Introduction
The diagnostic hallmark of acute promyelocytic
leukemia (APL) is the reciprocal translocation t(15;17)(q24;q21),
leading to the disruption of the promyelocytic leukemia (PML) and
retinoic acid receptor α (RARA) genes, resulting in PML-RARA and
RARA-PML fusion products in ~98% of cases (1–7). The
PML-RARA fusion transcript from der(15)t(15;17) serves a key role in
leukemogenesis, inhibiting the differentiation and promoting the
survival of myeloid precursor cells (8). Three regions of the PML locus are
primarily involved in the t(15;17) translocation breakpoint cluster
regions (bcrs): intron 6 (bcr1), exon 6 (bcr2) and intron 3 (bcr3),
whereas RARA breakpoints always occur in intron 2. As a
consequence, there are three possible PML-RARA isoforms, referred
to the as long (bcr1), variant (bcr2) and short (bcr3) isoforms
(9).
Assessment of PML-RARA formation, or variant RARA
gene rearrangements by means of conventional karyotyping,
fluorescence in situ hybridization (FISH) or reverse
transcription-polymerase chain reaction (RT-PCR), is required for
the diagnosis of APL (10). In rare
cytogenetically normal cases, FISH or molecular methods demonstrate
the presence of the PML-RARA fusion gene without the reciprocal
RARA-PML, resulting from a submicroscopic insertion of RARA into
PML. Since this cryptic insertion has rarely been reported, no
prognostic significance has been clearly established (9,11–22); however, a prompt diagnosis and the
administration of targeted therapies, including all-trans
retinoic acid (ATRA) and arsenic trioxide (ATO), are essential to
improve the outcome in these patients (21). Due to the use of contemporary targeted
therapy, APL has become a highly curable disease with complete
remission rates of >95% and cure rates of >80% (23–27).
To the best of our knowledge, the current case is
the first reported with two PML-RARA FISH fusion signals present on
the two copies of chromosome 15, as result of a cryptic insertion
of RARA into PML and chromosome 15 uniparental isodisomy (iUPD),
likely due to loss of the normal chromosome 15 and duplication of
the rearranged one (28,29). Written informed consent was obtained
from the patient.
Case report
Patient presentation
A 73-year-old female Caucasian patient was admitted
to the Humanitas Clinical and Research Center (Milan, Italy) in
January 2016 with monocytosis, anemia and thrombocytopenia
incidentally diagnosed during a knee replacement surgery. The
patient's medical history revealed β-thalassemia minor trait,
obesity, hypertension, mild fasting hyperglycemia and toxic
multinodular goiter. In 1982, the patient had undergone a bilateral
hysteroannessiectomy to remove fibroids, and in 2010 the patient
had undergone a cholecystectomy due to cholelithiasis. During the
admission, the peripheral blood examination revealed a hemoglobin
count of 8.8 g/dl (normal range, 12–16 g/dl) and a platelet count
of 4.7×1010/l (normal range,
13.0–40.0×1010/l), as well as a white blood cell count
of 5.24×109/l (normal range, 4–10×109/l). A
peripheral blood cell smear revealed prominent leukocytosis with
blast cells accounting for 94% of all nucleated cells,
characterized by hypogranular bilobed nuclei
(French-American-British classification M3 variant) (30). Peripheral blood flow cytometric
analysis revealed positivity for cluster of differentiation (CD)
13, CD33, myeloperoxidase, CD2 and CD9, and a negative result for
human leukocyte antigen-antigen D related, CD117, CD15, CD4, CD19,
CD14, CD10, CD3 and CD34. The patient was clinically diagnosed with
APL. The karyotype, as determined from the peripheral blood, was
46,XX. As Q-LAMP revealed positivity for the PML-RARA transcript,
FISH was performed and two fusion signals on the two copies of
chromosome 15 were observed.
Treatment
According to the ATRA and idarubicin (AIDA)
protocol, the patient was treated with induction chemotherapy and
received 45 mg/m2 ATRA twice a day and 12
mg/m2/day idarubicin for 3 days (one cycle). The patient
developed differentiation syndrome symptoms and disseminated
intravascular coagulation with intracranial bleeding. On day 20
post-therapy, the patient was in clinical remission. Molecular
analysis using microsatellites and performed on a peripheral blood
sample supported the hypothesis of chromosome 15 iUPD.
