Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent types of human malignancies worldwide, as well as the
primary cause of mortality in patients with chronic liver disease
(1). Despite recent advances in
therapeutics and surveillance programs for high-risk populations
(2,3),
the long-term prognosis for patients with HCC remains poor due to
the high incidence of intrahepatic recurrence (4). Therefore, it is important to elucidate
the molecular mechanisms underlying HCC progression and to identify
the risk factors for tumor recurrence following curative treatment,
in order to select appropriate therapies and accurately evaluate
patient prognosis.
The insulin-like growth factor (IGF) signaling
pathway is associated with HCC cell growth and is important during
the development and progression of HCC (5). IGF-I, IGF-II and their corresponding
receptors mediate the biological functions of the IGF signaling
pathway through the activation of the mitogen-activated protein
kinase signaling pathway, which is involved in cell growth and
metabolism (6,7). The ligands IGF-I and IGF-II, as well as
the abnormal stimulation of their receptors, have been implicated
in the early stages of human hepatocarcinogenesis (8,9). IGF
binding protein (IGFBP) 3 is a member of the IGFBP family, which
regulates components of the IGF signaling pathway (10). IGFBP-3 has been demonstrated to
inhibit cell proliferation, independently of its effects on
IGF-stimulated growth, in numerous types of malignancies (11,12).
Additionally, it has been reported that the expression levels of
IGFBP-3 correlate with the response of the patient to radiotherapy
and may serve as a marker for the chemosensitivity of glioblastoma
to semustine (13). A previous study
demonstrated that IGFBP-3 expression level in patients with
esophageal squamous cell carcinoma (ESCC) was a risk factor for
poor patient survival, as well as a marker for evaluating patient
prognosis (14). However, the
significance of IGFBP-3 expression levels, and their association
with the prognosis of patients with HCC, has yet to be
investigated.
The present study was conducted in order to
investigate the clinical and prognostic implications of IGFBP-3
expression levels in patients with HCC. Immunohistochemistry (IHC)
was used to examine whether the expression of IGFBP-3 in human
clinical tissue samples was associated with the clinicopathological
characteristics of HCC. Furthermore, the levels of IGFBP-3
expression and the clinical and prognostic factors of patients with
HCC were evaluated to determine if any correlations were present,
as well as whether the expression levels of IGFBP-3 are able to
accurately predict patient survival.
Materials and methods
Patients and tissue specimens
In the present retrospective study, a total of 120
HCC tissue samples and 50 matched adjacent non-malignant tissues
were obtained from 120 patients with HCC, following the receipt of
informed patient consent and ethical approval from The Institute
Research Ethics Committee of Xi'an Medical College Affiliated
Hospital (Xi'an, China). The tissue samples were collected between
January 2003 and December 2009 at The Xi'an Medical College
Affiliated Hospital. The HCC cases (summarized in Table I) were selected based on pathological
diagnosis and the availability of follow-up data. The diagnosis of
HCC was determined based on the 2002 American Joint Committee on
Cancer/International Union Against Cancer Tumor-Node-Metastasis
(TNM) classification system guidelines (15). The original clinical assessment
records were available and the tissue samples were assessed
histologically. Curative treatment was defined as the complete
removal of the tumor tissue, with no residual tumor visible in
three-phase dynamic computed tomography scans or gadoxetic
acid-enhanced magnetic resonance imaging scans at one month
following surgery. The follow-up period extended for >60
months.
 | Table I.Correlations between the IGFBP-3
expression levels in tumor tissue samples and the
clinicopathological characteristics of patients with hepatocellular
carcinoma. |
Table I.
Correlations between the IGFBP-3
expression levels in tumor tissue samples and the
clinicopathological characteristics of patients with hepatocellular
carcinoma.
|
|
| IGFBP-3 protein |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Number of cases | Low expression
(%) | High expression
(%) | P-value |
|---|
| Age, years |
|
|
| 0.436 |
|
≥50b | 63 | 31 (49.2)) | 32 (50.8 |
|
|
<50b | 57 | 24 (42.1) | 33 (57.9) |
|
| Gender |
|
|
| 0.677 |
| Male | 103 | 48 (46.6) | 55 (53.4) |
|
|
Female | 17 | 7 (41.1) | 10 (58.9) |
|
| AFP, ng/ml |
|
|
| 0.291 |
|
≤20 | 42 | 20 (47.6) | 22 (52.4) |
|
|
>20 | 78 | 35 (44.9) | 43 (54.1) |
|
| Liver
cirrhosis |
|
|
| 0.369 |
|
Yes | 75 | 32 (42.7) | 43 (57.3) |
|
| No | 45 | 23 (51.1) | 22 (48.9) |
|
| Tumor size, cm |
|
|
| 0.003a |
| ≥5 | 52 | 32 (61.5) | 20 (39.5) |
|
|
<5 | 68 | 23 (33.8) | 45 (66.2) |
|
| Tumor
multiplicity |
|
|
| 0.044a |
|
Single | 78 | 41 (52.6) | 37 (47.4) |
|
|
Multiple | 42 | 14 (33.3) | 28 (66.7) |
|
| N status |
|
|
| 0.008a |
| N0 | 33 | 21 (63.6) | 12 (36.4) |
|
| N1 | 87 | 32 (36.8) | 55 (63.2) |
|
| M status |
|
|
| 0.001a |
| M0 | 57 | 35 (61.4) | 22 (38.6) |
|
| M1 | 63 | 20 (31.7) | 43 (68.3) |
|
| Clinical stage |
|
|
| 0.001a |
| I | 13 | 10 (76.9) | 3 (23.1) |
|
| II | 47 | 28 (65.9) | 19 (34.1) |
|
|
III | 39 | 10 (51.5) | 29 (48.5) |
|
| IV | 21 | 7 (33.3) | 14 (66.7) |
|
| Survival
status |
|
|
| 0.015a |
|
Alive | 31 | 20 (64.5) | 11 (35.5) |
|
|
Succumbed | 89 | 35 (39.3) | 54 (61.7) |
|
IHC
IGFBP-3 expression was evaluated using a previously
established standard two-step IHC technique (14). Briefly, the tissue slides cut by
microtome (5-µm thick) were dried using a dryer overnight at 37°C,
dewaxed in xylene, rehydrated with graded ethanol and immersed in
3% hydrogen peroxide for 20 min at room temperature to block
endogenous peroxidase activity. For antigen retrieval, the tissue
slides were heated at 100°C in Tris (hydroxymethyl)
aminomethane-EDTA buffer (pH 8.0) in a pressure cooker for 10 min.
