Introduction
Osteosarcoma originates from primitive bone-forming
mesenchymal cells and has been identified as an aggressive sarcoma
of the bone (1,2). The incidence rate of osteosarcoma is
0.42% in inhabitants in USA, and osteosarcoma occurs most
frequently in adolescents and young adults (3,4).
Furthermore, distant metastases are common in patients with
osteosarcoma, with primary migration to the lungs and bones, and a
poor prognosis following recurrence and metastasis (5,6).
Current treatment strategies for osteosarcoma
include a presurgical window of carboplatin, macroscopic surgical
resection, multi-drug chemotherapy and radiotherapy (7,8). Despite
advances in the treatment of osteosarcoma, treatment efficacy and
short-term survival rates have not improved in recent years
(9,10). Thus, the development of a novel
therapeutic strategy for cancer treatment is warranted.
Notably, gene therapy holds great promise for
providing an innovative cancer treatment (11). Previous studies have identified
several candidate gene targets for osteosarcoma gene therapy
(12–14). For example, the proliferation
inhibition of osteosarcoma U2OS cells has been associated with the
repression of G1/S cell cycle transition mediated by the
overexpression of connexin43 (12).
Knockdown of S100 calcium binding protein A4 is correlated with the
reduced proliferation and invasiveness of osteosarcoma MG-63 cells
(13). Following the loss of MACC1
MET transcriptional regulator, osteosarcoma cells were demonstrated
to be less proliferative and more apoptotic, and exhibited lower
colony-forming and invasive abilities (14). However, there are only a few potential
therapeutic targets for osteosarcoma, thus the identification of
novel candidate genes is warranted to provide more viable clinical
therapeutic strategies for the treatment of the disease.
Calbindin 1 (CALB1) is a member of the EF-hand
helix-loop-helix intracellular Ca2+-binding protein
family and is mapped to human chromosome 8q21.3-q22.1 (15,16).
CALB1 is expressed normally in osteoblast cells and is
involved in the formation of mineralized bone matrix (17). Margolis et al (18) demonstrated that CALB1 serves an
essential role in bone remodeling, and that increased bone volume
was identified in CALB1-knockout mice. It has been
demonstrated that CALB1 is associated with an anti-apoptotic
function in several cell types, including bone cells (19,20). For
example, CALB1 inhibits apoptosis through preventing
caspase-3 activity in osteoblastic cells (21). A previous study also reported that, in
osteocytes and osteoblasts, CALB1 serves a protective role
against glucocorticoid-induced apoptosis (20). Furthermore, osteocytes are described
as terminally-differentiated osteoblasts, which are referred to as
osteosarcoma progenitors (22).
However, no clear association has been identified between
CALB1 and osteosarcoma.
In the present study, the function of CALB1
in osteosarcoma growth and progression was investigated. A
lentiviral-based system was used to functionally inhibit the
expression of CALB1 in osteosarcoma cells. The cell
viability of osteosarcoma cells was measured using MTT, crystal
violet staining and flow cytometry assays. This investigation may
provide clinicians a viable therapy for osteosarcoma in the
future.
Materials and methods
Cell lines
The U2OS osteosarcoma and 293T human embryonic
kidney cell lines were supplied by The Cell Bank of Type Culture
Collection of Chinese Academy of Sciences (Shanghai, China). The
cells were cultured in Dulbecco's modified Eagle's medium (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (Biological Industries, Cromwell, CT, USA). The
cells were incubated at 37°C in a humidified atmosphere consisting
of 5% CO2.
Construction of CALB1 shRNA expression
vector
The short hairpin RNA (shRNA) sequences targeting
CALB1 were as follows: S1,
5′-CGAACGGATCTTGCTCTTATTCTCGAGAATAAGAGCAAGATCCGTTCGTTTTT-3′; and
S2, 5′-GATTGGAGTTATCACCTGAAACTCGAGTTTCAGGTGATAACTCCAATCTTTTT-3′,
which were designed based on the human CALB1 gene (National
Center for Biotechnology Information accession no., 004929.2). The
sequence used as a negative control was as follows:
5′-GCGGAGGGTTTGAAAGAATATCTCGAGATATTCTTTCAAACCCTCCGCTTTTTT-3′. The
three oligonucleotides were inserted into pGP vectors (Shanghai
Hollybio, Shanghai, China) expressing green fluorescent protein
(GFP) at the EcoRI and BamHI cleavage sites.
Packaging and infection for
shRNA-expressing lentivirus vectors
To package lentivirus vectors, the reconstructed
vectors pGP-CALB1-shRNA 1, pGP-CALB1-shRNA 2 or pGP-Con-shRNA were
transfected into 293T cells at a confluence of 90% along with two
helper plasmids pVSVG-I and pCMV∆R8.92 (Shanghai Hollybio) using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The supernatant was collected at 48 h
post-transfection and the lentiviral particles were harvested
through ultracentrifugation at 4,000 × g for 10 min at 4°C, prior
to subsequently being passed through a 45-µm filter. U2OS cells
were cultured for 72 h at 37°C in 6-well plates at an inoculation
density of 5×104 cells/well and infected with the lentivirus
containing CALB1-shRNA 1, CALB1-shRNA 2 or Con-shRNA at a
multiplicity of infection of 20. The infection efficiency was
observed at 72 h post-infection using fluorescence microscopy.