On day 48 post-therapy, cytogenetic, FISH and RT-PCR
analyses were performed, with normal results. The patient received
consolidation therapy with 1,000 mg/m2/day cytarabine
for 5 days, 5 mg/m2/day idarubicin for 5 days and 45
mg/m2 ATRA twice a day for 15 days, and is presently
under maintenance therapy. During the clinical course no
substantial difference compared with classical APL patients was
observed.
Cytogenetic analysis
Cytogenetic analysis was performed on peripheral
blood samples incubated for 24 h according to standard procedures
(31). A total of 27 spontaneous
quinacrine-banded metaphases were analyzed and the karyotype
described according to the International System for Human
Cytogenetic Nomenclature 2013 criteria (32). Following induction therapy on day 48,
the karyotype was obtained from a bone marrow sample following a
24-h incubation period. A total of 25 spontaneous metaphases were
analyzed.
FISH analysis
FISH was performed according to the manufacturer's
protocol on metaphase and interphase nuclei using the commercially
available SureFISH PML-RARA dual-color dual-fusion DNA probe,
specific for the PML (15q24; spectrum red) and RARA (17q21;
spectrum green) loci (Agilent Technologies, Inc., Santa Clara, CA,
USA). A total of 15 metaphases and 100 nuclei were scored for the
peripheral blood and the bone marrow samples.
Molecular methods
The presence of the PML-RARA transcript was
evaluated using a commercial kit (DiaSorin, Saluggia, Italy) based
