VM, Asim M,
Hafeez BB, Sarfaraz S and Mukhtar H: Green tea polyphenol EGCG
sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated
apoptosis and synergistically inhibits biomarkers associated with
angiogenesis and metastasis. Oncogene. 27:2055–2063. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szliszka E and Krol W: The role of dietary
polyphenols in tumor necrosis factor-related apoptosis inducing
ligand (TRAIL)-induced apoptosis for cancer chemoprevention. Eur J
Cancer Prev. 20:63–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia YF, Ye BQ, Li YD, Wang JG, He XJ, Lin
X, Yao X, Ma D, Slungaard A, Hebbel RP, et al: Andrographolide
attenuates inflammation by inhibition of NF-kappa B activation
through covalent modification of reduced cysteine 62 of p50. J
Immunol. 173:4207–4217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YJ, Wang JT, Fan QX and Geng JG:
Andrographolide inhibits NF-kappaBeta activation and attenuates
neointimal hyperplasia in arterial restenosis. Cell Res.
17:933–941. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang QQ, Zhou DL, Ding Y, Liu HY, Lei Y,
Fang HY, Gu QL, He XD, Qi CL, Yang Y, et al: Andrographolide
inhibits melanoma tumor growth by inactivating the TLR4/NF-κB
signaling pathway. Melanoma Res. 24:545–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang QQ, Ding Y, Lei Y, Qi CL, He XD, Lan
T, Li JC, Gong P, Yang X, Geng JG and Wang LJ: Andrographolide
suppress tumor growth by inhibiting TLR4/NF-κB signaling activation
in insulinoma. Int J Biol Sci. 10:404–414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu SH, Lin CH, Liang FP, Chen PF, Kuo CD,
Alam MM, Maiti B, Hung SK, Chi CW, Sun CM and Fu SL:
Andrographolide downregulates the v-Src and Bcr-Abl oncoproteins
and induces Hsp90 cleavage in the ROS-dependent suppression of
cancer malignancy. Biochem Pharmacol. 87:229–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen K, Ji L, Lu B, Xu C, Gong C, Morahan
G and Wang Z: Andrographolide inhibits tumor angiogenesis via
blocking VEGFA/VEGFR2-MAPKs signaling cascade. Chem Biol Interact.
218:99–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang SH, Wang SM, Syu JP, Chen Y, Wang SD,
Peng YS, Kuo MF and Kung HN: Andrographolide induces apoptosis of
C6 glioma cells via the ERK-p53-caspase 7-PARP pathway. Biomed Res
Int. 2014:3128472014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Zhang C, Jiang H and Cheng J:
Andrographolide inhibits hypoxia-inducible factor-1 through
phosphatidylinositol 3-kinase/AKT pathway and suppresses breast
cancer growth. Onco Targets Ther. 8:427–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nateewattana J, Dutta S, Reabroi S, Saeeng
R, Kasemsook S, Chairoungdua A, Weerachayaphorn J, Wongkham S and
Piyachaturawat P: Induction of apoptosis in cholangiocarcinoma by
an andrographolide analogue is mediated through topoisomerase II
alpha inhibition. Eur J Pharmacol. 723:148–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu CY, Yang YC, Li CC, Liu KL, Lii CK and
Chen HW: Andrographolide inhibits TNFα-induced ICAM-1 expression
via suppression of NADPH oxidase activation and induction of HO-1
and GCLM expression through the PI3K/Akt/Nrf2 and PI3K/Akt/AP-1
pathways in human endothelial cells. Biochem Pharmacol. 91:40–50.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J, Ong CN, Hur GM and Shen HM:
Inhibition of the JAK-STAT3 pathway by andrographolide enhances
chemosensitivity of cancer cells to doxorubicin. Biochem Pharmacol.
79:1242–1250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin HH, Shi MD, Tseng HC and Chen JH:
Andrographolide sensitizes the cytotoxicity of human colorectal
carcinoma cells toward cisplatin via enhancing apoptosis pathways
in vitro and in vivo. Toxicol Sci. 139:108–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin JN, Park SY, Cha JH, Park JY, Lee BR,
Jung SA, Lee ST, Yun CW, Seol DW and Kim TH: Generation of a novel
proform of tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL) protein that can be reactivated by matrix
metalloproteinases. Exp Cell Res. 312:3892–3898. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rafehi H, Orlowski C, Georgiadis GT,
Ververis K, El-Osta A and Karagiannis TC: Clonogenic assay:
Adherent cells. J Vis Exp. pii: 2573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
el-Deiry WS, Harper JW, O'Connor PM,
Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill
DE, Wang Y, et al: WAF1/CIP1 is induced in p53-mediated G1 arrest
and apoptosis. Cancer Res. 54:1169–1174. 1994.PubMed/NCBI
|
|
28
|
Wu GS, Burns TF, McDonald ER III, Jiang W,
Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, et al:
KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor
gene. Nat Genet. 17:141–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheikh MS, Burns TF, Huang Y, Wu GS,
Amundson S, Brooks KS, Fornace AJ Jr and el-Deiry WS: p53-dependent
and -independent regulation of the death receptor KILLER/DR5 gene
expression in response to genotoxic stress and tumor necrosis
factor alpha. Cancer Res. 58:1593–1598. 1998.PubMed/NCBI
|
|
30
|
Park JG, Yang HK, Kim WH, Chung JK, Kang
MS, Lee JH, Oh JH, Park HS, Yeo KS, Kang SH, et al: Establishment
and characterization of human gastric carcinoma cell lines. Int J
Cancer. 70:443–449. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruiz-González R, Milán P, Bresolí-Obach R,
Stockert JC, Villanueva A, Cañete M and Nonell S: Photodynamic
Synergistic Effect of Pheophorbide a and Doxorubicin in Combined
Treatment against Tumoral Cells. Cancers (Basel). 9:pii: E18. 2017.
View Article : Google Scholar
|
|
32
|
Jerzak KJ, Berry S, Ko YJ, Earle C and
Chan KK: Cetuximab plus Irinotecan versus Panitumumab in Patients
with refractory metastatic colorectal cancer in Ontario, Canada.
Int J Cancer. 2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang W and Tung CH: Cisplatin
Cross-linked Multifunctional Nanodrugplexes for Combination
Therapy. ACS Appl Mater Interfaces. 2017.(Epub ahead of print).
|
|
34
|
Zhang H, Dong L, Chen Q, Kong L, Meng B,
Wang H, Fu K and Wang X, Pan-Hammarström Q, Wang P and Wang X:
Synergistic antitumor effect of histone deacetylase inhibitor and
Doxorubicin in peripheral T-cell lymphoma. Leuk Res. 56:29–35.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jung EM, Lim JH, Lee TJ, Park JW, Choi KS
and Kwon TK: Curcumin sensitizes tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis through
reactive oxygen species-mediated upregulation of death receptor 5
(DR5). Carcinogenesis. 26:1905–1913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang SY, Zhong MZ, Yuan GJ, Hou SP, Yin
LL, Jiang H and Yu Z: Casticin, a flavonoid, potentiates
TRAIL-induced apoptosis through modulation of anti-apoptotic
proteins and death receptor 5 in colon cancer cells. Oncol Rep.
29:474–480. 2013.PubMed/NCBI
|
|
37
|
Zhou Y, Tian L, Long L, Quan M, Liu F and
Cao J: Casticin potentiates TRAIL-induced apoptosis of gastric
cancer cells through endoplasmic reticulum stress. PLoS One.
8:e588552013. View Article : Google Scholar : PubMed/NCBI
|