Introduction
Breast cancer is the second most prevalent cause of
cancer-associated mortality in females; metastasis in breast cancer
is the primary cause of mortality and is a crucial factor in
treatment (1–3). Constitutive activation of nuclear
factor-κB (NF-κB), a family of transcription factors (4), stimulates proliferation and metastasis,
and inhibits apoptosis in breast cancer (5). These proteins form homo- or heterodimers
and have similar structural characteristics, including the Rel
homology domain, which is necessary for dimerization, binding to
cognate DNA elements and nuclear localization signals (4,6). In
non-stimulated cells, NF-κB complexes in an inactive form interact
with a monomer of an inhibitory protein called inhibitor of NF-κB
(IκB) (6). NF-κB activity stimulating
signals cause dissociation of IκB, allowing NF-κB dimers to locate
to the nucleus and alter gene expression (6). Additionally, NF-κB signaling is
essential for epithelial-mesenchymal transition (EMT), and the
therapeutic inhibition of NF-κB may be an effective strategy to
control tumor invasion and metastasis (7).
Transforming growth factor β (TGF-β) is a
pleiotropic cytokine that is found in three isoforms (TGF-β1,
TGF-β2 and TGF-β3), which are structurally and functionally
associated (8). TGF-β isoforms are
secreted as biologically latent precursor molecules and are
stimulated by proteolytic cleavage interactions with integrins or
by pH alterations in the local microenvironment; intracellular
TGF-β signaling is complex and is activated by numerous signaling
pathways (9). TGF-β1 is involved in
the occurrence and development of breast cancer (10) and the TGF-β signaling pathway is
deregulated in breast cancer progression and metastasis (11,12).
In invasive breast cancer, certain alterations have
been observed in the stromal structure, including a reduction in
the expression of two small leucine rich proteoglycans,
fibromodulin (Fmod) and decorin (Dcn), and the acquisition of TGF-β
antagonist activity in vitro and in vivo has been
identified (13–16). Dcn is a dermatansulfate proteoglycan
that reduces the growth of tumors, including gliomas, breast, lung,
colon and squamous cell carcinoma, and has an anti-angiogenic role
through the binding TGF-β, epidermal growth factor receptor (EGFR)
and inducing the expression of p21 (13,17–21). Fmod,
a keratan sulfate proteoglycan, functions as a modulator of TGF-β
activity in scarless wound healing and is involved in collagen
assembly in skin development (22,23). In
addition, Fmod exerts a potent TGF-β-antagonist activity, compared
with Dcn, in the inhibition of neointimal hyperplasia in saphenous
vein graft (15). Fmod is also
implicated in the inhibitory effect of NF-κB signaling thorough the
suppression of IκBα protein in 3T3-L1 fibroblasts (24).
In the current study, the inhibitory effects of Fmod
and Dcn overexpression on NF-κB and TGF-β1 were investigated using
adenovirus-mediated gene transfer in the 4T1 breast cancer cell
line.
Materials and methods
Recombinant adenovirus construct
The recombinant adenovirus (Rad) Fmod and Dcn
expression cassettes were constructed by Dr Paul Kingston (Gene
Therapy Unit, University of Manchester, Manchester, UK), and
contain the major immediate-early murine cytomegalovirus
enhancer/promoter, Woodchuck hepatitis virus regulatory element and
a fragment of the rabbit smooth muscle myosin heavy chain promoter,
which produces increased transgene expression compared with other
expression vectors. Rad vectors are E1/E3-deleted first-generation
adenoviruses that have a recombinant transgene and promoter
inserted instead of possessing an E1 region (13). The efficiency of these vectors was
confirmed in a previous study (13).
Cell culture
The highly metastatic 4T1 breast cancer cell line
was obtained from Pasture Institute of Iran (Tehran, Iran) and
cultured in RPMI-1,640 (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) in 5% CO2 at
37°C. Cells were not allowed to be >80% confluent, and
4×105 cells were treated with Rad-Fmod, Rad-Dcn or
Rad-LacZ at a multiplicity of infection (MOI) of 1,000, which was
considered an appropriate MOI for this adenovirus (25). Cells were incubated for 4 h at 37°C
and medium was regularly replaced with fresh RPMI-1,640
(Sigma-Aldrich; Merck KGaA). After 72 h, cells were used for
further analysis. Uninfected cells cultured in the same conditions
were used as a negative control.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The 4T1 cell line total RNA was extracted using an
RNeasy Mini kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's protocol. RNA was reverse-transcribed with a OneStep
RT-PCR kit (Qiagen GmbH, Germany) at 50°C for 30 min with initial
PCR activation at 95°C for 15 min. cDNA was amplified by 35 cycles,
each consisting of three steps: Denaturation at 94°C for 45 sec,
annealing at 63°C for 45 sec, extension at 72°C for 1 min and final
extension at 72°C for 10 min. Specific primers (Metabion GmbH,
Steinkirchen, Germany) for Dcn (forward primer,
5′-CCCAGAAGTTCCTGATGAC-3′; reverse primer,
5′-CAGAGCGCACGTAGACAC-3′), Fmod (forward primer,
5′-TGAAGGCAGCACCTGACCGC-3′; reverse primer,
5′-ACGCCTTGGCTTCTCCTGCC-3′) and β-actin as a control (forward
primer, 5′-ATATCGCTGCGCTGGTCGTC-3′; reverse primer,
5′-AGGATGGCGTGAGGGAGAGC-3′) were used in this experiment. PCR
products were separated on 1% agarose gel with 0.5 µg/ml ethidium
bromide for Dcn and 2% agarose gel for Fmod.