Quantification of CALB1 mRNA using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
To elucidate the CALB1 mRNA-knockdown
efficiency in U2OS cells, total RNA was extracted at 5 days
post-infection using Trizol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and treated with recombinant DNase I (Takara
Biotechnology Co., Ltd., Dalian, China). A total of 2 µg RNA was
then reversed transcribed into cDNA using the SuperScript™ II
Reverse Transcriptase kit (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The primer
sequences used were as follows: CLAB1 forward,
5′-TGGCATCGGAAGAGCAGCAG-3′ and reverse,
5′-TGACGGAAGTGGTTACCTGGAAG-3′; and β-actin forward,
5′-GTGGACATCCGCAAAGAC-3′ and reverse, 5′-AAAGGGTGTAACGCAACTA-3′.
The qPCR (20 µl), consisting of 10 µl 2X SYBR Premix Ex Taq™, 0.8
µl forward and reverse primers (2.5 µM), 5 µl cDNA (150 ng), and
4.2 µl double distilled (dd)H2O, was performed using the
CFX Connect™ Real-Time PCR system (BioRad Laboratories, Inc.,
Hercules, CA, USA) with the following thermocycling conditions:
Initial denaturation at 95°C for 1 min and denaturation at 95°C for
5 sec, followed by 20 sec of annealing and extension at 60°C for 40
cycles. The 2ΔΔCq method was used to calculate the
CALB1 mRNA expression between different groups (23). All mRNA expression values were
normalized to β-actin.
Western blot assay for CALB1 protein
expression
U2OS cells were washed with ice-cold PBS at 7 days
post-infection and solubilized in 2X SDS Sample Buffer [100 mM
Tris-HCl (pH 6.8), 10 mM EDTA, 4% SDS and 10% glycine]. The
precipitated protein was collected through centrifugation at 4°C
for 10 min at 12,000 × g. The protein concentrations were then
determined using the bicinchoninic BCA protein assay kit (Thermo
Fisher Scientific, Inc.). Subsequently, equal amounts of total
protein (30 µg/lane) were separated by 10% SDS-PAGE and transferred
onto a polyvinylidene difluoride membrane. The membranes were
blocked with 5% skimmed milk in Tris-buffered saline with Tween-20
[TBST; 150 nmol/l NaCl, 100 m mol/l Tris-base, 0.1% Tween-20 (pH
7.6)] for 30 min at room temperature. The membranes were then
incubated with rabbit anti-CALB1 (cat. no. 14479-1-AP; 1:500
dilution) or rabbit anti-GAPDH (cat. no. 10494-1-AP; 1:100,000
dilution) primary polyclonal antibodies (both ProteinTech Group,
Inc., Chicago, IL, USA) overnight at 4°C. Following washing with
TBST, the blots were incubated with goat anti-rabbit immunoglobulin
G horseradish peroxidase-conjugated secondary antibody (cat. no.
sc-2054; 1:5,000 dilution; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) at room temperature for 2 h and developed with an Amersham
ECL Prime Western Blotting Detection Reagent (GE Healthcare Life
Sciences, Shanghai, China).
MTT assay
The lentivirus-infected osteosarcoma U2OS cells were
seeded into 96-well tissue culture plates at a density of 2×103
cells/well and incubated at 37°C for between 1 and 5 days. Each
day, 20 µl MTT solution (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was added to each well. Following 4 h of incubation at
37°C, the medium was discarded and 100 µl DMSO was added to each
well to dissolve the MTT crystals. Optical density was measured
during growth at a wavelength of 595 nm using a microplate
reader.
Colony-forming assay
To evaluate the colony formation capacity of the
cells, a low number of 96 h post-infected osteosarcoma U2OS cells
were plated into 6-well plates at a density of 5×102 cells/well.
The cells were observed daily, and the medium was changed on the
2nd, 4th, 6th and 8th days. On day 9, the cells were washed and
then fixed with 4% paraformaldehyde. Subsequently, the cells were
washed twice in PBS and stained with freshly prepared crystal
violet for 10 min at room temperature. Following washing with
ddH2O, colonies (>50 cells/colony) were counted by
eye.
Cell cycle analysis
To monitor cell cycle progression, flow cytometry
was performed following staining with propidium iodide (PI). At 3
days post-infection with lentiviral vectors, the U2OS cells were
seeded at a density of 7×104 cells/6-cm dish and serum starved for
72 h. The cells were harvested by centrifugation at 4°C for 5 min
at 2,000 × g following digestion with pancreatin, washed 3 times in
ice-cold PBS and then fixed in 70% ethanol, prior to incubation at
4°C for 25 min. The ethanol was removed through centrifugation at
4°C for 5 min at 2,000 × g and discarded, and the cell pellet was
subsequently resuspended in 10 µg/ml DNAse-free RNase A and
incubated for 30 min at 37°C. For flow cytometry analysis, 100 µl
PI solutions were added to each sample and analyzed using a FACS
Calibur II sorter and BD FACSCalibur flow cytometer and CellQuest
Pro Software (BD Biosciences, Franklin Lakes, NJ, USA).
Apoptosis detection
Apoptotic cells were quantified according to the
protocol of the Annexin V-APC/7-AAD Detection kit (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China). The cells were divided into
four groups: i) Unstained cells, APC-/7-AAD-, viable; ii) cells
stained with Annexin V-FITC and no 7-AAD, APC+/7-AAD-, early
apoptotic; iii) cells stained with 7-AAD and no Annexin V-FITC,
APC-/7-AAD+, necrotic; and iv) cells stained with Annexin V-FITC
and 7-AAD, APC+/7-AAD+, late apoptotic.