Subsequently, the tissue slides were incubated with 10% normal
rabbit serum (catalog no. 18140; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at room temperature for 20 min to reduce
nonspecific interactions. Tissue sections were then incubated with
a 1:50 dilution of anti-IGFBP-3 polyclonal antibody (directed
against amino acids 113–210 of human IGFBP-3; catalog no. sc-9028;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at 37°C in
a humidified chamber. Following washing five times with 0.01 mol/l
PBS (pH 7.4) for 10 min, the slides were incubated with a secondary
rabbit anti-mouse antibody (catalog no. F0232; Envision; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) at a dilution of
1:100 for 30 min at 37°C. This was followed by washing 3 times with
PBS and staining with 50% 3,3-diaminobenzidine for 20 sec at room
temperature. Nuclei were counterstained with Meyer's hematoxylin.
PBS alone was used as a negative control.
Evaluation of IGFBP-3 expression using
IHC
IGFBP-3 expression levels were evaluated using IHC
by randomly selecting and counting the percentage of positive cells
in five fields under a light microscope (DM2000; Leica Microsystems
GmbH, Wetzlar, Germany) (x400) from each tissue slide, and then
calculating the mean score for each slide from these values. The
nuclear immunoreactivity of the IGFBP-3 was scored
semi-quantitatively by comparing the number of IGFBP-3 positive
tumor cells with the total number of tumor cells. Scores were
assigned in 5% increments (0–100%). The reproducibility of this
scoring method between pathologists has been described previously
(16,17). Three independent pathologists who were
blinded to the clinical follow-up data evaluated IGFBP-3 expression
levels. Their conclusions were concordant in 85% of the cases,
suggesting that this scoring method is reproducible.
Selection of cut-off scores
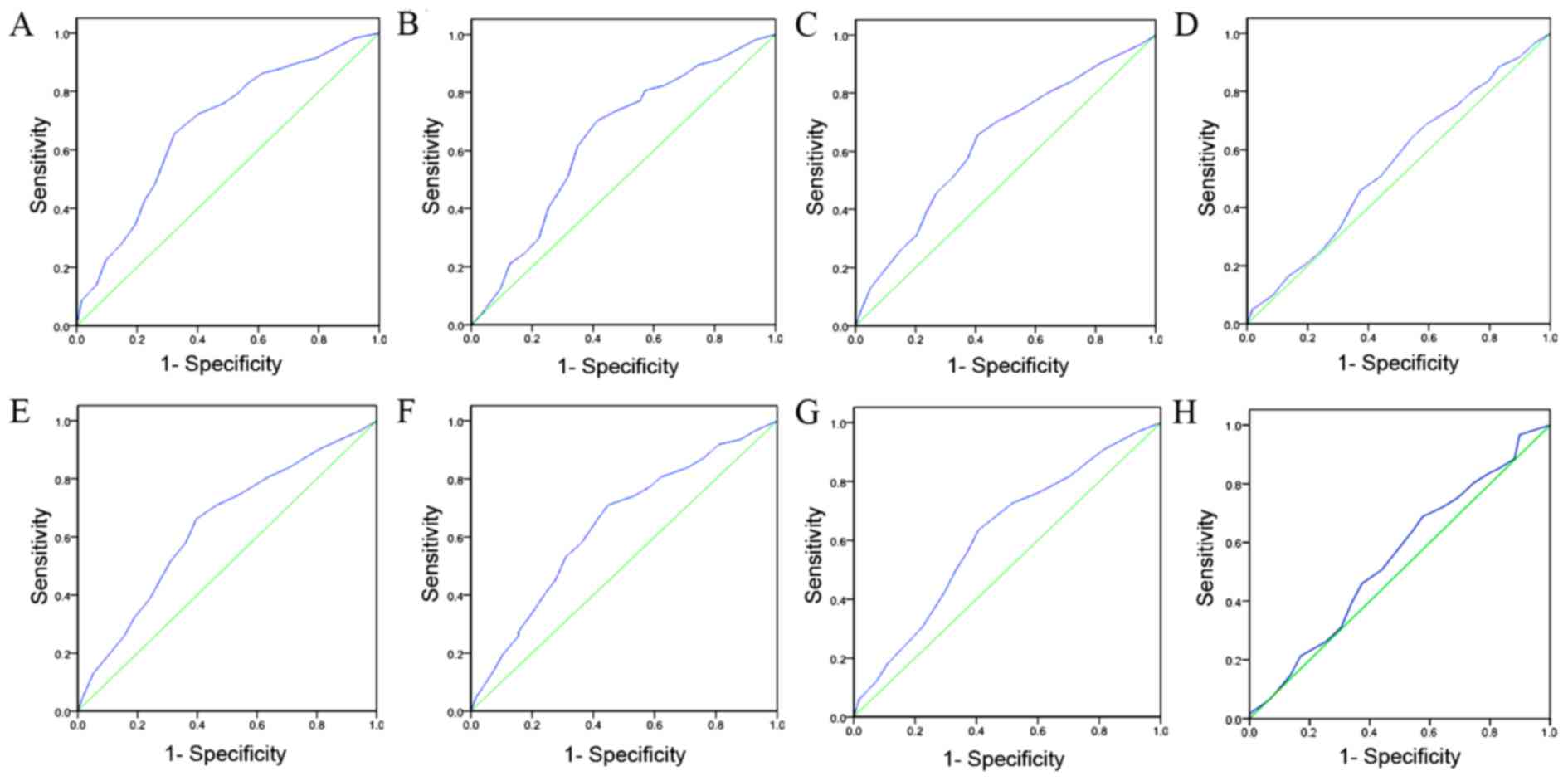
ROC analysis was used to determine the cut-off
scores for positive IGFBP-3 expression in tissue specimens using
the 0, 1 positivity-criterion (18).