RT-quantitative PCR (RT-qPCR)
RT-qPCR was used for the detection of Fmod and Dcn
inhibitory effects on TGF-β1 expression in the 4T1 breast cancer
cell line. Total RNA was extracted using an RNeasy Mini kit (Qiagen
GmbH), then cDNA was amplified with the QuantiTec Reverse
transcription kit (Qiagen GmbH) and TGF-β1 mRNA expression was
quantified using a QuantiFast SYBR-Green Master PCR kit (Qiagen
GmbH) in triplicate on an Applied Biosystems 7300 using the
‘standard curve method’ (26).
Standard curves for TGF-β1 and GAPDH were generated via five serial
dilutions with cDNA. Cq values from each gene were measured from
the curve and were quantified relative to GAPDH as the control
housekeeping gene (27). All
experiments were performed in three independent experiments with
60°C as the annealing temperature. The amplification process
included 95°C for 5 min, followed by 35 cycles at 95°C for 10 sec
and 60°C for 30 sec. The primers were as follows: Mouse TGF-β1
forward, 5′-GGTAACCGGCTGCTGACC-3; mouse TGF-β1 reverse,
5′-GCCCTGTATTCCGTCTCCTTG-3′; mouse GAPDH forward,
5′-GGCCTTCCGTGTTCCTAC-3′; mouse GAPDH reverse,
5′-TGTCATCATACTTGGCAGGTT-3′.
Nuclear extract and NF-κB transbinding
assay
A total of 1×106 cells/ml 4T1 cells were collected
and nuclear extracts were isolated using the Nuclear Extract kit
from Active Motif GmbH (Regensburg, Germany) according to the
manufacturer's protocol. Nuclear extracts were stored at −80°C
until they were used, and their concentration was measured using a
Bradford assay. NF-κB DNA binding activity was determined using an
Trans-AM P65-NF-κB ELISA-based kit (cat number 40096; Active Motif
GmbH), which is a sensitive assay that measures the quantity of
activated NF-κB in the nuclear extracts from the 4T1 breast cancer
cell line, prior to and following Fmod and Dcn expression. In
total, 10 µg nuclear extract was added to a biotinylated
oligonucleotide containing the NF-κB consensus site attached to the
streptavidin-coated 96-well plates. Plates were washed with wash
buffer (cat number 40096; Active Motif GmbH) to remove all the
unbound reagents; to visualize NF-κB DNA binding, an anti-p65
primary antibody (dilution, 1:2,000; cat number 40096; Active Motif
GmbH) was added for 1 h at room temperature without agitation,
followed by a goat anti-rabbit secondary antibody conjugated with
horseradish peroxidase (dilution, 1:5,000; cat number 40096; Active
Motif GmbH) at room temperature without agitation. Subsequent to an
incubation for 1 h at room temperature, 100 µl developing solution
(cat number 40096; Active Motif GmbH) was added to all wells for 5
min at room temperature protected from direct light. The blue color
development in the sample wells was monitored until it turned
medium to dark blue. Subsequently, 100 µl stop solution (cat number
40096; Active Motif GmbH) was added and the blue color turned
yellow. Finally, the absorbance value was ascertained using a
spectrophotometer at a wavelength of 450 nm. For the p65 positive
control: 2.5 µg of Jurkat nuclear extract was provided (1 µl of
nuclear extract in 19 µl of complete lysis buffer per well
according to the Active Motif kit protocol). For blank wells: 20 µl
complete lysis buffer was added per well (according to the Active
Motif kit protocol).
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance to compare replicates with GraphPad Prism 6
(GraphPad Software, Inc., La Jolla, CA, USA). Results are presented
as mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Rad-Fmod and Rad-Dcn expression levels
in the 4T1 breast cancer cell line as evaluated using RT-PCR
Expression of replication-defective adenovirus
containing bovine Fmod cDNA (Rad-Fmod) and human Dcn cDNA (Rad-Dcn)
were identified in the 4T1 breast cancer cell line using RT-PCR.
The 4T1 breast cancer cell line is highly metastatic and was
established to be negative for Fmod and Dcn transcripts (28,29).