Statistical analysis
Statistical analysis was performed using SPSS
software package (version 13.0; SPSS, Inc., Chicago, IL, USA). All
data are presented as the mean ± standard deviation following three
independent experiments. Student's t-test (unpaired) was conducted
for statistical comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Lentiviral-mediated delivery of
shCALB1 s results in the knockdown of CALB1 gene and protein
expression in osteosarcoma U2OS cells
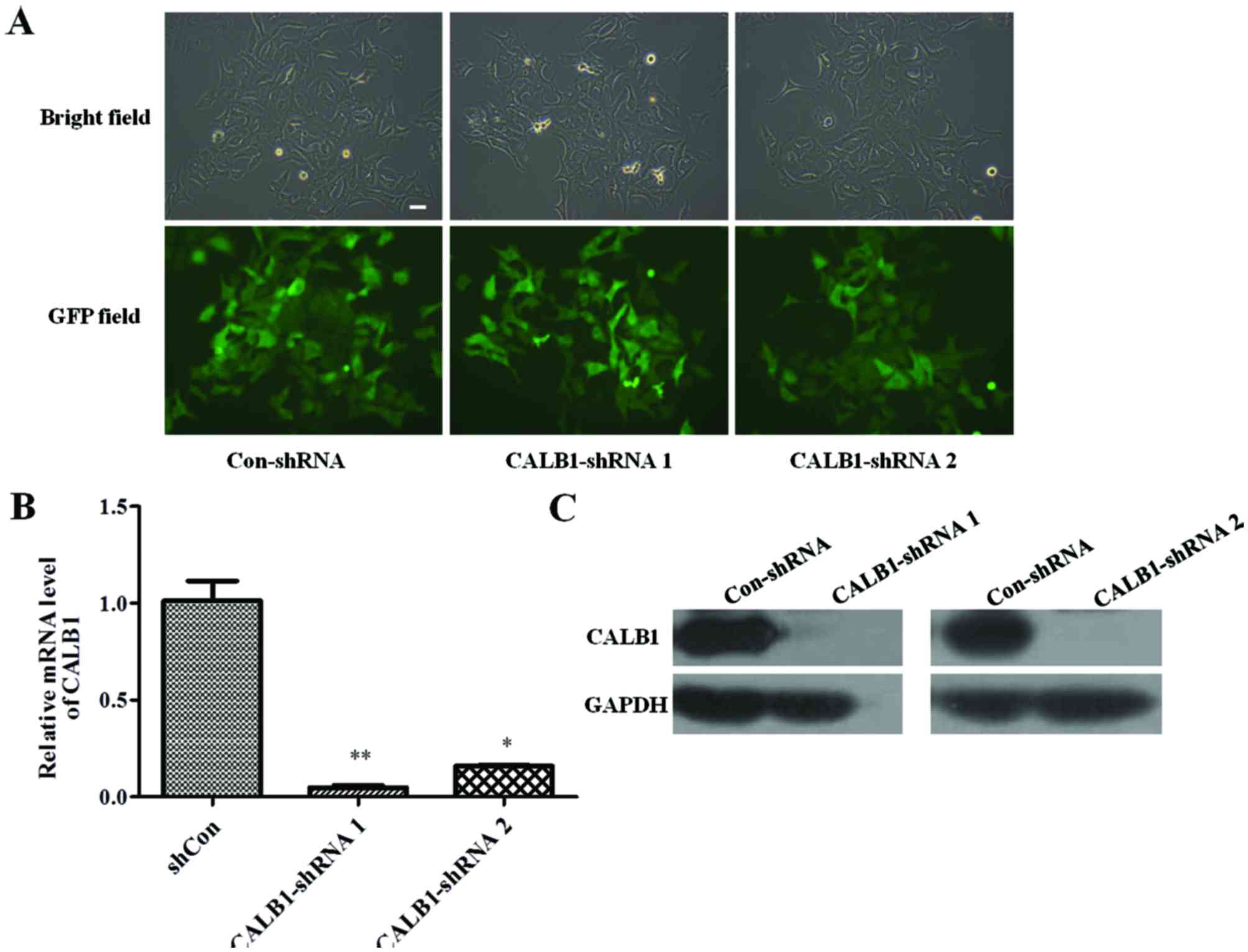
To identify whether the Con-shRNA and shCALB1 s were
successfully infected into U2OS cells, the cellular green
fluorescence was observed under a fluorescent microscope. The
efficiency of infection was determined by counting the number of
cells expressing GFP. The U2OS cells were successfully infected
with lentivirus CALB1-shRNA 1, CALB1-shRNA 2, or Con-shRNA, all
exhibiting an infection efficiency of >80% (Fig. 1A).
The effect of CALB1-shRNA 1 or 2 lentiviral
infection on CALB1 gene and protein expression levels was
then investigated. RT-qPCR results demonstrated that the silencing
shRNA-infected groups exhibited significantly lower expression of
the CALB1 gene compared with the Con-shRNA group
(CALB1-shRNA 1, P<0.01; CALB1-shRNA 2, P<0.05; Fig. 1B). Infection of U2OS cells with
CALB1-shRNA 1 or 2 reduced CALB1 protein expression compared with
that in cells infected with Con-shRNA (Fig. 1C). These results indicate that
lentiviral vectors carrying CALB1-shRNA 1 or 2 can functionally
depress CALB1 gene and protein expression in U2OS cells.