on the non-PCR quenching loop-mediated isothermal amplification
(Q-LAMP) method modified to introduce fluorescent oligonucleotides
and a new polymerase with RNA reverse transcription and DNA
amplification activity, as previously described by Spinelli et
al (33). To investigate the iUPD
hypothesis, the analysis of short tandem repeats (STR) present on
15 different alleles comprising the Penta E locus on chromosome 15
was performed on 0.5 ng of DNA at diagnosis and on day 20
post-therapy using a commercial kit (PowerPlex® 16
System; Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol.
Results
Cytogenetics and FISH results
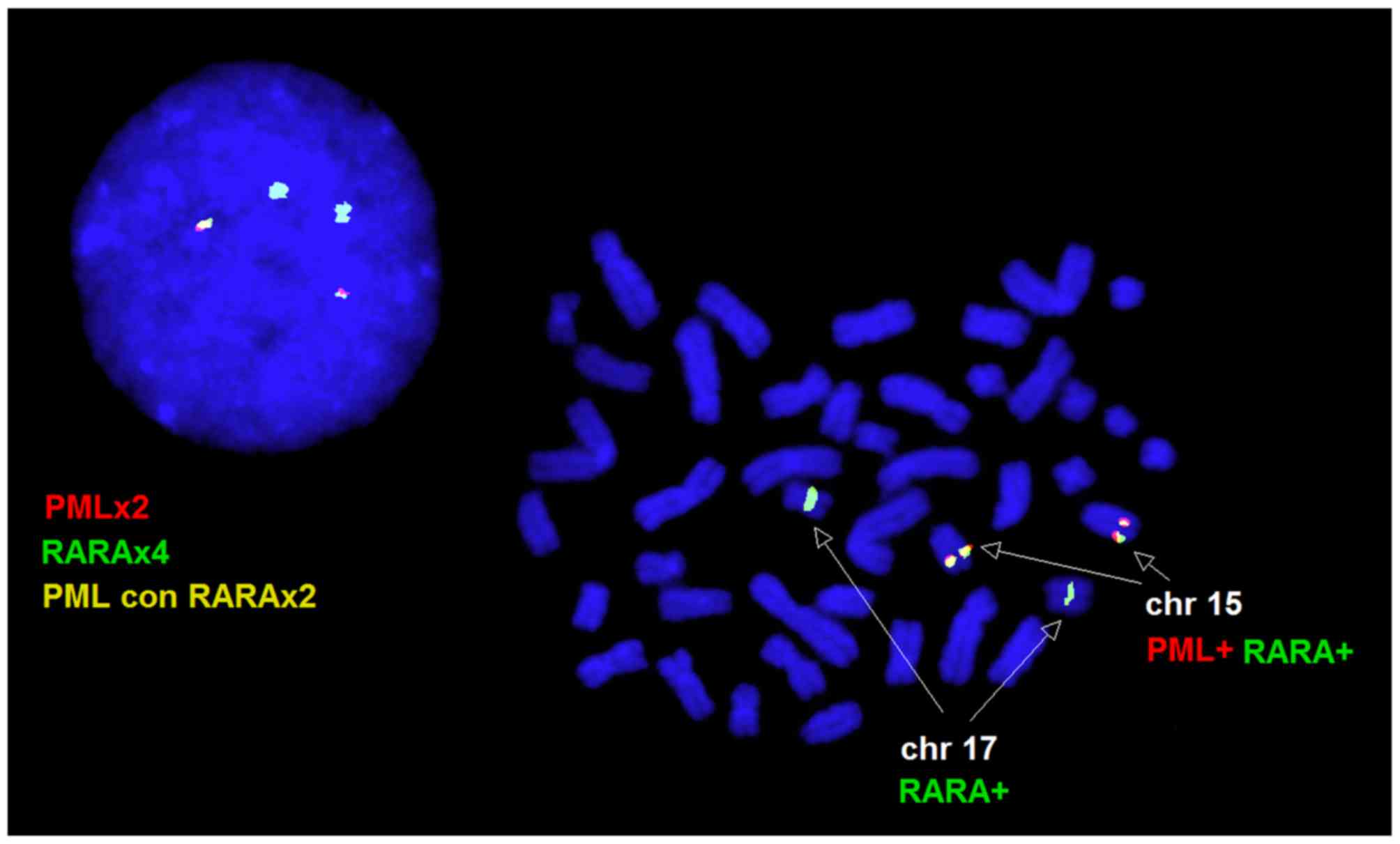
A 46,XX karyotype was observed in the peripheral
blood and bone marrow samples. In the peripheral blood sample,
interphase FISH revealed normal cells with two red PML and two
green RARA signals in 12% of the nuclei, and a variant fusion
pattern characterized by two green RARA and two yellow PML-RARA
fusion signals in 88% of nuclei. All the metaphase cells analyzed
by FISH exhibited two PML/RARA fusion signals, one on each copy of
chromosome 15, and two normal RARA signals on the two copies of
chromosome 17, consistent with the interphase FISH pattern
(Fig. 1). A normal FISH pattern was
observed on the bone marrow specimen following induction
therapy.
Molecular results
The Q-LAMP assay assessed the presence of the
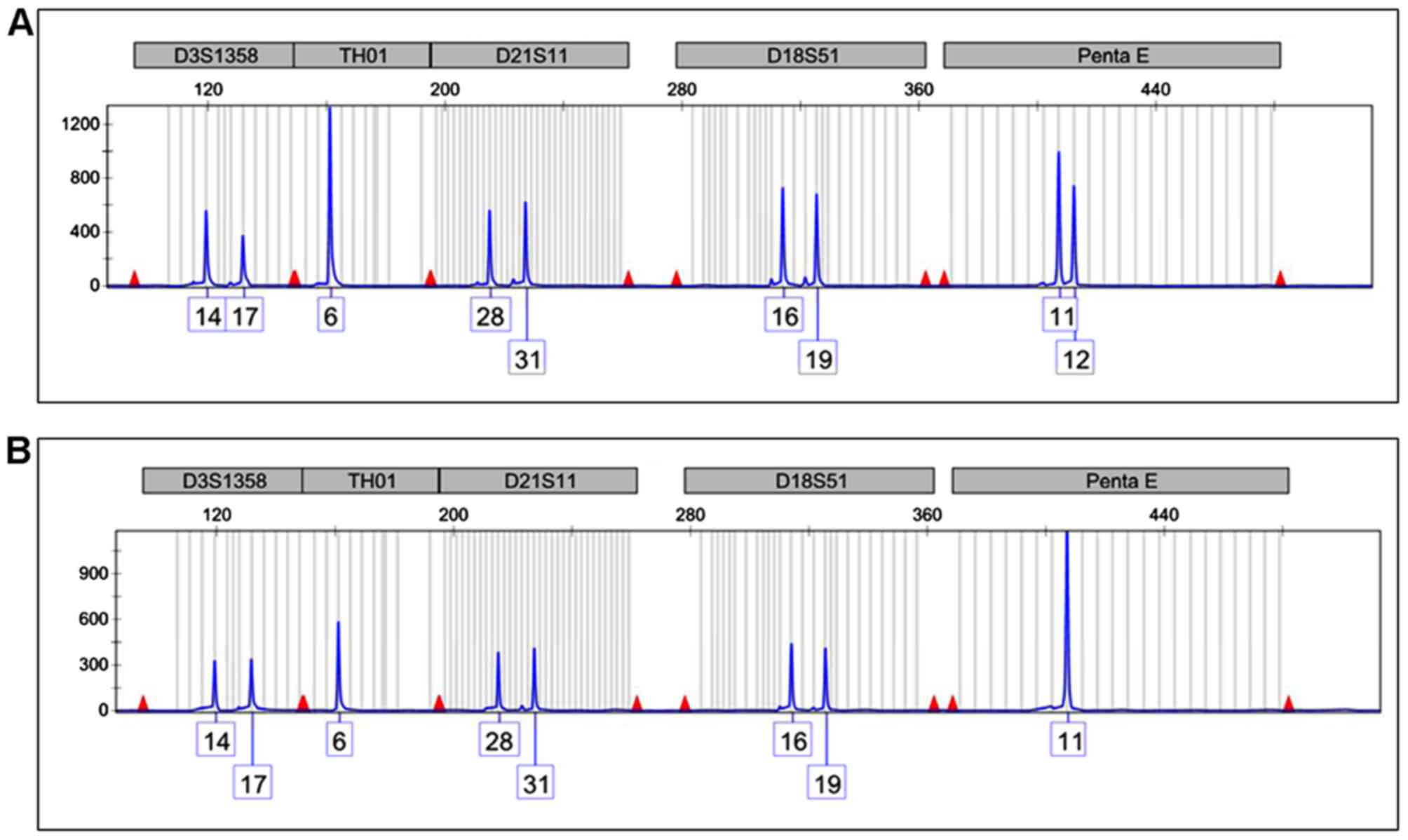
bcr3/short form PML-RARA fusion transcript. The STR analysis of the