Following 4T1 cell line adenoviral infection, mRNA was extracted
and RT-PCR using specific PCR primers was performed. Fmod and Dcn
expression signals were detected in Rad-Fmod and Rad-Dcn infected
cell lines, but were not identified in Rad-LacZ-transfected cells
(control; Fig. 1). Therefore, these
results demonstrate the lack of Fmod and Dcn expression in the
extracellular matrix in 4T1 highly metastatic breast cancer
cells.
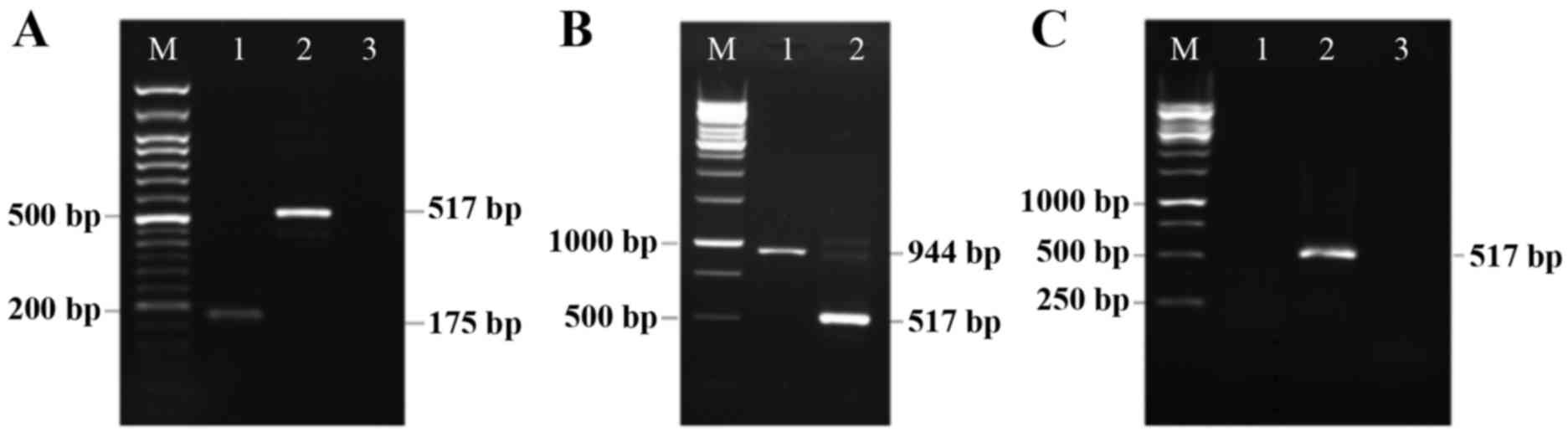 | Figure 1.Detection of Rad-Fmod and Rad-Dcn
expression in the 4T1 breast cancer cell line. (A) Rad-Fmod is
expressed in the 4T1 breast cancer cell line (175 bp, lane 1). This
image presents β-actin functioning as an internal control (517 bp,
lane 2), the non-template control (lane 3) and the DNA marker (lane
M). (B) This image presents Rad-Dcn gene expression (944 bp, lane
1), β-actin (517 bp, lane 2) and the DNA marker (lane M). (C) This
image presents the 4T1 cell line infected with Rad-LacZ as a
control, and was evaluated for fibromodulin (lane 1), β-actin (lane
2) and decorin (lane 3) expression. Rad-Fmod, recombinant
adenovirus fibromodulin; Rad-Dcn, recombinant adenovirus decorin;
Rad-LacZ, recombinant adenovirus LacZ. |
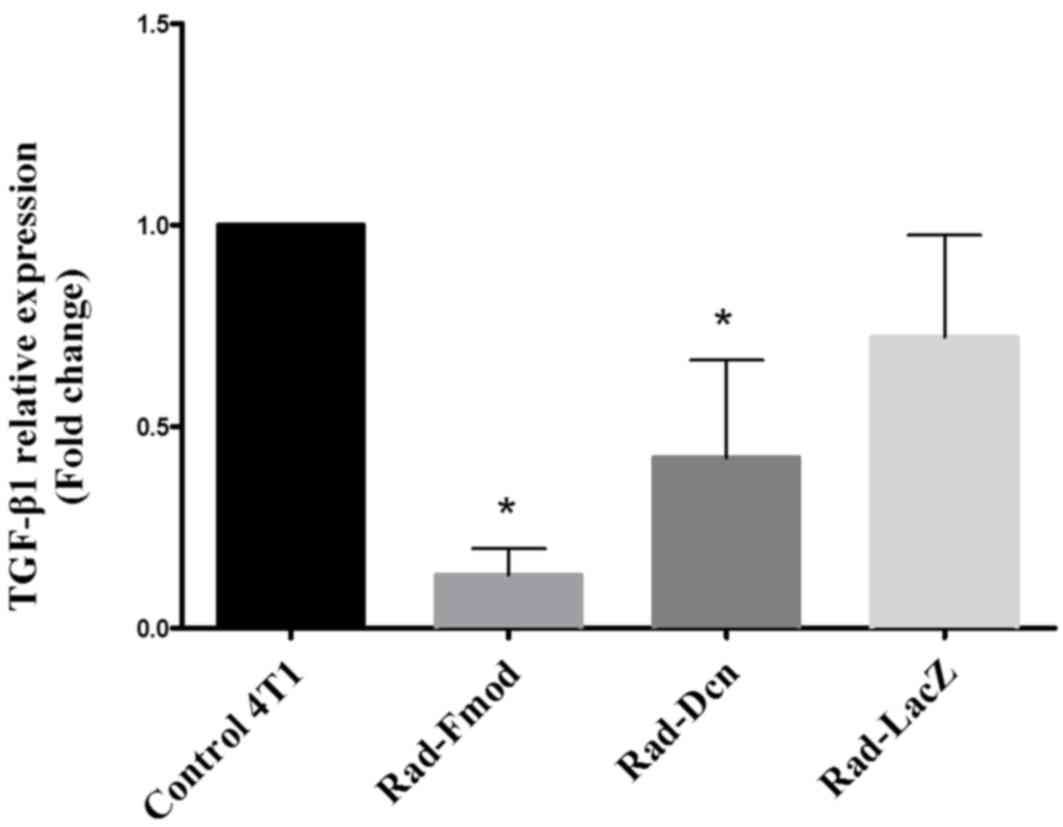
TGF-β1 is suppressed by Rad-fmod and
Rad-dcn expression in the 4T1 breast cancer cell line
Increased expression levels of TGF-β1 have been
associated with malignant conversion and progression in breast
cancer (30). In the present study,
the inhibitory effects of Rad-Fmod, Rad-Dcn and Rad-LacZ (control)
on TGF-β1 expression were examined using RT-qPCR. The 4T1 cell line
was infected with recombinant adenoviral vectors for 72 h at an MOI
