Introduction
Combined renal cell carcinoma (RCC) and urothelial
carcinoma (UC) of the renal pelvis is a rare type of multiple
primary malignant tumor, which is characterized by the coexistence
of two histologically distinct malignant tumors in the same organ
with a shorter median time to relapse and mortality compared with a
solitary tumor (1). There are only a
few such cases in the world (2,3). Research
into prognostic markers of multiple primary malignant tumors is
important to establish adequate therapeutic strategies. Cancer
stem-like cells (CSCs) are a small population of cancer cells that
have the properties of tumor-initiating ability, self-renewal and
differentiation (4). CSCs are more
resistant to chemotherapy and radiotherapy than non-CSC populations
via various mechanisms (5). Several
studies have indicated that detection of CSC markers such as
cluster of differentiation 44 (CD44) and aldehyde dehydrogenase 1
A1 (ALDH1A1) in urologic neoplasms can provide useful prognostic
information (6–8). Furthermore, CD44 and ALDH1A1 have
demonstrated high levels of activity in several types of solid
cancer (9).
The present study reports a case of synchronous RCC
and UC of the left kidney with poor prognosis. Abnormal expression
of CD44 and ALDH1A1 CSC markers investigated prior to and
subsequent to chemotherapy may indicate poor prognosis.
Materials and methods
Patient
A 76-year-old female with a 5-month history of left
flank pain presented to the Department of Urology, Nanjing Drum
Tower Hospital, The Affiliated Hospital of Nanjing University
Medical School (Nanjing, China) without a history of fever,
fatigue, weight loss or gross hematuria in March 2014. Her medical