To determine the IGFBP-3 score, the sensitivity and specificity for
each outcome (clinicopathological features) was plotted, generating
an ROC curve. In ROC analysis, the optimal cut-off score was chosen
as the value with the most similar maximum sensitivity and
specificity [the point (0.0, 1.0) on the curve]. According to the
ROC analysis, the threshold value was defined 65%. Tissue specimens
designated as ‘negative’ for the protein were those with scores
below or equal to the threshold value, whereas ‘positive’ specimens
were those with scores above the threshold (19,20). In
order to use ROC analysis, the clinicopathological features were
categorized as follows: α-fetoprotein (AFP) level (≥20 or <20),
liver cirrhosis (serious hepatocyte necrosis or no hepatocyte
necrosis), tumor size (tumor diameter, ≥5 or <5 cm), tumor
multiplicity (single or multiple), N stage (N0, no lymph node
involvement; N1, lymph node involvement), M stage (M0, absence of
metastasis; M1, presence of metastasis), clinical stage (low, I+II;
high, III+IV) and length of survival [mortality due to HCC or
censored (lost to follow-up, alive, or mortality due to other
causes)]. IGFBP-3 immunoreactivity was classified using ROC curve
analysis, with a low expression rate defined as <65% IGFBP-3
positive cells and a high expression rate defined as ≥65% IGFBP-3
positive cells.
Western blot analysis
Protein concentration was determined by
bicinchoninic acid protein quantification. Total protein (20 µg)
was isolated from 10 paired HCC and adjacent non-malignant tissue
samples using TRIzol® buffer (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) (21) as previously described (22). Briefly, equal quantities of whole cell
lysate and tissue lysate were separated by 8% SDS-PAGE and
transferred to a polyvinylidene difluoride membrane (Pall Life
Sciences, Port Washington, NY, USA) and blocked in 5% milk powder.
The membranes were washed three times with PBS and incubated with
primary mouse monoclonal antibodies against human IGFBP-3
(dilution, 1:200; catalog no. sc-9028; Santa Cruz Biotechnology,
Inc.) at 4°C overnight. Subsequent to washing three times with PBS,
blots were incubated with secondary rabbit anti-mouse antibody
(catalog no. F0232; Envision; Dako; Agilent Technologies, Inc.) at
a dilution of 1:5,000 for 2 h at room temperature. Immunoreactivity
was then detected using an Amersham enhanced chemiluminescence
western blotting detection kit (GE Healthcare Life Sciences,
Uppsala, Sweden) and analyzed using Image lab Software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). All experiments were
conducted according to the manufacturer's protocol where
applicable.
Statistical analysis
All statistical analyses were performed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). Measurement data are
presented by continuous variable and ranked data are presented by
classified variable. The correlation between the protein expression