of 1,000. Notably, the overexpression of Rad-Fmod and Rad-Dcn
demonstrated a significant reduction (P<0.05) of TGF-β1
expression compared with the non-transfected 4T1 cell line
(Fig. 2). Fmod may be considered as a
more effective inhibitor than Dcn compared with the non-transfected
4T1 cell line and Rad-LacZ.
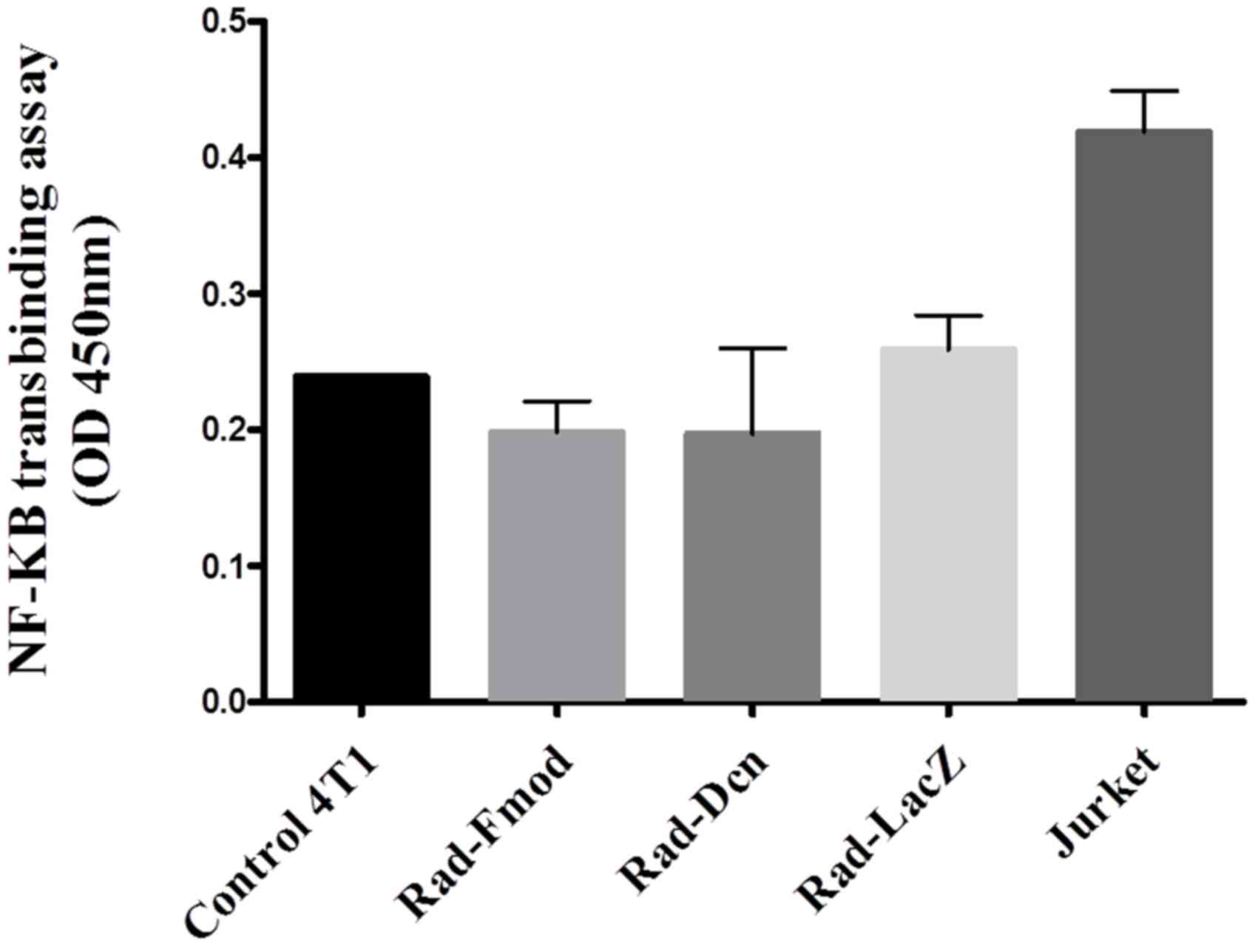
Fmod suppresses NF-κB DNA binding
NF-κB has been established to have an important role
in breast cancer progression, control of cell proliferation and
oncogenesis (7). To study the effects
of Fmod and Dcn on NF-κB activity, the 4T1 cell line was
transfected with Rad-Fmod, Rad-Dcn and Rad-LacZ (control) for 72 h
at an MOI of 1,000, nuclear protein extraction was performed and
p65 DNA binding activity was measured using a NF-κB transbinding
assay. Extracellular signals stimulate NF-κB via the
phosphorylation and degradation of IκB, and subsequent NF-κB
nuclear translocation promotes the expression of numerous target
genes (31). Rad-Fmod and Rad-Dcn
infected cells demonstrated a 31 and 27% reduction, respectively,
in NF-κB DNA binding activity compared with Rad-LacZ infected
cells. These results suggest that Fmod expression may have the
ability to reduce NF-κB activity more effectively compared with Dcn
expression (P>0.05) (Fig. 3).
Discussion
NF-κB is a transcription factor that regulates the
transcription of numerous target genes involved in angiogenesis,
invasion, migration and apoptosis (32,33). NF-κB
is normally sequestered in the cytoplasm by the inhibitory
molecules of the IκB family (IκBα, IκBβ and IκBε) (32). In response to certain stimulatory
agents, including viral infection, inflammatory cytokines and
bacterial lipopolysaccharide, IκBs are rapidly phosphorylated and