history included an 8-year history of hypertension, and there was
no family history of urologic malignancies. Physical examination
revealed that her vital signs were stable, and no palpable
abdominal mass could be detected. Results from routine
examinations, including electrocardiogram, chest radiography,
pulmonary function test and laboratory tests of blood and urine,
were all within the normal limits, with the exception of 8.4 red
blood cells (RBC)/µl in urine (normal range, 0–5 RBC/µl).
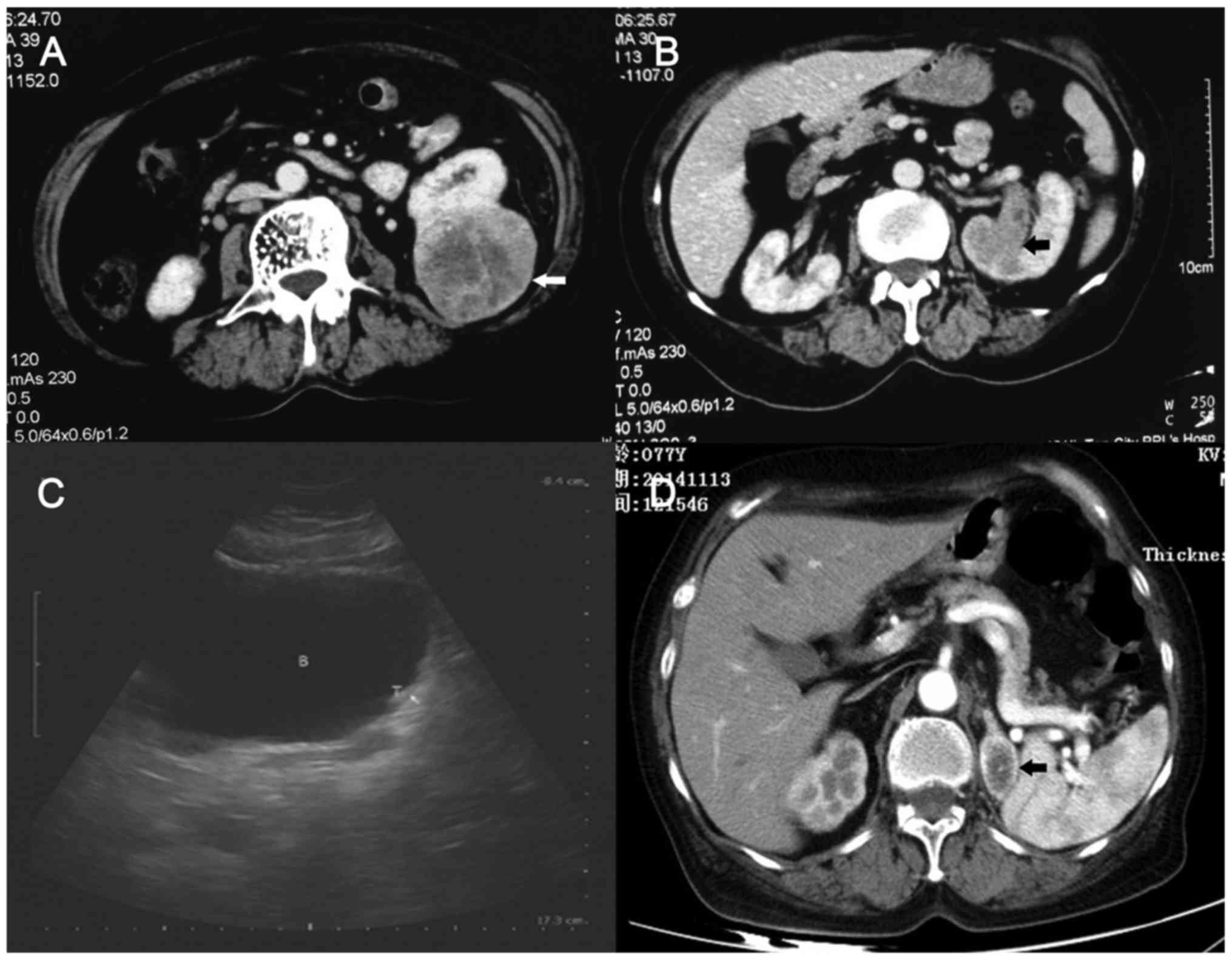
Ultrasonography suggested a left renal mass. A computed tomography
(CT) scan revealed a 7.5-cm solid mass on the posterior aspect of
the lower pole of the left kidney (Fig.
1A). In addition, a solid mass protruding into the upper
collecting system was suspicious for RCC with invasion into the
collecting system or for UC of the renal pelvis (Fig. 1B). The contralateral kidney was
normal. The glomerular filtration rates of the left and right
kidneys were 21.4 and 38.2 ml/min, respectively (normal range,
>36.5 ml/min).
The patient underwent transperitoneal laparoscopic
left radical nephrectomy. Upon dissecting the kidney, it was
obvious that there were two morphologically distinct masses in the
kidney. Intraoperative frozen section of the suspicious mass
confirmed a UC of the renal pelvis; thus, transperitoneal
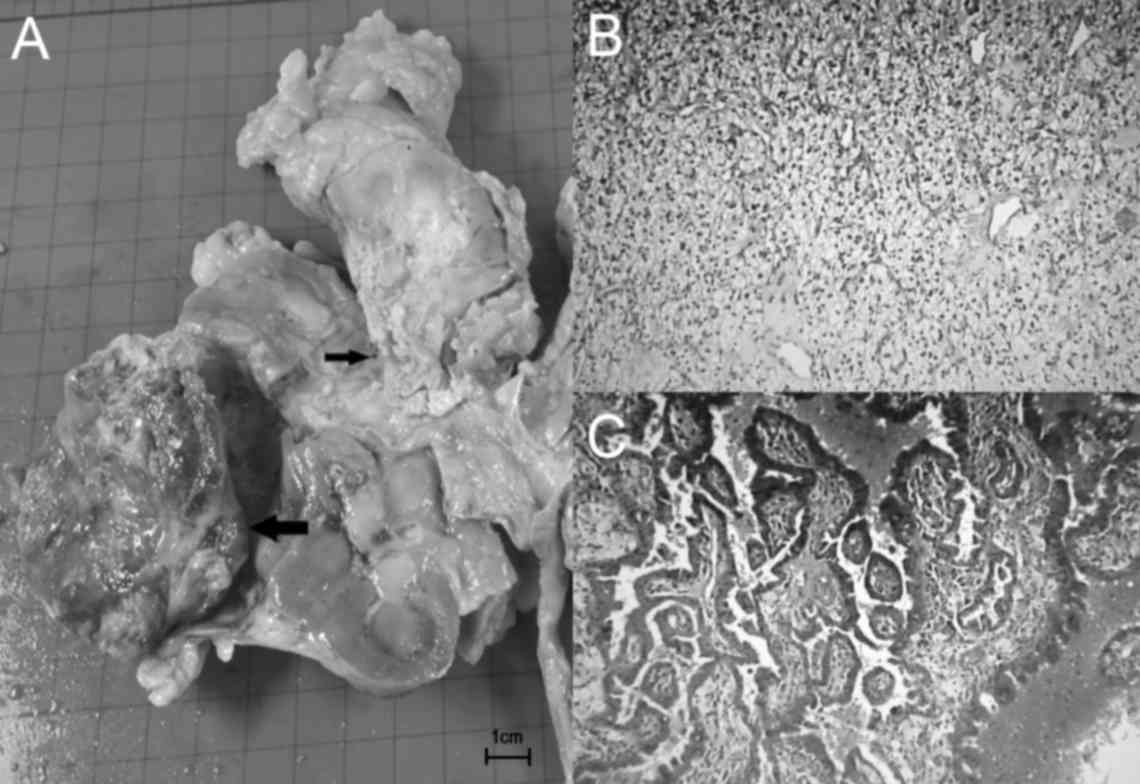
laparoscopic left ureterectomy was performed. The cut surface of
the gross specimen displayed two masses: A 7.5×5.0×4.5-cm
yellowish, sharply marginated solid tumor in the lower pole of the
kidney; and a 4.0×3.0×2.5-cm mass in the superior aspect of the
renal pelvis (Fig. 2A). Tissue blocks
were embedded in paraffin, sectioned and stained with hematoxylin