levels of IGFBP-3 and the clinicopathological data from patients
with HCC was evaluated using the χ2 test. ROC analysis was
performed to determine the cut-off score for positive IGFBP-3
expression. The association between the length of patient survival
and each variable was evaluated using the log-rank test. Multiple
Cox proportional hazards regression analysis was performed to
determine whether IGFBP-3 expression levels were an independent
predictor of patient survival. P<0.05 was considered to indicate
a statistically significant difference.
Results
IGFBP-3 expression levels in HCC and
non-malignant patient tissue samples
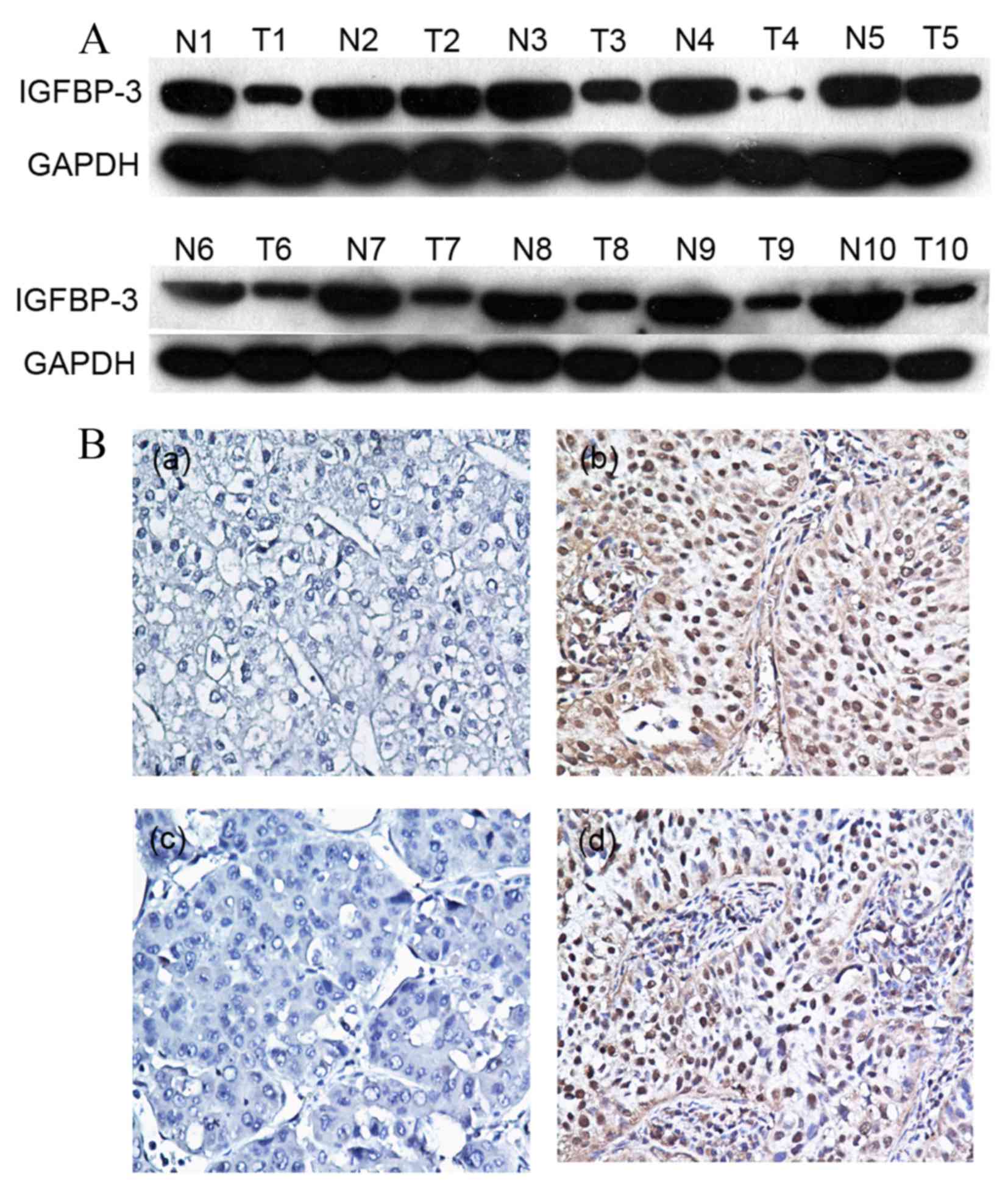
Initially, IGFBP-3 expression was examined in 10
pairs of fresh HCC and adjacent non-malignant tissue specimens
obtained from fresh tissue database using western blot analysis. In
the primary HCC tissue samples, 7/10 (70%) exhibited markedly
reduced levels of IGFBP-3 expression compared with in the adjacent
non-malignant liver tissue samples (Fig.
1A). Protein expression was subsequently evaluated using IHC in
120 pairs of HCC and 50 adjacent non-malignant tissue specimens
(Fig. 1B). The immunoreactivity
scores ranged from 0 to 100%. According to ROC analysis, tissues
with IGFBP-3 expression levels that were above the critical
threshold of 65% were defined as positive (Fig. 2). High expression levels of IGFBP-3
were detected in 65/120 (54.2%) of the HCC tissue samples, and in
36/50 (72%) of the adjacent non-malignant liver tissues. Therefore,
IHC analysis demonstrated that IGFBP-3 expression levels were
significantly decreased in the primary cancer tissues, as compared
with in the matched adjacent non-malignant tissues (P=0.031).
Associations between the levels of
IGFBP-3 expression and the clinicopathological parameters
The expression levels of IGFBP-3 in patients with
HCC, with respect to several standard clinicopathological features,
are presented in Table I. Analysis of
120 patients with HCC revealed that the expression levels of
IGFBP-3 were correlated with the following clinicopathological
parameters: T stage, N stage, M stage, tumor multiplicity, clinical
stage and the length of survival (χ2 test, P<0.05; Table I). No significant differences were
identified between IGFBP-3 expression levels and patient age,
gender, AFP levels or liver cirrhosis (P>0.05; Table I). These results suggest a correlation
between decreased IGFBP-3 expression levels and clinical
progression in patients with HCC.
Associations between
clinicopathological variables and IGFBP-3 expression levels and
survival in patients with HCC
Univariate Cox regression analyses and Kaplan-Meier
survival curves were evaluated using a log-rank test. The χ2
analysis indicated a correlation between the protein expression
levels of IGFBP-3 in tumor tissues and the survival time of
patients with HCC (P=0.015; Table I).