Penta E locus on chromosome 15 revealed the presence of two peaks
of 11 and 12 STR repeats in the remission sample (Fig. 2A) demonstrating that two chromosomes
with varying STR numbers were present in the patient's normal
cells. Conversely, only the 11 repeat signal was present in the
diagnostic sample, representing the APL cells (Fig. 2B). This latter feature is consistent
with the presence of only one chromosome 15 or with the presence of
two identical copies of chromosome 15 (iUPD).
Discussion
Approximately 9% of APL patients do not harbor the
classic t(15;17) translocation; however, certain patients still
express the PML-RARA fusion gene. These cases are considered to
have a ‘cryptic’ transcript resulting from sub-microscopic
insertions of PML or RARA or more complex rearrangements, thus
escaping detection with conventional cytogenetic analysis. In these
rare cases, the cryptic transcript is usually detected by RT-PCR.
As ATRA and ATO are targeted therapies against the action of the
PML-RARA protein, patients who lack the classic translocation but
present the fusion product may also benefit from these therapies
(14,15,20,21). There
have been a number of previous studies describing patients with
morphological features of APL and a normal karyotype who are
FISH-negative for t(15;17), but RT-PCR-positive for PML-RARA. The
treatment with ATRA reveals similar good responses and favorable
prognoses compared with those observed in patients harboring the
classic t(15;17) variant (14,15,20,21).