CALB1-knockdown results in the
inhibition of proliferative and colony-forming capacity
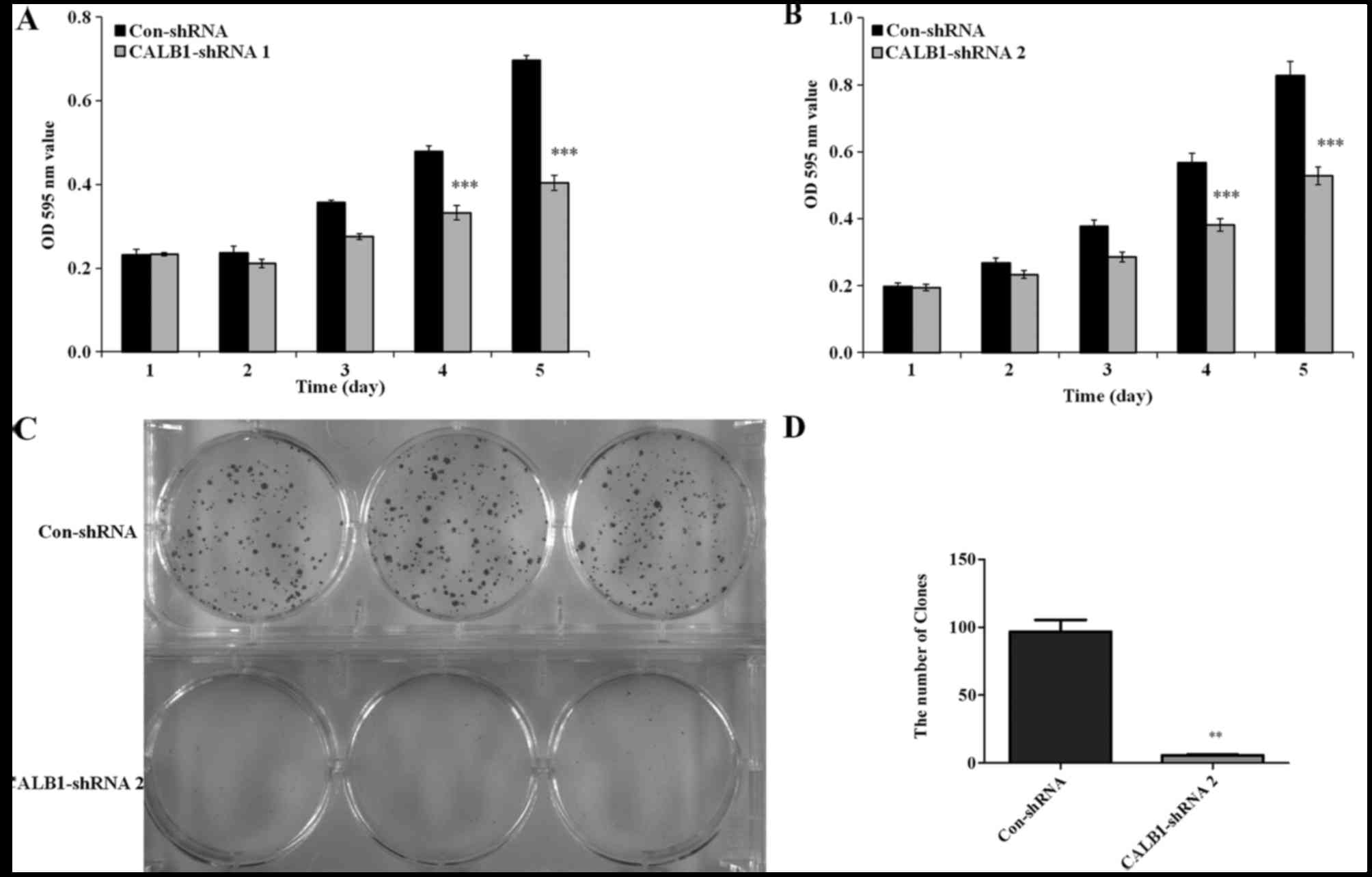
To detect whether the proliferation of osteosarcoma
U2OS cells was affected by CALB1-knockdown, an MTT viability
assay was performed. U2OS cells with the CALB1-knockdown exhibited
decreased cell proliferation from day 3 compared with the control
(Fig. 2). Following culturing,
significantly reduced proliferation of CALB1-shRNA 1-infected U2OS
cells were observed on the 4th (32.6%) and 5th (36.2%) days
compared with that in the Con-shRNA-infected cells (P<0.001;
Fig. 2A). Additionally, the
proliferation of the U2OS cells infected with CALB1-shRNA 2 was
significantly decreased by the 4th (30.7%) and 5th (42.0%) days
compared with that in the Con-shRNA-infected group (P<0.001;
Fig. 2B).
Since the proliferative ability of shRNA 1 and shRNA
2 were both markedly impaired in the MTT assay, only one shRNA
(shRNA 2) was used in the subsequent experiments. The
colony-forming ability of CALB1-shRNA 2 and Con-shRNA cells was
measured using the crystal violet staining method. Following 9 days
in culture, a decline in the number and size of the colonies was
detected in CALB1-shRNA 2-infected U2OS cells compared with the
control group (Fig. 2C). The number
of colonies was calculated in the CALB1-shRNA 2 and Con-shRNA
groups. Fig. 2D illustrates that
CALB1-knockdown resulted in a 94.1% decrease in colony cell
numbers compared with the control group (P<0.01). These results
suggest that CALB1-knockdown can significantly suppress the
proliferative and colony-forming abilities of osteosarcoma U2OS
cells.