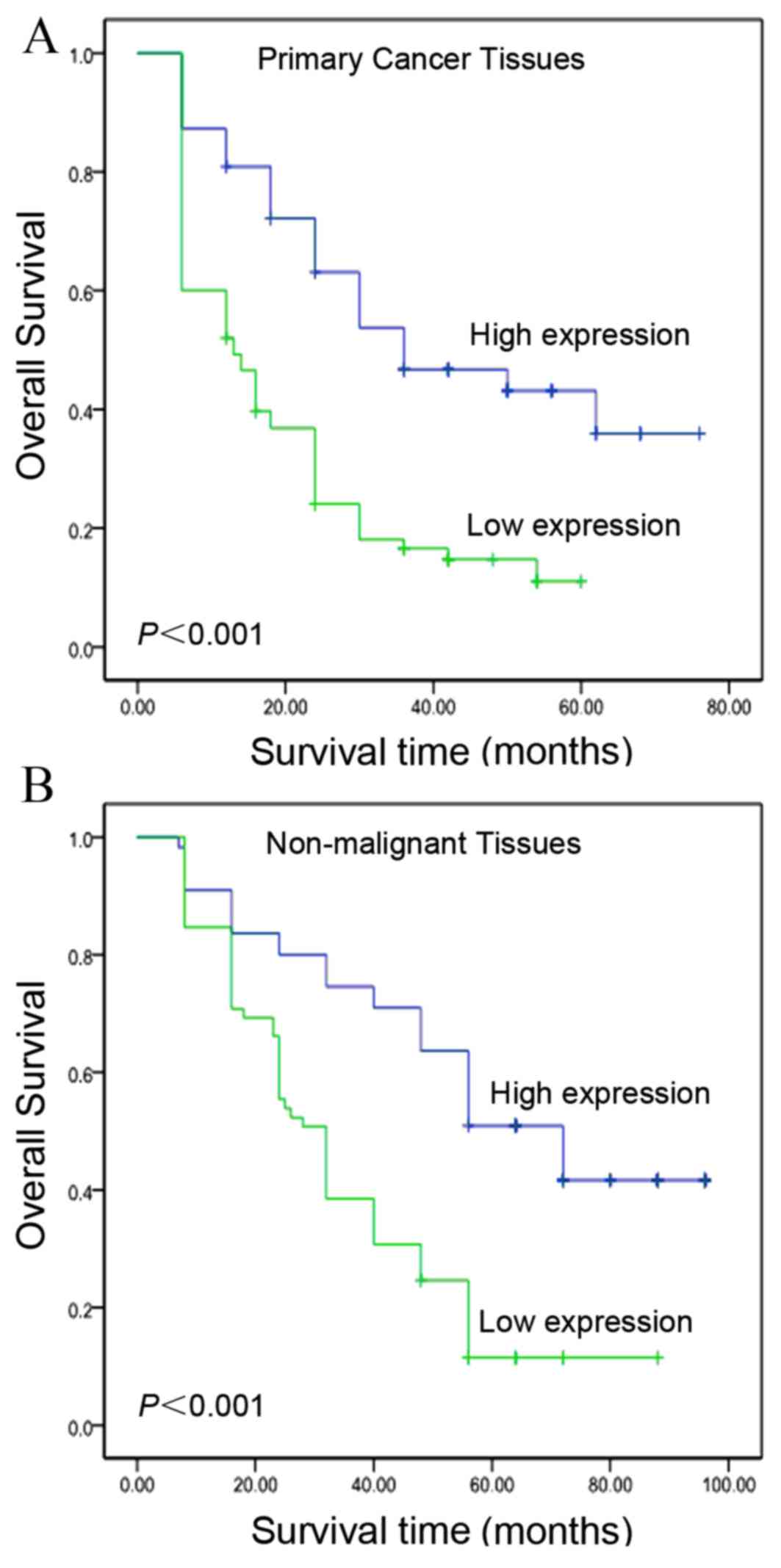
Kaplan-Meier curves revealed that, within the primary HCC category,
patients with high levels of IGFBP-3 expression exhibited a longer
overall survival time (median, 39.4 months), as compared with
patients with low levels of IGFBP-3 expression (median, 18.7
months; log-rank test, P<0.001; Fig.
3A). In addition, patient survival was analyzed to determine
the associations between survival time and IGFBP-3 expression
levels in adjacent non-malignant tissues. As presented in Fig. 3B, the overall survival time was
greater (median, 57.4 months) for patients with high basal
expression levels of IGFBP-3, compared with for patients expressing
low levels of IGFBP-3 (median, 37.6 months; log-rank test,
P<0.001; Fig. 3B). In addition,
Kaplan-Meier analysis demonstrated the significant impact of
established clinicopathological prognostic parameters, including
tumor size (P=0.003), T stage (P=0.002), N stage (P=0.001) and
clinical stage (P<0.001), on patient survival. Furthermore,
IGFBP-3 expression levels in the analyzed patient tissue specimens
were directly correlated with survival time (P<0.001), even
following patient stratification based on clinicopathological
classifications (Table II).
 | Table II.Univariate Cox regression analysis
(log-rank test) of the association between IGFBP-3 expression
levels and clinicopathological features. |
Table II.
Univariate Cox regression analysis
(log-rank test) of the association between IGFBP-3 expression
levels and clinicopathological features.
| Patient
characteristics | Relative risk (95%
CI) | P-value |
|---|
| Age, years |
| 0.656 |
|
≤51.2b | 1.000 |
|
|
>51.2b | 1.086
(0.698–1.698) |
|
| Gender |
| 0.542 |
|
Male | 1.000 |
|
|
Female | 0.886
(0.732–1.732) |
|
| AFP, ng/ml |
| 0.124 |
|
≤20 | 1.000 |
|
|
>20 | 1.853
(0.987–2.987) |
|
| Liver
cirrhosis |
| 0.952 |
|
Yes | 1.000 |
|
| No | 1.008
(0.558–1.558) |
|
| Tumor size, cm |
| 0.003a |
| ≥5 | 1.000 |
|
|
<5 | 3.689
(1.856–7.856) |
|
| T status |
| 0.002a |
|
T2–3 | 1.000 |
|
| T4 | 2.864
(1.862–4.862) |
|
| N status |
| 0.001a |
| N0 | 1.000 |
|
| N1 | 3.869
(1.684–5.684) |
|
| Clinical stage |
|
<0.001a |
|
I/II | 1.000 |
|
|
III/IV | 3.965
(2.589–8.589) |
|
| IGFBP-3
expression |
|
<0.001a |
|
High | 1.000 |
|
|
Low | 3.542
(1.622–7.622) |
|
Independent prognostic factors for HCC
according to multivariate Cox regression analysis
A multivariate progression analysis based on the Cox
proportional hazards model was applied to determine the independent
value of certain clinical parameters when predicting the overall
survival of patients with HCC. As presented in Table III, IGFBP-3 expression levels and
other clinicopathological features that were identified as
significant by univariate analysis, including tumor size, T stage,
N stage and clinical stage, were included in the multivariate
analysis. Decreased levels of IGFBP-3 expression were determined to
be an independent prognostic factor for favorable overall survival
(95% confidence interval, 3.642–13.568; P=0.003). Of the other
parameters evaluated, T stage (P=0.015), N stage (P=0.006) and
clinical stage (P=0.002) were also demonstrated to be independent
prognostic factors for overall survival.
 | Table III.Multivariate Cox regression analysis
of potential prognostic factors for patients with hepatocellular
carcinoma. |
Table III.