The increasing number of reported cases with cryptic
rearrangements supports the requirement for varied integrated
diagnostic approaches in order to recognize the presence of fusion
products that respond to targeted therapy. In particular, the
current case demonstrated the importance of FISH evaluation not
only on interphase nuclei, but also on metaphase cells, which
allowed the identification of the underlying mechanism leading to
the rearrangement. Q-LAMP revealed the presence of the PML-RARA
transcript, but only FISH detected the presence of the double
insertion of RARA into each copy of the PML gene, supporting the
hypothesis of chromosome 15 iUPD, which was then confirmed using
microsatellite analysis.
Acquired UPD (aUPD), most frequently segmental, has
been reported in 15–20% of patients affected by acute myeloid
leukemia, resulting in gene dosage alterations and homozygosity for
mutated genes that can provide a proliferative advantage or
increased chemoresistance (34,35).
Chromosomes 11 and 13 have the highest number of aUPDs, but aUPDs
on Xq, 1p, 2p, 2q, 6p, 9p, 17p, 17q, 19q and 21q have also been
reported (34,35). To the best of our knowledge, no aUPD
has been reported for chromosome 15; however, it is well
established that chromosome 15 is imprinted and constitutional UPD
causes specific syndromes (28,29).
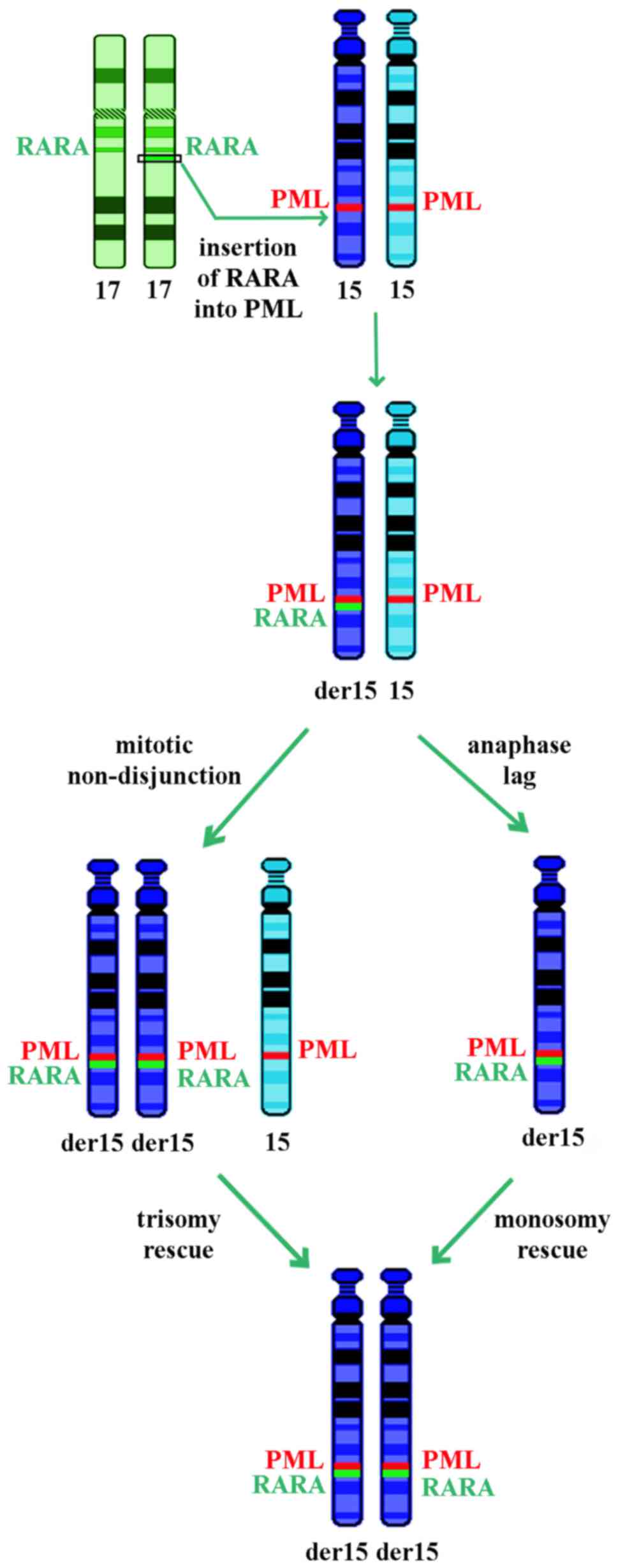
Numerical somatic UPDs may occur due to mitotic
errors, including non-disjunction or loss of a chromosome due to
anaphase lag followed by duplication of the remaining chromosome
(28,29). The chromosome 15 iUPD observed in the
current case may be the result of an insertion of RARA into PML,
loss of the normal chromosome 15 and monosomy rescue by duplication
of the rearranged one or trisomy consequent to non-disjunction
followed by loss of the homolog, as reported in constitutional
cases (Fig. 3) (28,29). These
two mechanisms may be possible in acute myeloid leukemia, and
chromosome 15 trisomy, despite being rare, is more frequently
reported (36).