degraded to promote the nuclear transfer of NF-κB and the
activation of a number of genes (34,35).
Activation of NF-κB has been demonstrated in a variety of
inflammatory, autoimmune diseases and human disorders (24,36), and
the failure of cancer treatment due to the activation of NF-κB and
resistance to chemotherapeutic agents, has been demonstrated
(37,38). Therefore, NF-κB may be a potential
therapeutic target.
TGF-β has exhibited bifunctional activities through
its role in the regulation of cell growth, differentiation and
migration (39), and it been
established as important for cancer progression and EMT (39). The functional polymorphic TGF-β genes
that are expressed in humans include TGF-β1–3 and TGF-β receptor
(TGF-βR) types I–III (39). A number
of previous studies have demonstrated an association between allele
variants of TGF-β1 and invasive breast cancer (30,39,40). TGF-β
is deregulated during tumor progression and its enhancement has
been identified in numerous tumor types, including glioblastomas,
melanoma cells, colorectal and prostate carcinoma (41). The TGF-β ligand is released in a
latent form in the extracellular matrix and when activated binds to
the TGF-βR types I–III (39). The
phosphorylation of TGF-β receptors initiates a cascade of
Smad-dependent and -independent proteins locating to the nucleus
for transcriptional regulation (42).
Extracellular matrix macromolecules are involved in
cellular physiologic events and pathological processes (43). Among these, Fmod and Dcn,
extracellular matrix proteoglycans, have been designated as potent
antitumor molecules and the lack of these proteins is permissive
for tumorigenesis (44).