CALB1-knockdown significantly disrupts
the cell cycle progression of U2OS cells
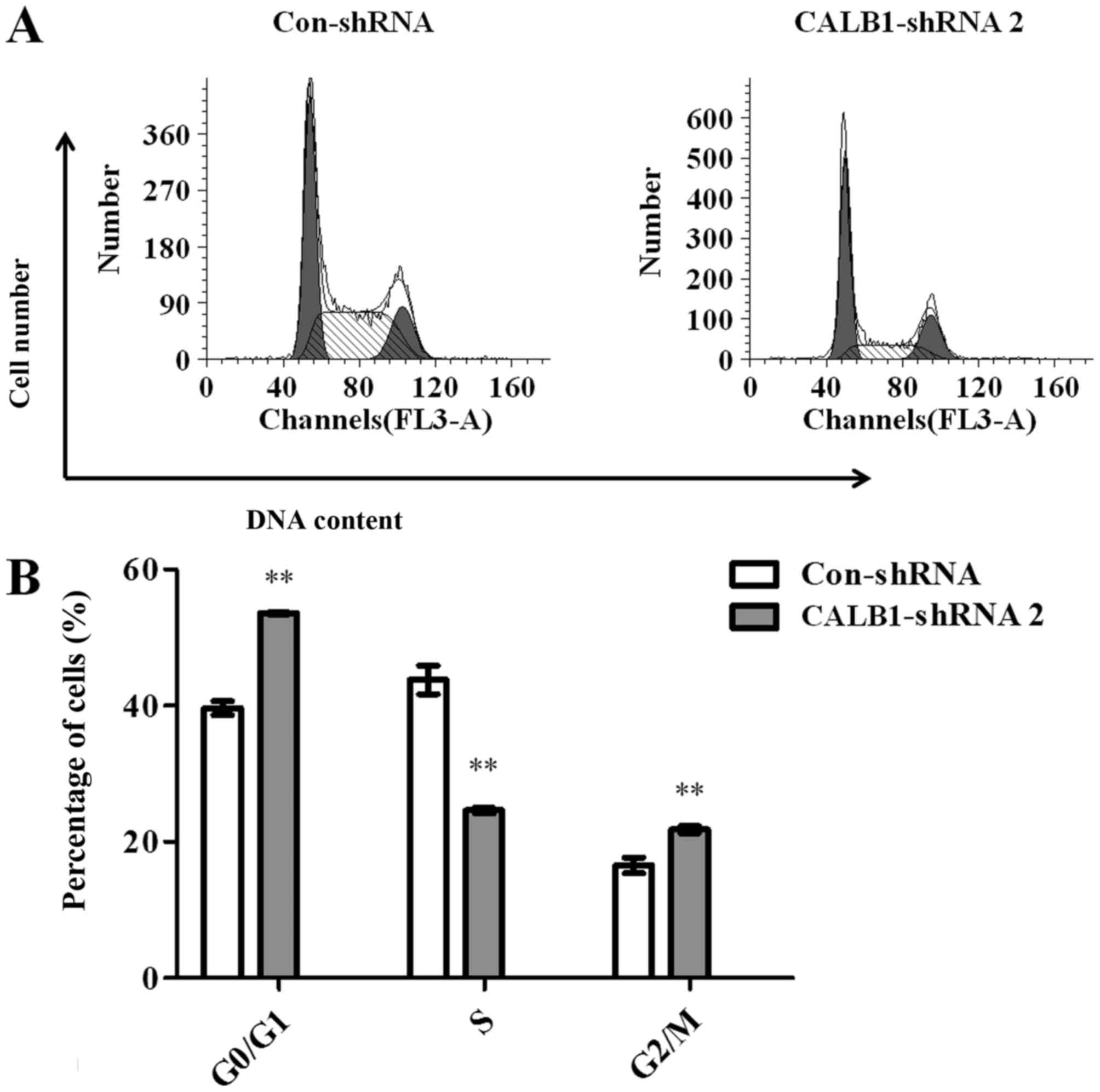
To investigate the potential mechanisms underlying
CALB1-knockdown induced growth inhibition in U2OS cells, the
cell cycle progression of Con-shRNA- and CALB1-shRNA 2-infected
cells was examined using flow cytometry (Fig. 3A). The results demonstrated that
53.567±0.137, 24.603±0.420 and 21.827±0.508% of CALB1-shRNA 2 cells
were in the G0/G1, S, and G2/M
phases, respectively. However, 39.650±1.002, 43.840±2.105 and
16.513±1.154% of Con-shRNA cells entered the
G0/G1, S, and G2/M phases.
Calculations revealed that, following the knockdown of
CALB1, the proportion of cells was significantly increased
in the G0/G1 (35.1%) and G2/M
(32.2%) stages, and significantly decreased in the S stage (43.9%)
compared with the control group (all P<0.01) (Fig. 3B). These data suggest that the
knockdown of CALB1 induced cell cycle arrest at the
G0/G1 and G2/M stages in U2OS
cells.