Multivariate Cox regression analysis
of potential prognostic factors for patients with hepatocellular
carcinoma.
| Patient
characteristics | Relative risk
(95%CI) | P-value |
|---|
| Tumor size, cm |
| 0.042a |
| ≥5 | 1.000 |
|
|
<5 | 2.346
(0.817–5.817) |
|
| T status |
| 0.015a |
|
T2–3 | 1.000 |
|
| T4 | 3.568
(1.325–6.325) |
|
| N status |
| 0.006a |
| N0 | 1.000 |
|
| N1 | 2.359
(1.234-73694) |
|
| Clinical stage |
| 0.002a |
|
I/II | 1.000 |
|
|
III/IV | 5.367
(2.689–11.689) |
|
| IGFBP-3
expression |
| 0.003a |
|
High | 1.000 |
|
|
Low | 3.568
(3.642–13.642) |
|
Discussion
Disease progression and survival in patients with
HCC with similar clinicopathological classifications often exhibits
considerable variability, and the conventional grading system may
have reached its limits for providing information regarding patient
prognosis and treatment strategies (23,24).
Therefore, it is important to establish criteria to allow the
development of novel diagnostics and risk assessments. IGFBP-3 was
initially first recognized as a protein carrier and cell signaling
pathway messenger, and has an established association with the
growth of HCC cells (25). IGFBP-3 is
able to bind to IGF-I and IGF-II and regulate the concentrations of
these proteins in circulation; therefore, it has been demonstrated
to inhibit cell proliferation, promote apoptosis and reduce growth
in numerous types of solid tumor, including breast cancer and
prostate cancer (25–28). In addition, IGFBP-3 has been reported
to possess pro-apoptotic and anti-proliferative functions, via its
interactions with other signaling receptors or proteins, to
regulate cell apoptosis, cell proliferation and the bioavailability
of insulin and IGFs (29). A previous
study revealed that IGFBP-3 exerts no direct effect on Hs578T
breast cancer cells, but is able enhance apoptosis induced by the
physiological trigger ceramide in an IGF-independent manner
(30). In addition, increased protein
expression levels of IGFBP-3 are associated with the upregulation
of the pro-apoptotic proteins B-cell lymphoma 2 (Bcl-2) -associated
death promoter and Bcl-2-associated X-protein (Bax), as well as
increased apoptosis through the modulation of the Bax/Bcl-2 protein
ratio in response to quercetin (a flavonoid present in food
products, including onion, grapes and green vegetables) in human
prostate cancer cells (31). Another
previous study demonstrated that IGFBP-3 is able to increase
ceramide-induced apoptosis in breast cancer cells, and enhance
tumor protein (p)53-dependent and p53-independent apoptosis in
various cancer cell lines, including MCF7 and A549 (32). These observations collectively suggest
that IGFBP-3 may function as a tumor suppressor.
Additionally, a previous study revealed that
EGF-induced EGFR activation is able to reduce IGFBP-3 levels
(33). Therefore, IGFBP-3, an EGFR
downstream target molecule, may serve as a radiosensitizer to
enhance the sensitivity of ESCC to radiotherapy in primary and
immortalized human esophageal epithelial cells (33). A previous study identified a potential
association between IGFBP-3 levels in EGFR-overexpressing ESCC
cells and the increased chemosensitivity of cells to nimotuzumab
(34). In addition, reduced levels of
IGFBP-3 expression may be a risk factor for advanced
clinicopathological classification and poor prognosis in patients
with ESCC and may, therefore, serve as a useful marker for
prognostic evaluation (14).
Furthermore, Adamek et al (35) reported that an estimation of the
IGF-1:IGFBP-3 ratio may provide additional non-invasive markers for
hepatitis C virus (HCV)-associated liver injury. Aleem et al
(36) demonstrated that serum IGFBP-3
levels are reduced as hepatic dysfunction progresses, and may be
correlated with the development of HCC in patients with chronic HCV
and liver cirrhosis. Taken together, these findings suggest that
IGFBP-3 expression may be a key mediator of HCC tumorigenesis.
In the present study, IHC probing for IGFBP-3 was
performed on a large cohort of HCC tumor samples, totaling 120
cases with complete clinicopathological and follow-up data. IGFBP-3
immunoreactivity was evaluated using a scoring system based on the
proportion of IGFBP-3 positive tumor cells present in each tissue
sample. This method was assessed independently by three
pathologists and observed to be reproducible, resulting in a more
complete evaluation of the prognostic or predictive value of
various markers in liver cancer. In order to avoid the use of
predetermined and often arbitrarily set values when selecting IHC
cut-off scores for positive IGFBP-3 expression, ROC analysis was
performed for each of the clinicopathological parameters, including
AFP levels, liver cirrhosis, tumor size, tumor multiplicity, N
stage, M stage, clinical stage and survival time. The IHC results
for IGFBP-3 expression revealed that the majority of matched
non-malignant tissues (72.0%) stained intensely for cytoplasmic
IGFBP-3, whereas only 54.2% of primary HCC tissues exhibited
intense cytoplasmic IGFBP-3 staining. The IGFBP-3 expression level
in HCC cells also demonstrated a correlation with tumor size, tumor
multiplicity, N stage, M stage, clinical stage and survival time.
Overall, these results suggest that IGFBP-3 may be a novel
prognostic marker for HCC.