and eosin. Histologically, the larger tumor exhibited the
characteristics of stage T2a clear cell carcinoma, with Fuhrman's
nuclear grade 3 of 4 and without invasion of the renal capsule or
pelvis (Fig. 2B). The following key
was used to assess immunohistochemical staining: -, negative; ±,
weak positive; +, moderate positive; and ++, strong positive
Immunohistochemical staining (2 h at room temperature) of RCC
demonstrated 30% Ki-67+ (1:400; cat. no., 19972-1-AP;
ProteinTech Group, Inc., Chicago, IL, USA), P53±
(1:1,000; cat. no., 10442-1-AP; ProteinTech Group, Inc.),
cyclooxygenase 2++ (1:200; cat. no., 38024; Signalway
Antibody LLC, College Park, MD, USA), vascular endothelial growth
factor± (1;200; cat. no., 19003-1-AP; ProteinTech Group,
Inc.), epidermal growth factor receptor− (1:100; cat.
no., 42520; Signalway Antibody LLC) and O6-methylguanine DNA
methyltransferase+ (1:100; cat. no., 17195-1-AP;
ProteinTech Group, Inc.). The second tumor was a stage T3,
high-grade papillary UC with invasion of the renal parenchyma
(Fig. 2C). The surgical margins were
negative, and no metastasis of lymph nodes was detected. The
patient received gemcitabine (1000 mg/m2; day 1 and day
8) and cisplatin (70 mg/m2; day 2) chemotherapy
following surgery, every 3 weeks. Bladder recurrence of UC occurred
at the follow-up of 5 months (Fig.
1C), and left adrenal metastasis of RCC occurred at the
follow-up of 15 months (Fig. 1D). The
patient accordingly underwent transurethral resection of a bladder
tumor and laparoscopic left adrenalectomy.
At present, the patient remains under follow-up. The
patient gave informed consent for their data to be published as
part of the present study.