Induction of necrosis and apoptosis by
depletion of CALB1 in U2OS cells
To understand the mechanisms underlying the
suppression of U2OS cell proliferation and colony formation
following CALB1 depletion, cells were probed with Annexin
V-FITC/7-AAD and apoptotic cells were quantified using flow
cytometry (Fig. 4A). Following
infection, 3.07±0.14% of Con-shRNA-infected cells entered the early
apoptotic stage, while 5.22±0.18 and 5.17±0.35% of CALB1-shRNA 1
and 2, respectively, entered this stage (Fig. 4B). The percentage of cells that
entered the late apoptotic stage were as follows: 20.43±2.2%,
Con-shRNA; 33.90±0.36%, CALB1-shRNA 1; and 30.13±0.64%, CALB1-shRNA
2. The percentage of cells that entered the necrotic stage were as
follows: 10.98±0.29%, Con-shRNA; 14.76±0.14%, CALB1-shRNA 1; and
12.00±0.57%, CALB1-shRNA 2. A significantly higher percentage of
Con-shRNA viable cells (65.52±2.12%) were identified compared with
CALB1-shRNA 1 (46.13±0.15%; P<0.01) and CALB1-shRNA 2
(52.69±0.50%; P<0.001) cells (Fig.
4B). These results indicate that CALB1-knockdown induces
the apoptosis and necrosis of osteosarcoma U2OS cells.
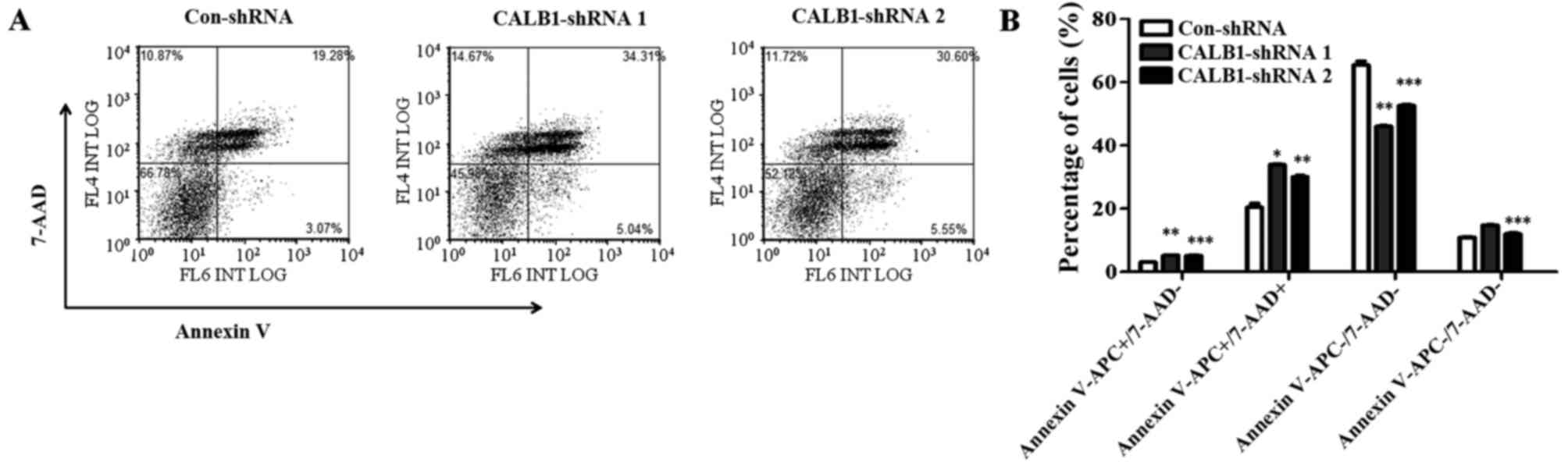 | Figure 4.CALB1 depletion causes
necrosis and apoptosis of osteosarcoma U2OS cells. (A) The
apoptotic death of U2OS cells administered with three treatments,
Con-shRNA, CALB1-shRNA 1 and CALB1-shRNA 2, was determined through
Annexin V-APC/7-AAD double staining and analyzed using flow
cytometry. (B) Deficiency of CALB1 in U2OS cells could
trigger necrosis, and early and late apoptosis.
APC−/7-AAD− and
APC−/7-AAD+ along the horizontal axis
represent viable and necrotic cells, while
APC+/7-AAD− and
APC+/7-AAD+ represent early and late
apoptotic cells respectively. *P<0.05, **P<0.01, and
***P<0.001 vs. Con-shRNA. CALB1, calbindin 1; sh, short hairpin;
con, negative control. |
Discussion
Osteosarcoma is the most common type of malignant
bone tumor, with a peak incidence in children and adolescents
(24,25). CALB1 is a 28-kDa calcium-binding
protein and functions to rescue neuronal, osteocyte, osteoblast and
lymphocyte cells from apoptosis (20,21).
CALB1 is commonly expressed in classic medulloblastomas, the
human medulloblastoma D283 MED cell line and in lung carcinomas
(26,27). The present study aimed to investigate
the potential role of CALB1 in osteosarcoma tumor
progression. The results revealed that the proliferation and colony
formation abilities were inhibited in osteosarcoma U2OS cells
following CALB1-knockdown. Notably, accelerated apoptosis of
osteosarcoma U2OS cells was observed following the knockdown of
CALB1.
Uncontrolled proliferation and escape of cells from
apoptotic death are characteristics of cancer cells (28,29). It
has been previously demonstrated that anti-growth signals prevent
cell proliferation through two different mechanisms: Cells may
enter the quiescent state (G0) and not participate in
the active proliferative cycle; or cells may be induced to enter
post-mitotic states and lose the potential to differentiate
(28). Fang et al (30) reported that the upregulation of
caveolin 1 resulted in G0/G1 phase arrest in
endothelial cells, contributing to the suppression of their
proliferation. The results of a previous study demonstrated that
the mediator complex subunit 19-knockdown through lentivirus vector
shRNA inhibits human osteosarcoma cell proliferation by inducing
cell cycle arrest at the G0/G1 phase
(31). Furthermore, a previous study
observed decreased proliferation of human esophageal cancer cells
following the inhibition of β-catenin expression (32). In the present study, the absence of
CALB1 expression in human osteosarcoma U2OS cells may result
in the activation of anti-growth signals, inducing cell cycle
arrest at the G0/G1 phase and disturbance to
the G1 to S phase transition.