The presence of iUPD in the present case apparently
did not affect the good outcome of therapy and the prognosis.
However, the consequent loss of heterozygosity for the entire
chromosome may have introduced homozygosity for coding
polymorphisms with variable functional activity, including those in
the drug metabolizing enzymes, non-coding regulatory polymorphisms
that result in differential expression of alleles and
haploinsufficiency or overexpression of important proteins
(37–39). A close follow-up is required for this
patient in order to monitor the possible prognostic negative effect
due to the iUPD.
References
|
1
|
Lo Coco F, Nervi C, Avvisati G and
Mandelli F: Acute promyelocytic leukemia: A curable disease.
Leukemia. 12:1866–1880. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kadam PR, Merchant AA and Advani SH:
Cytogenetic findings in patients with acute promyelocytic leukemia
and a case of cml blast crisis with promyelocytic proliferation.
Cancer Genet Cytogenet. 50:109–117. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bapna A, Nair R, Tapan KS, Nair CN, Kadam
P, Gladstone B and Advani SH: All-trans-retinoic acid (ATRA):
Pediatric acute promyelocytic leukemia. Pediatr Hematol Oncol.
15:243–248. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Advani SH, Nair R, Bapna A, Gladstone B,
Kadam P, Saikia TK, Parekh PM, Gopal R and Nair CN: Acute
promyelocytic leukemia: All-trans retinoic acid (ATRA) along with
chemotherapy is superior to ATRA alone. Am J Hematol. 60:87–93.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burnett AK, Grimwade D, Solomon E,
Wheatley K and Goldstone AH: Presenting white blood cell count and
kinetics of molecular remission predict prognosis in acute
promyelocytic leukemia treated with all-trans retinoic acid: Result
of the Randomized MRC Trial. Blood. 93:4131–4143. 1999.PubMed/NCBI
|
|
6
|
Amare PS, Baisane C, Saikia T, Nair R,
Gawade H and Advani S: Fluorescence in situ hybridization: A highly
efficient technique of molecular diagnosis and prediction for
disease course in patients with myeloid leukemias. Cancer Genet
Cytogenet. 131:125–134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brockman SR, Paternoster SF, Ketterling RP
and Dewald GW: New highly sensitive fluorescence in situ
hybridization method to detect PML/RARA fusion in acute
promyelocytic leukemia. Cancer Genet Cytogenet. 145:144–151. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mistry AR, Pedersen EW, Solomon E and
Grimwade D: The molecular pathogenesis of acute promyelocytic
leukaemia: Implications for the clinical management of the disease.
Blood Rev. 17:71–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park TS, Kim JS, Song J, Lee KA, Yoon S,
Suh B, Lee JH, Lee HJ, Kim JK and Choi JR: Acute promyelocytic
leukemia with insertion of PML exon 7a and partial deletion of exon
3 of RARA: A novel variant transcript related to aggressive course
and not detected with real-time polymerase chain reaction analysis.
Cancer Genet Cytogenet. 188:103–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. 4th Edition. IARC;
Lyon: 2008
|
|
11
|
Tirado CA, Jahn JA, Scheerle J, Eid M,
Meister RJ, Christie RJ, Croft CD, Wallingford S, Heritage DW,
Mowrey PN and Meloni-Ehrig AM: Variant acute promyelocytic leukemia
translocation (15;17) originating from two subsequent balanced
translocations involving the same chromosomes 15 and 17 while
preserving the PML/RARA fusion. Cancer Genet Cytogenet. 161:70–73.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang HY, Ding J, Vasef MA and Wilson KF: A
bcr3/short form PML-RARalpha transcript in an acute promyelocytic
leukemia resulted from a derivative chromosome 17 due to
submicroscopic insertion of the PML gene into the RARalpha locus.