In the current study, the lack of expression of Fmod
and Dcn has been demonstrated in non-transfected and Rad-LacZ
infected 4T1 cells, and the expression of Rad-Fmod and Rad-Dcn was
examined using RT-PCR. The lack of expression of Fmod and Dcn in
this highly metastatic cell line demonstrated an inhibitory role in
acquisition of metastatic phenotypes and the increased expression
of Rad-Fmod and Rad-Dcn in transfected cells. In addition, the
effects of Fmod and Dcn on NF-κB and TGF-β1 expression were
analyzed in the highly metastatic 4T1 breast cancer cell line. Fmod
and Dcn may control TGF-β antagonist activity with differing
affinities for the isoforms of TGF-β (15). Using RT-qPCR, the present study
demonstrated that Fmod is a more potent inhibitor of TGF-β1
expression in 4T1 breast cancer cells compared with Dcn. Fmod
contributes to a significant reduction in TGF-β1 expression levels
compared with Rad-LacZ. Therefore, Fmod-deficiency in adult animals
may lead to delayed wound healing and increased scar size, and Fmod
overexpression may contribute to a decrease in TGF-β1 expression
levels (45,46). Fmod was considered as a dominant
inhibitor of TGF-β1 compared with Dcn in cultured human saphenous
vein cells, and TGF-β1 was identified at a lower expression level
in cells treated with Ad5-Fmod or Ad5-Dcn compared with those
receiving Ad5-LacZ or vehicle only (15). In addition, a previous study evaluated
the effects of recombinant adenoviral Dcn gene transfer in the rat
CNS-1 glioma model (13). These
results determined that ectopic Dcn expression has the potential to
slow glioma development (13).
Additionally, it was demonstrated that exogenous TGF-β ligands
inhibit lung branch morphogenesis in mouse embryonic lungs in ex
vivo culture, and treatment with a recombinant adenovirus
containing Dcn cDNA abrogated this effect (21). This study indicated that Dcn is
involved in suppressing TGF-β-mediated negative regulation and may
be used as a potential therapeutic approach to optimize the levels
of TGF-β signaling (21).
A potential explanation of why Fmod has improved
inhibitory activity compared with Dcn is that Fmod may have
effective structural characteristics (47). Fmod and Dcn core proteins are composed
of two disulfide-bonded domains flanking 10 leucine-rich repeats
(LRR) (47). Dcn with a
dermatansulfate chain in its amino terminal region may be secreted
and has been identified to interact with EGFR through the
activation of mitogen-activated protein kinase signaling pathway
and this induces p21 cell cycle arrest (17). Additionally, Dcn interacts with
collagen through the inner concave surface in the center of its
arch-shaped structure with high affinity (48). In the case of Fmod, one to four
keratan-sulfate chains may reside between the LRR domains and there
are two collagen binding sites; therefore, LRRs 7 and 11 each may
bind one collagen monomer (47). LRRs
11 on the C-terminus exhibited a higher affinity for collagen that
may produce less spatial limitation for TGF-β1 binding in the
center of the Fmod protein (49).
Notably, five potential sites for sulfation of tyrosine residues,
as a post-translational modification, are indicated in the
N-terminus of bovine Fmod (50).
Sulfation has been recognized to influence the half-life of Fmod
and increase its stability (50).
The effect of Fmod and Dcn NF-κB activity was
evaluated using a highly sensitive NF-κB transbinding assay.
Despite the efficient expression of Fmod and Dcn proteins, there
were no significant alterations in the levels of NF-κB in the
nuclear extracts in comparison with the β-galactosidase transgene
and the non-transfected 4T1 cell line. By contrast, in parental
3T3-L1 fibroblasts with high levels of NF-κB activity, Fmod
inhibits NF-κB signaling by delaying the degradation of IκBα
protein through the activation of c-Jun N-terminal kinase, the
suppression of calpain and casein kinase 2 activity and the
induction of fibroblast apoptosis (24).
In conclusion, the results of the present study
indicate that Fmod binding to TGF-β1 is more effective compared
with Dcn in vitro, but to further evaluate its effectiveness
and therapeutic potential, this must be investigated in vivo
and in combination with other antitumor agents.
Acknowledgements
The authors would like to thank Dr Paul Kingston
(Manchester Academic Health Science Centre, University of
Manchester, Manchester, UK) for the gifted recombinant adenoviral
vectors and the Zanjan University of Medical Sciences (Zanjan,
Iran) for financial support.
References
|
1
|
Huber MA, Maier HJ, Alacakaptan M,
Wiedemann E, Braunger J, Boehmelt G, Madwed JB, Young ER, Marshall
DR, Pehamberger H, et al: BI 5700, a selective chemical inhibitor
of I B kinase 2, specifically suppresses epithelial-mesenchymal
transition and metastasis in mouse models of tumor progression.
Genes Cancer. 1:101–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keklikoglou I, Koerner C, Schmidt C, Zhang
JD, Heckmann D, Shavinskaya A, Allgayer H, Gückel B, Fehm T,
Schneeweiss A, et al: MicroRNA-520/373 family functions as a tumor
suppressor in estrogen receptor negative breast cancer by targeting
NF-κB and TGF-β signaling pathways. Oncogene. 31:4150–4163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reed CC, Waterhouse A, Kirby S, Kay P,
Owens RT, McQuillan DJ and Iozzo RV: Decorin prevents metastatic
spreading of breast cancer. Oncogene. 24:1104–1110. 2004.