Univariate Cox proportional hazards analysis
revealed that T, N, M and clinical stage classifications may be
risk factors for cancer-associated mortality. IGFBP-3 expression
levels were significantly correlated with survival time (mean, 39.4
months vs. 18.7 months; P<0.001). Furthermore, decreased levels
of IGFBP-3 expression in patients with HCC were demonstrated to be
an independent predictor of shorter survival time, as evaluated by
multivariable Cox proportional hazards regression analysis. These
results suggest that the reduction of IGFBP-3 expression levels in
HCC cells may facilitate cancer cell invasion and metastasis. By
contrast, patients that retained higher levels of IGFBP-3
expression exhibited a significantly more favorable prognosis.
These findings demonstrate the importance of IGFBP-3 expression
levels for the survival and prognosis of patients with HCC. The
results also raise the possibility that IGFBP-3 possesses an
important function within the underlying biological mechanisms that
promote the growth and development of human cancer. Therefore,
further studies must investigate the correlation between the
expression levels of IGFBP-3 and the treatment outcomes following
chemotherapy and radiofrequency ablation therapy in patients with
HCC. Although further investigation is required, an evaluation of
the IGFBP-3 expression profile may be useful when assessing the
prognosis of patients with HCC.
In conclusion, the present study demonstrated that
low levels of IGFBP-3 expression correlate with certain
clinicopathological features and the poor overall survival of
patients with HCC. Therefore, evaluation of IGFBP-3 expression
patterns using IHC may be used as a novel approach for
identification of those patients with HCC who have increased risk
of tumor invasion and progression. Overall, these results suggest
that IGFBP-3 may be used as a novel marker to aid the assessment of
prognosis for patients with HCC.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant nos. 81225018, 81172340 and
30901769), the 973 Project of China (grant nos. 2010CB912802 and
2010CB529404) and the Ph.D. Programs Foundation of The Ministry of
Education of China (grant no. 20110171110078).
References
|
1
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bolondi L, Sofia S, Siringo S, Gaiani S,
Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M and
Sherman M: Surveillance programme of cirrhotic patients for early
diagnosis and treatment of hepatocellular carcinoma: A cost
effectiveness analysis. Gut. 48:251–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi
CG and Lai CL: Early detection of hepatocellular carcinoma
increases the chance of treatment: Hong Kong experience.
Hepatology. 31:330–335. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mikami E, Kanno N, Ueno Y and Shimosegawa
T: Retrospective evaluation of tumor-mass-reduction therapy for the
prognosis of recurrent hepatocellular carcinoma. Hepatol Int.
1:460–468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho EJ, Lee JH, Yoo JJ, Choi WM, Lee MJ,
Cho Y, Lee DH, Lee YB, Kwon JH, Yu SJ, et al: Serum insulin-like
growth factor-I level is an independent predictor of recurrence and
survival in early hepatocellular carcinoma: A prospective cohort
study. Clin Cancer Res. 19:4218–4227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao L, Wang X, Wang X, Zhang L, Qiang C,
Chang S, Ren W, Li S, Yang Y, Tong D, et al: IGF-1R, a target of
let-7b, mediates crosstalk between IRS-2/Akt and MAPK pathways to
promote proliferation of oral squamous cell carcinoma. Oncotarget.
5:2562–2574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin C and Guo J, Qiu X, Ma K, Xiang M, Zhu
X and Guo J: IGF-1 induces iNOS expression via the p38 MAPK signal
pathway in the anti-apoptotic process in pulmonary artery smooth
muscle cells during PAH. J Recept Signal Transduct Res. 34:325–331.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan XD, Yao M, Wang L, Zhang HJ, Yan MJ,
Gu X, Shi Y, Chen J, Dong ZZ and Yao DF: Overexpression of
insulin-like growth factor-I receptor as a pertinent biomarker for
hepatocytes malignant transformation. World J Gastroenterol.
19:6084–6092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sedlaczek N, Hasilik A, Neuhaus P,
Schuppan D and Herbst H: Focal overexpression of insulin-like
growth factor 2 by hepatocytes and cholangiocytes in viral liver
cirrhosis. Br J Cancer. 88:733–739. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jogie-Brahim S, Min HK and Oh Y: Potential
of proteomics towards the investigation of the IGF-independent
actions of IGFBP-3. Expert Rev Proteomics. 2:71–86. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akturk M, Arslan M, Altinova A, Ozdemir A,
Ersoy R, Yetkin I, Ayvali E, Gonen S and Toruner F: Association of
serum levels of IGF-I and IGFBP-1 with renal function in patients
with type 2 diabetes mellitus. Growth Horm Igf Res. 17:186–193.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeschke MG, Barrow RE, Suzuki F, Rai J,
Benjamin D and Herndon DN: IGF-I/IGFBP-3 equilibrates ratios of
pro- to anti-inflammatory cytokines, which are predictors for organ
function in severely burned pediatric patients. Mol Med. 8:238–246.
2002.PubMed/NCBI
|
|
13
|
Zhao Z, Liu Y, He H, Chen X, Chen J and Lu
YC: Candidate genes influencing sensitivity and resistance of human
glioblastoma to Semustine. Brain Res Bull. 86:189–194. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao L, He LR, Zhang R, Cai MY, Liao YJ,
Qian D, Xi M, Zeng YX, Xie D and Liu MZ: Low expression of IGFBP-3
predicts poor prognosis in patients with esophageal squamous cell
carcinoma. Med Oncol. 29:2669–2676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varotti G, Ramacciato G, Ercolani G, Grazi
GL, Vetrone G, Cescon M, Del Gaudio M, Ravaioli M, Ziparo V, Lauro
A and Pinna A: Comparison between the fifth and sixth editions of
the AJCC/UICC TNM staging systems for hepatocellular carcinoma:
Multicentric study on 393 cirrhotic respected patients. Eur J Surg
Oncol. 31:760–767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie D, Zeng YX, Wang HJ, Wen JM, Tao Y,
Sham JS and Guan XY: Expression of cytoplasmic and nuclear Survivin
in primary and secondary human glioblastoma. Br J Cancer.