The results of the present study also revealed that
the infected U2OS cells underwent cell cycle arrest at the
G2/M and G0/G1 phases. This was
also demonstrated to occur in bladder cancer cells following the
knockdown of survivin, which resulted in G2/M arrest and
the induction of apoptosis (33). In
addition, a previous study demonstrated that decreased expression
of ALG2 α-1,3/1,6-mannosyltransferase in HeLa cells induced
G2/M cell cycle phase accumulation, and early and late
apoptosis (34). The results of the
present study indicate that CALB1-knockdown may regulate
mitosis genes to inhibit mitotic progression at the latter
stage.
It has been typically demonstrated that apoptosis is
an important component in cancer pathogenesis (35). The inhibition of apoptosis facilitates
the growth of tumors (34). CALB1 is
a calcium-binding protein and is responsible for maintaining low
levels of intracellular calcium (36). Calbindin, by buffering calcium,
functions to inhibit nerve cell apoptosis when induced by high
levels of intracellular calcium (21). The present study revealed that the
number of early apoptotic, late apoptotic and necrotic cells were
significantly reduced in CALB1-knockdown U2OS cells. These
results suggest that the knockdown of CALB1 may increase the
concentrations of intracellular calcium, resulting in a promotion
of apoptosis in U2OS cells, and contributing to the decrease in
cell proliferation and colony formation.
CALB1 inhibits the activity of caspase-3, a
downstream effector of multiple apoptotic signaling pathways, and
that inhibition results in an inhibition of apoptosis in
osteoblastic cells (21,37). CALB1 may also protect against
apoptosis in dopaminergic neurons through activation of the
phosphoinositide 3 (PI3) kinase-Akt signaling pathway (38). In the present study, the knockdown of
CALB1 may have accelerated U2OS apoptosis via the activation
of caspase-3 and/or by the inactivation of the PI3-kinase-Akt
signaling pathway, which is dependent on the calcium binding
activity of CALB1.
In conclusion, the present study demonstrated that
CALB1-knockdown inhibits the proliferation and colony
formation of osteosarcoma U2OS cells. The suppression of
CALB1 inhibited human osteosarcoma cell growth through
modulation of the cell cycle and the induction of apoptosis, and
may be a potential therapeutic target for the treatment of patients
with osteosarcoma.
References
|
1
|
Maire G, Martin JW, Yoshimoto M,
Chilton-MacNeill S, Zielenska M and Squire JA: Analysis of
miRNA-gene expression-genomic profiles reveals complex mechanisms
of microRNA deregulation in osteosarcoma. Cancer Genet.
204:138–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcomaPediatric and Adolescent Osteosarcoma. Jaffe N,
Bruland OS and Bielack S: Springer; New York: pp. 3–13. 2010
|
|
3
|
Kobayashi E, Hornicek FJ and Duan Z:
MicroRNA involvement in osteosarcoma. Sarcoma. 2012:3597392012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leithner A, Maurer-Ertl W, Glehr M,
Friesenbichler J, Leithner K and Windhager R: Wikipedia and
osteosarcoma: A trustworthy patients' information? J Am Med Inform
Assoc. 17:373–374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ternovoi VV, Curiel DT, Smith BF and
Siegal GP: Adenovirus-mediated p53 tumor suppressor gene therapy of
osteosarcoma. Lab Invest. 86:748–766. 2006.PubMed/NCBI
|
|
6
|
Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs
JJ, Gitelis S, O'Keefe RJ, Konttinen YT, Yin G and Li TF:
Inhibition of the Wnt-β-catenin and Notch signaling pathways
sensitizes osteosarcoma cells to chemotherapy. Biochem Biophys Res
Commun. 431:274–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ando K, Mori K, Corradini N, Redini F and
Heymann D: Mifamurtide for the treatment of nonmetastatic
osteosarcoma. Expert Opin Pharmacother. 12:285–292. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferguson WS, Harris MB, Goorin AM,
Gebhardt MC, Link MP, Shochat SJ, Siegal GP, Devidas M and Grier
HE: Presurgical window of carboplatin and surgery and multidrug
chemotherapy for the treatment of newly diagnosed metastatic or
unresectable osteosarcoma: Pediatric Oncology Group Trial. J
Pediatr Hematol Oncol. 23:340–348. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaffe N: Osteosarcoma: Review of the Past,
Impact on the Future. The American ExperiencePediatric and
Adolescent Osteosarcoma. Jaffe N, Bruland OS and Bielack S:
Springer; NY: pp. 239–262. 2010
|
|
10
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cross D and Burmester JK: Gene therapy for
cancer treatment: Past, present and future. Clin Med Res.
4:218–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang YW, Morita I, Ikeda M, Ma KW and
Murota S: Connexin43 suppresses proliferation of osteosarcoma U2OS
cells through post-transcriptional regulation of p27. Oncogene.
20:4138–4149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma X, Yang Y, Wang Y, An G and Lv G: Small
interfering RNA-directed knockdown of S100A4 decreases
proliferation and invasiveness of osteosarcoma cells. Cancer Lett.