View Article : Google Scholar
|
|
4
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shostak K and Chariot A: NF-κB, stem cells
and breast cancer: The links get stronger. Breast Cancer Res.
13:2142011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
May MJ and Ghosh S: Rel/NF-kappa B and I
kappa B proteins: An overview. Semin Cancer Biol. 8:63–73. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Syed V: TGF-β Signaling in Cancer. J Cell
Biochem. 117:1279–1287. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buck MB and Knabbe C: TGF-Beta Signaling
in Breast Cancer. Ann N Y Acad Sci. 1089:119–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Zhu H, Chen T, Dai G and Zou L:
TGF-β1 and BRCA2 expression are associated with clinical factors in
breast cancer. Cell Biochem Biophys. 60:245–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buck MB: Prognostic significance of
transforming growth factor beta receptor II in estrogen
receptor-negative breast cancer patients. Clin Cancer Res.
10:491–498. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Figueroa JD, Flanders KC, Garcia-Closas M,
Anderson WF, Yang XR, Matsuno RK, Duggan MA, Pfeiffer RM, Ooshima
A, Cornelison R, et al: Expression of TGF-beta signaling factors in
invasive breast cancers: Relationships with age at diagnosis and
tumor characteristics. Breast Cancer Res Treat. 121:727–735. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biglari A, Bataille D, Naumann U, Weller
M, Zirger J, Castro MG and Lowenstein PR: Effects of ectopic
decorin in modulating intracranial glioma progression in vivo, in a
rat syngeneic model. Cancer Gene Ther. 11:721–732. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hildebrand A, Romarís M, Rasmussen LM,
Heinegård D, Twardzik DR, Border WA and Ruoslahti E: Interaction of
the small interstitial proteoglycans biglycan, decorin and
fibromodulin with transforming growth factor beta. Biochem. J.
302:527–534. 1994.
|
|
15
|
Ranjzad P, Salem HK and Kingston PA:
Adenovirus-mediated gene transfer of fibromodulin inhibits
neointimal hyperplasia in an organ culture model of human saphenous
vein graft disease. Gene Ther. 16:1154–1162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Troup S, Njue C, Kliewer EV, Parisien M,
Roskelley C, Chakravarti S, Roughley PJ, Murphy LC and Watson PH:
Reduced expression of the small leucine-rich proteoglycans,
lumican, and decorin is associated with poor outcome in
node-negative invasive breast cancer. Clin Cancer Res. 9:207–214.
2003.PubMed/NCBI
|
|
17
|
Goldoni S and Iozzo RV: Tumor
microenvironment: Modulation by decorin and related molecules
harboring leucine-rich tandem motifs. Int J Cancer. 123:2473–2479.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldoni S, Seidler DG, Heath J, Fassan M,
Baffa R, Thakur ML, Owens RT, McQuillan DJ and Iozzo RV: An
antimetastatic role for decorin in breast cancer. Am J Pathol.
173:844–855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mohan RR, Tovey JC, Sharma A, Schultz GS,
Cowden JW and Tandon A: Targeted decorin gene therapy delivered
with adeno-associated virus effectively retards corneal
neovascularization in vivo. PLoS One. 6:e264322011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reed CC, Gauldie J and Iozzo RV:
Suppression of tumorigenicity by adenovirus-mediated gene transfer
of decorin. Oncogene. 21:3688–3695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao J, Sime PJ, Bringas P Jr, Gauldie J
and Warburton D: Adenovirus-mediated decorin gene transfer prevents
TGF-beta-induced inhibition of lung morphogenesis. Am J Physiol.
277:L412–L422. 1999.PubMed/NCBI
|
|
22
|
Rydell-Törmänen K, Andréasson K,
Hesselstrand R and Westergren-Thorsson G: Absence of fibromodulin
affects matrix composition, collagen deposition and cell turnover
in healthy and fibrotic lung parenchyma. Sci Rep. 4:63832014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soo C, Hu FY, Zhang X, Wang Y, Beanes SR,
Lorenz HP, Hedrick MH, Mackool RJ, Plaas A, Kim SJ, et al:
Differential expression of fibromodulin, a transforming growth
factor-beta modulator, in fetal skin development and scarless
repair. Am J Pathol. 157:423–433. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee YH and Schiemann WP: Fibromodulin
suppresses nuclear factor-kappaB activity by inducing the delayed
degradation of IKBA via a JNK-dependent pathway coupled to
fibroblast apoptosis. J Biol Chem. 286:6414–6422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Appleby CE, Kingston PA, David A, Gerdes
CA, Umaña P, Castro MG, Lowenstein PR and Heagerty AM: A novel
combination of promoter and enhancers increases transgene
expression in vascular smooth muscle cells in vitro and coronary
arteries in vivo after adenovirus-mediated gene transfer. Gene
Ther. 10:1616–1622. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Creating Standard Curves with Genomic DNA
or Plasmid DNA Templates for Use in Quantitative PCR. Applied
Biosystem support.