Am J Clin Pathol. 131:64–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldschmidt N, Yehuda-Gafni O, Abeliovich
D, Slyusarevsky E and Rund D: Interstitial insertion of RARα gene
into PML gene in a patient with acute promyelocytic leukemia (APL)
lacking the classic t(15;17). Hematology. 15:332–337. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim MJ, Cho SY, Kim MH, Lee JJ, Kang SY,
Cho EH, Huh J, Yoon HJ, Park TS, Lee WI, et al: FISH-negative
cryptic PML-RARA rearrangement detected by long-distance polymerase
chain reaction and sequencing analyses: A case study and review of
the literature. Cancer Genet Cytogenet. 203:278–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewis C, Patel V, Abhyankar S, Zhang D,
Ketterling RP, McClure RF and Persons DL: Microgranular variant of
acute promyelocytic leukemia with normal conventional cytogenetics,
negative PML/RARA FISH and positive PML/RARA transcripts by RT-PCR.
Cancer Genet. 204:522–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Welch JS, Westervelt P, Ding L, Larson DE,
Klco JM, Kulkarni S, Wallis J, Chen K, Payton JE, Fulton RS, et al:
Use of whole-genome sequencing to diagnose a cryptic fusion
oncogene. JAMA. 305:1577–1584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amare PK, Baisane C, Nair R, Menon H,
Banavali S, Kabre S, Gujral S and Subramaniam P: Characterization
of cryptic rearrangements, deletion, complex variants of PML, RARA
in acute promyelocytic leukemia. Indian J Hum Genet. 17:54–58.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koshy J, Qian YW, Bhagwath G, Willis M,
Kelley TW and Papenhausen P: Microarray, gene sequencing, and
reverse transcriptase-polymerase chain reaction analyses of a
cryptic PML-RARA translocation. Cancer Genet. 205:537–540. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gruver AM, Rogers HJ, Cook JR, Ballif BC,
Schultz RA, Batanian JR, Fesler MJ and Tubbs RR: Modified
array-based comparative genomic hybridization detects cryptic and
variant PML-RARA rearrangements in acute promyelocytic leukemia
lacking classic translocations. Diagn Mol Pathol. 22:10–21. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blanco EM, Curry CV, Lu XY, Sarabia SF,
Redell MS, Lopez-Terrada DH and Roy A: Cytogenetically cryptic and
FISH-negative PML/RARA rearrangement in acute promyelocytic
leukemia detected only by PCR: An exceedingly rare phenomenon.
Cancer Genet. 207:48–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan H, Ortega V, Fanasch HM, Wang Y,
Holder KN, Higgins RA, Mendiola C, Mohamed G, Vadlamudi K and
Velagaleti G: PML-RARA fusion resulting from a cryptic insertion of
RARA gene into PML gene without the reciprocal RARA-PML fusion:
Clinical, cytogenetic and molecular characterization and prognosis.