299:171–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang K, Tian F, Zhang Y, Zhu Q, Xue N,
Zhu H, Wang H and Guo X: MACC1 is involved in the regulation of
proliferation, colony formation, invasion ability, cell cycle
distribution, apoptosis and tumorigenicity by altering Akt
signaling pathway in human osteosarcoma. Tumor Biol. 35:2537–2548.
2014. View Article : Google Scholar
|
|
15
|
Reiche D, Pfannkuche H, Michel K, Hoppe S
and Schemann M: Immunohistochemical evidence for the presence of
calbindin containing neurones in the myenteric plexus of the
guinea-pig stomach. Neurosci Lett. 270:71–74. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parmentier M, Passage E, Vassart G and
Mattei MG: The human calbindin D28k (CALB1) and calretinin (CALB2)
genes are located at 8q21.3----q22.1 and 16q22----q23,
respectively, suggesting a common duplication with the carbonic
anhydrase isozyme loci. Cytogenet Cell Genet. 57:41–43. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee CT, Huynh VM, Lai LW and Lien YH:
Cyclosporine A-induced hypercalciuria in calbindin-D28k knockout
and wild-type mice. Kidney Int. 62:2055–2061. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Margolis DS, Kim D, Szivek JA, Lai LW and
Lien YH: Functionally improved bone in calbindin-D28k knockout
mice. Bone. 39:477–484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rabinovitch A, Suarez-Pinzon WL, Sooy K,
Strynadka K and Christakos S: Expression of calbindin-D28k in a
pancreatic Isletβ-Cell line protects against cytokine-induced
apoptosis and necrosis. Endocrinology. 142:3649–3655. 2001.
View Article : Google Scholar
|
|
20
|
Liu Y, Porta A, Peng X, Gengaro K,
Cunningham EB, Li H, Dominguez LA, Bellido T and Christakos S:
Prevention of Glucocorticoid-Induced Apoptosis in Osteocytes and
Osteoblasts by Calbindin-D28k. J Bone Miner Res. 19:479–490. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bellido T, Huening M, Raval-Pandya M,
Manolagas SC and Christakos S: Calbindin-D28k is expressed in
osteoblastic cells and suppresses their apoptosis by inhibiting
caspase-3 activity. J Biol Chem. 275:26328–26332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sottnik JL, Campbell B, Mehra R,
Behbahani-Nejad O, Hall CL and Keller ET: Osteocytes serve as a
progenitor cell of osteosarcoma. J Cell Biochem. 115:1420–1429.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trieb K, Lehner R, Stulnig T, Sulzbacher I
and Shroyer KR: Survivin expression in human osteosarcoma is a
marker for survival. Eur J Surg Oncol. 29:379–382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katsetos CD, Herman MM, Krishna L, Vender
JR, Vinores SA, Agamanolis DP, Schiffer D, Burger PC and Urich H:
Calbindin-D28k in subsets of medulloblastomas and in the human
medulloblastoma cell line D283 Med. Arch Pathol Lab Med.
119:734–743. 1995.PubMed/NCBI
|
|
27
|
Castro CY, Stephenson M, Gondo MM,
Medeiros LJ and Cagle PT: Prognostic implications of calbindin-D28k
expression in lung cancer: Analysis of 452 cases. Mod Pathol.
13:808–813. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fang K, Fu W, Beardsley AR, Sun X, Lisanti
MP and Liu J: Overexpression of caveolin-1 inhibits endothelial
cell proliferation by arresting the cell cycle at G0/G1 phase. Cell
Cycle. 6:199–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang T, Hao L, Feng Y, Wang G, Qin D and
Gu G: Knockdown of MED19 by lentivirus-mediated shRNA in human
osteosarcoma cells inhibits cell proliferation by inducing cell
cycle arrest in the G0/G1 phase. Oncol Res. 19:193–201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang JS, Ji AF, Wan HJ, Lu YL, Yang JZ, Ma
LL, Wang YJ and Wei W: Gene Silencing of ß-catenin by RNAi inhibits
proliferation of human esophageal cancer cells by inducing G0/G1
cell cycle arrest. Asian Pac J Cancer Prev. 13:2527–2532. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ning S, Fuessel S, Kotzsch M, Kraemer K,
Kappler M, Schmidt U, Taubert H, Wirth MP and Meye A:
siRNA-mediated down-regulation of survivin inhibits bladder cancer
cell growth. Int J Oncol. 25:1065–1071. 2004.PubMed/NCBI
|
|
34
|
Høj BR, la Cour JM, Mollerup J and
Berchtold MW: ALG-2 knockdown in HeLa cells results in G2/M cell
cycle phase accumulation and cell death. Biochem Biophys Res
Commun. 378:145–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rintoul GL, Raymond LA and Baimbridge KG:
Calcium buffering and protection from excitotoxic cell death by
exogenous calbindin-D28k in HEK 293 cells. Cell calcium.
29:277–287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Christakos S and Liu Y: Biological actions
and mechanism of action of calbindin in the process of apoptosis. J
Steroid Biochem Mol Biol. 89–90:401–404. 2004. View Article : Google Scholar
|
|
38
|
Sun S, Li F, Gao X, Zhu Y, Chen J, Zhu X,
Yuan H and Gao D: Calbindin-D28K inhibits apoptosis in dopaminergic
neurons by activation of the PI3-kinase-Akt signaling pathway.
Neuroscience. 199:359–367. 2011. View Article : Google Scholar : PubMed/NCBI
|