Immunoblotting
Tumor samples were lysed in radioimmunoprecipitation
assay buffer containing cOmplete™, Mini Protease Inhibitor Cocktail
(Roche Diagnostics, Basel, Switzerland) following surgery. The
proteins in the lysates (20 µg) were separated by 30% SDS-PAGE and
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA). Upon blocking with 5% non-fat milk in PBS
containing Tween 20 (PBST), primary antibodies against CD44 (Cat#
3570S; Cell Signaling Technology, Inc., Danvers, MA, USA), ALDH1A1
(Cat# 22109-1-AP; ProteinTech Group, Inc., Chicago, IL, USA) and
β-actin (Cat# 05-0079; AbMax, Beijing, China) were used. Dilutions
for all antibodies were 1:1,000, and membranes were incubated for
16 h at 4°C. The membranes were then washed with PBST three times
(5 min, room temperature) and incubated (1 h at room temperature)
with a horseradish peroxidase-conjugated secondary antibody
(1;1,000; cat. no., L3052-2; Signalway Antibody LLC). The western
blots were visualized using enhanced chemiluminescence reagents
(Cat# WBKLS0100; EMD Millipore).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNAs were extracted using TRIzol®
(Cat# 15596018; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol. RT was
conducted using random primers provided in Takara system
(PrimeScript RT Reagent kit with gDNA Eraser; Takara Biotechnology
Co., Ltd., Dalian, China). The expression of relative genes was
measured by RT-qPCR using SYBR Green (Takara Biotechnology Co.,
Ltd.) in an ABI 7500 StepOnePlus Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primers used were
as follows: CD44 forward, 5′-ATCGCTCTCCTGCTAACAGTC-3′ and reverse,
5′-CTCGTACTGGATGGGTGAACT-3′; ALDH1A1 forward,
5′-CACCACGTACAAGGGTCAGGTGC-3′ and reverse,
5′-CAGCCTCCCACGCTGGGGTAT-3′; and β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGA-3′. The thermocycling conditions were as
follows: Pre-denaturation at 95°C for 10 sec, followed by
denaturation at 95°C for 5 sec, and annealing and extension at 60°C
for 31 sec. The expression of target genes was calculated based on
the quantification cycle (Cq) values compared with a reference gene
β-actin, using the formula 2−ΔΔCq (?). RT-qPCR was
performed in triplicate for each sample in a 10-µl reaction
mixture, which consisted of template complementary DNA (0.2 µl),
primers (0.4 µl, l.0 M), ROX Reference Dye II (0.2 µl;
SYBR® Premix Ex Taq kit; Takara Biotechnology Co.,
Ltd.), distilled H2O (4.2 µl) and SYBR Premix Ex Taq (5
µl; SYBR® Premix Ex Taq kit; Takara Biotechnology Co.,
Ltd.). All reactions were performed in triplicate.