Eur J Haematol. 93:354–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shepshelovich D, Oniashvili N, Parnes D,
Klein A, Muchtar E, Yeshaya J, Aviram A, Rabizadeh E and Raanani P:
Acute promyelocytic leukemia with isochromosome 17q and cryptic
PML-RARA successfully treated with all-trans retinoic acid and
arsenic trioxide. Cancer Genet. 208:575–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tallman MS, Andersen JW, Schiffer CA,
Appelbaum FR, Feusner JH, Woods WG, Ogden A, Weinstein H, Shepherd
L, Willman C, et al: All-trans retinoic acid in acute promyelocytic
leukemia: long-term outcome and prognostic factor analysis from the
North American Intergroup protocol. Blood. 100:4298–4302. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adès L, Guerci A, Raffoux E, Sanz M,
Chevallier P, Lapusan S, Recher C, Thomas X, Rayon C, Castaigne S,
et al: Very long-term outcome of acute promyelocytic leukemia after
treatment with all-trans retinoic acid and chemotherapy: The
European APL Group experience. Blood. 115:1690–1696. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lo-Coco F, Avvisati G, Vignetti M, Breccia
M, Gallo E, Rambaldi A, Paoloni F, Fioritoni G, Ferrara F, Specchia
G, et al: Front-line treatment of acute promyelocytic leukemia with
AIDA induction followed by risk-adapted consolidation for adults
younger than 61 years: Results of the AIDA-2000 trial of the GIMEMA
Group. Blood. 116:3171–3179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iland HJ, Bradstock K, Supple SG, Catalano
A, Collins M, Hertzberg M, Browett P, Grigg A, Firkin F, Hugman A,
et al: All-trans-retinoic acid, idarubicin, and IV arsenic trioxide
as initial therapy in acute promyelocytic leukemia (APML4). Blood.
120:1570–1580, quiz 1752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burnett AK, Russel NH, Hills RK, Bowen D,
Kell J, Knapper S, Morgan YG, Lok J, Grech A, Jones G, et al:
Arsenic trioxide and all-trans retinoic acid treatment for acute
promyelocytic leukaemia in all risk groups (AML17): Results of a
randomised, controlled, phase 3 trial. Lancet Oncol. 16:1295–1305.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ledbetter DH and Engel E: Uniparental
disomy in humans: Development of an imprinting map and its
implications for prenatal diagnosis. Hum Mol Genet 4 Spec.
1757–1764. 1995. View Article : Google Scholar
|
|
29
|
Liher T: Cytogenetic contribution to
uniparental disomy (UPD). Mol Cytogenet. 3:82010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–481. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barch MJ, Knutsen T and Spurbeck JL: The
AGT cytogenetics laboratory manual. 3rd edition. Lippincott-Raven
Publishers; Philadelphia: 1997
|
|
32
|
Shaffer LG, McGowan-Jordan J and Schmid:
ISCN 2013 An International System for Human Cytogenetic
Nomenclature. Karger Medical and Scientific Publishers; Basel:
2013
|
|
33
|
Spinelli O, Rambaldi A, Rigo F, Zanghì P,
D'Agostini E, Amicarelli G, Colotta F, Divona M, Ciardi C, Coco FL,
et al: Simple, rapid and accurate molecular diagnosis of acute
promyelocytic leukemia by loop mediated amplification technology.
Oncoscience. 2:50–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raghavan M, Smith LL, Lillington DM,
Chaplin T, Kakkas I, Molloy G, Chelala C, Cazier JB, Cavenagh JD,
Fitzgibbon J, et al: Segmental uniparental disomy is a commonly
acquired genetic event in relapsed acute myeloid leukemia. Blood.
112:814–821. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta M, Raghavan M, Gale R, Chelala C,
Allen C, Molloy G, Chaplin T, Linch DC, Cazier JB and Young BD:
Novel regions of acquired uniparental disomy discovered in acute
myeloid leukemia. Genes Chromosomes Cancer. 47:729–739. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mitelman Database of Chromosome
Aberrations and Gene Fusions in Cancer. 2016 Mitelman F, Johansson
B and Mertens F: http://cgap.nci.nih.gov/Chromosomes/MitelmanOctober
6–2016.
|
|
37
|
Stebbing J, Bower M, Syed N, Smith P, Yu V
and Crook T: Epigenetics: An emerging technology in the diagnosis
and treatment of cancer. Pharmacogenomics. 7:747–757. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schwahn B and Rozen R: Polymorphisms in
the methylenetetrahydrofolate reductase gene: Clinical
consequences. Am J Pharmacogenomics. 1:189–201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Milani L, Gupta M, Andersen M, Dhar S,
Fryknäs M, Isaksson A, Larsson R and Syvänen AC: Allelic imbalance
in gene expression as a guide to cis-acting regulatory single
nucleotide polymorphisms in cancer cells. Nucleic Acids Res.
35:e342007. View Article : Google Scholar : PubMed/NCBI
|