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pulaski BA and Ostrand-Rosenberg S: Mouse
4T1 breast tumor model. Curr Protoc Immunol Chapter. 20:Unit 20.2.
2001. View Article : Google Scholar
|
|
29
|
Sainio A, Nyman M, Lund R, Vuorikoski S,
Boström P, Laato M, Boström PJ and Järveläinen H: Lack of decorin
expression by human bladder cancer cells offers new tools in the
therapy of urothelial malignancies. PLoS One. 8:e761902013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barcellos-Hoff MH and Akhurst RJ:
Transforming growth factor-beta in breast cancer: Too much, too
late. Breast Cancer Res. 11:2022009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Israël A: The IKK complex: An integrator
of all signals that activate NF-kappaB? Trends in Cell Biol.
10:129–133. 2000. View Article : Google Scholar
|
|
32
|
Lee CH, Jeon YT, Kim SH and Song YS:
NF-kappaB as a potential molecular target for cancer therapy.
Biofactors. 29:19–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pahl HL: Activators and target genes of
Rel/NF-kappaB transcription factors. Oncogene. 18:6853–6866. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Courtois G: The NF-kappaB signaling
pathway in human genetic diseases. Cell Mol Life Sci. 62:1682–1691.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karin M and Ben-Neriah Y: Phosphorylation
Meets Ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baldwin AS Jr: Series Introduction: The
transcription factor NF-kappaB and human disease. J Clin Invest.
107:3–6. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rahman KW, Ali S, Aboukameel A, Sarkar SH,
Wang Z, Philip PA, Sakr WA and Raz A: Inactivation of NF-kappaB by
3,3′-diindolylmethane contributes to increased apoptosis induced by
chemotherapeutic agent in breast cancer cells. Mol Cancer Ther.
6:2757–2765. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tergaonkar V, Pando M, Vafa O, Wahl G and
Verma I: p53 stabilization is decreased upon NFkappaB activation: A
role for NFkappaB in acquisition of resistance to chemotherapy.
Cancer Cell. 1:493–503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pickup M, Novitskiy S and Moses HL: The
roles of TGFβ in the tumour microenvironment. Nat Rev Cancer.
13:788–799. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hishida A, Iwata H, Hamajima N, Matsuo K,
Mizutani M, Iwase T, Miura S, Emi N, Hirose K and Tajima K:
Transforming growth factor B1 T29C polymorphism and breast cancer
risk in Japanese women. Breast Cancer. 10:63–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ständer M, Naumann U, Wick W and Weller M:
Transforming growth factor-beta and P-21: Multiple molecular
targets of decorin-mediated suppression of neoplastic growth. Cell
Tissue Res. 296:221–227. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stover DG, Bierie B and Moses HL: A
delicate balance: TGF-beta and the tumor microenvironment. J Cell
Biochem. 101:851–861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Boström P, Sainio A, Kakko T, Savontaus M,
Söderström M and Järveläinen H: Localization of decorin gene
expression in normal human breast tissue and in benign and
malignant tumors of the human breast. Histochem Cell Biol.
139:161–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Iozzo RV and Schaefer L: Proteoglycans in
health and disease: Novel regulatory signaling mechanisms evoked by
the small leucine-rich proteoglycans. FEBS J. 277:3864–3875. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng Z, Lee KS, Zhang X, Nguyen C, Hsu C,
Wang JZ, Rackohn TM, Enjamuri DR, Murphy M, Ting K and Soo C:
Fibromodulin-deficiency alters temporospatial expression patterns
of transforming growth factor-β ligands and receptors during adult
mouse skin wound healing. PLoS One. 9:e908172014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng Z, Nguyen C, Zhang X, Khorasani H,
Wang JZ, Zara JN, Chu F, Yin W, Pang S, Le A, et al: Delayed wound
closure in fibromodulin-deficient mice is associated with increased
TGF-β3 signaling. J Invest Dermatol. 131:769–778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roughley PJ: The structure and function of
cartilage proteoglycans. Eur Cells Mater. 12:92–101. 2006.
View Article : Google Scholar
|
|
48
|
Iozzo RV: Matrix proteoglycans: From
molecular design to cellular function. Annu Rev Biochem.
67:609–652. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kalamajski S and Oldberg Å: The role of
small leucine-rich proteoglycans in collagen fibrillogenesis.
Matrix Biol. 29:248–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Antonsson P, Heinegård D and Oldberg Å:
Structure and deduced amino acid sequence of the human fibromodulin
gene. Biochim Biophys Acta. 1174:204–206. 1993. View Article : Google Scholar : PubMed/NCBI
|