94:108–114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zlobec I, Steele R, Terracciano L, Jass JR
and Lugli A: Selecting immunohistochemical cut-off scores for novel
biomarkers of progression and survival in colorectal cancer. J Clin
Pathol. 60:1112–1116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai MY, Zhang B, He WP, Yang GF, Rao HL,
Rao ZY, Wu QL, Guan XY, Kung HF, Zeng YX and Xie D: Decreased
expression of PinX1 protein is correlated with tumor development
and is a new independent poor prognostic factor in ovarian
carcinoma. Cancer Sci. 101:1543–1549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mesa MS, Marrodán MD, Lomaglio DB,
López-Ejeda N, Moreno-Romero S, Bejarano JI, Dipierri JE and
Pacheco JL: Anthropometric parameters in screening for excess of
adiposity in argentinian and spanish adolescents: Evaluation using
receiver operating characteristic (ROC) methodology. Ann Hum Biol.
40:396–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanley JA: Receiver operating
characteristic (ROC) methodology: The state of the art. Crit Rev
Diagn Imaging. 29:307–335. 1989.PubMed/NCBI
|
|
21
|
Taylor SC and Posch A: The design of a
quantitative western blot experiment. Biomed Res Int.
2014:3615902014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshino K, Motoyama S, Koyota S, Shibuya
K, Usami S, Maruyama K, Saito H, Minamiya Y, Sugiyama T and Ogawa
J: IGFBP3 and BAG1 enhance radiation-induced apoptosis in squamous
esophageal cancer cells. Biochem Biophys Res Commun. 404:1070–1075.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanaka H, Iijima H, Higashiura A, Yoh K,
Ishii A, Takashima T, Sakai Y, Aizawa N, Iwata K, Ikeda N, et al:
New malignant grading system for hepatocellular carcinoma using the
Sonazoid contrast agent for ultrasonography. J Gastroenterol.
49:755–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han DH, Choi GH, Kim KS, Choi JS, Park YN,
Kim SU, Park JY, Ahn SH and Han KH: Prognostic significance of the
worst grade in hepatocellular carcinoma with heterogeneous
histologic grades of differentiation. J Gastroenterol Hepatol.
28:1384–1390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shahjee HM and Bhattacharyya N: Activation
of various downstream signaling molecules by IGFBP-3. J Cancer
Ther. 5:830–835. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tas F, Karabulut S, Bilgin E, Tastekin D
and Duranyildiz D: Clinical significance of serum insulin-like
growth factor-1 (IGF-1) and insulin-like growth factor binding
protein-3 (IGFBP-3) in patients with breast cancer. Tumour Biol.
35:9303–9309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ingermann AR, Yang YF, Han J, Mikami A,
Garza AE, Mohanraj L, Fan L, Idowu M, Ware JL, Kim HS, et al:
Identification of a novel cell death receptor mediating
IGFBP-3-induced anti-tumor effects in breast and prostate cancer. J
Biol Chem. 285:30233–30246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Butt AJ and Williams AC: IGFBP-3 and
apoptosis-a license to kill? Apoptosis. 6:199–205. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang XP, Zhou WH and Zhang YF: Genetic
variations in the IGF-IGFR-IGFBP axis confer susceptibility to lung
and esophageal cancer. Genet Mol Res. 13:2107–2119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gill ZP, Perks CM, Newcomb PV and Holly
JM: Insulin-like growth factor-binding protein (IGFBP-3)
predisposes breast cancer cells to programmed cell death in a
non-IGF-dependent manner. J Biol Chem. 272:25602–25607. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vijayababu MR, Kanagaraj P, Arunkumar A,
Ilangovan R, Dharmarajan A and Arunakaran J: Quercetin induces
p53-independent apoptosis in human prostate cancer cells by
modulating Bcl-2-related proteins: A possible mediation by IGFBP-3.
Oncol Res. 16:67–74. 2006.PubMed/NCBI
|
|
32
|
Butt AJ, Firth SM, King MA and Baxter RC:
Insulin-like growth factor-binding protein-3 modulates expression
of Bax and Bcl-2 and potentiates p53-independent radiation-induced
apoptosis in human breast cancer cells. J Biol Chem.
275:39174–39181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takaoka M, Harada H, Andl CD, Oyama K,
Naomoto Y, Dempsey KL, Klein-Szanto AJ, El-Deiry WS, Grimberg A and
Nakagawa H: Epidermal growth factor receptor regulates aberrant
expression of insulin-like growth factor-binding protein 3. Cancer
Res. 64:7711–7723. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao L, He LR, Xi M, Cai MY, Shen JX, Li
QQ, Liao YJ, Qian D, Feng ZZ, Zeng YX, et al: Nimotuzumab promotes
radiosensitivity of EGFR-overexpression esophageal squamous cell
carcinoma cells by upregulating IGFBP-3. J Transl Med. 10:2492012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Adamek A, Kasprzak A, Mikoś H,
Przybyszewska W, Seraszek-Jaros A, Czajka A, Sterzyńska K and
Mozer-Lisewska I: The insulin-like growth factor-1 and expression
of its binding protein-3 in chronic hepatitis C and hepatocellular
carcinoma. Oncol Rep. 30:1337–1345. 2013.PubMed/NCBI
|
|
36
|
Aleem E, Elshayeb A, Elhabachi N, Mansour
AR, Gowily A and Hela A: Serum IGFBP-3 is a more effective
predictor than IGF-1 and IGF-2 for the development of
hepatocellular carcinoma in patients with chronic HCV infection.
Oncol Lett. 3:704–712. 2012.PubMed/NCBI
|