Results
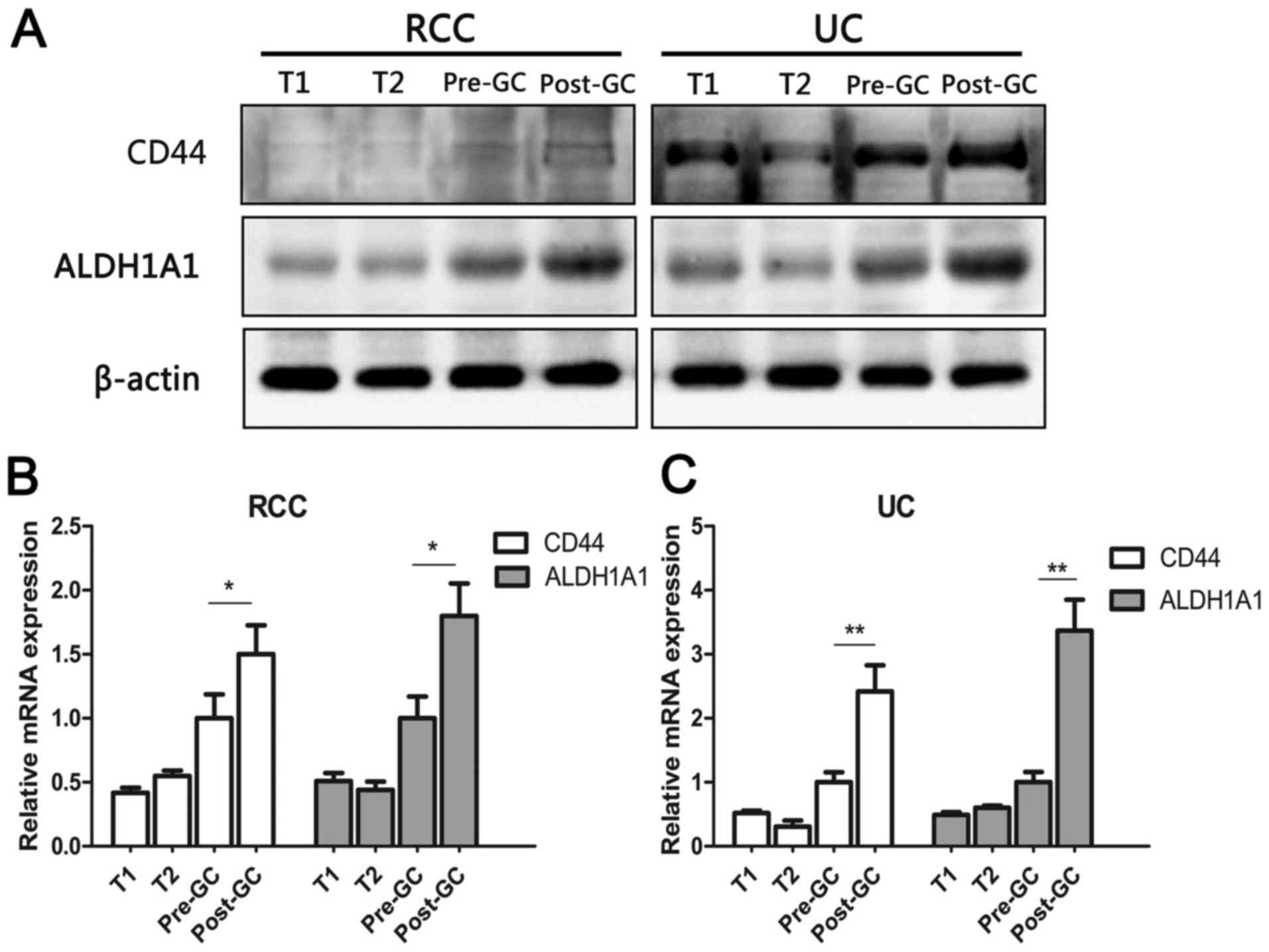
Western blotting revealed that the protein levels of
CD44 and ALDH1A1 were higher when RCC and UC of the renal pelvis
occurred simultaneously. In addition, the expression level of
cancer stem cell markers in metastatic lesions was higher than that
in primary lesions following chemotherapy in the present case
(Fig. 3A), although RCC was not
sensitive to chemotherapy according to the guidelines (10).
RT-qPCR analysis revealed that gene expression of
CD44 and ALDH1A1 in UC of the present case increased more
significantly than that of RCC following chemotherapy (Fig. 3B and C).
Discussion
Multiple primary malignant neoplasms are
characterized by the coexistence of two adjacent but histologically
distinct malignant tumors (1). The
incidence of this kind of tumor in the kidney is lower than that in
other organs (11). RCC is the most
common lesion of the kidney, and accounts for ~70% of renal
malignancies (12). Primary UC of the
renal pelvis is a relatively rare disease, which accounts for 5–7%
of urinary tract tumors (13). The
combination of these two types of tumor has rarely been reported
previously in the literature. The earliest case was reported by
Graves and Templeton (12) in 1921,
and the most recent one was reported by Atilgan et al
(2) in 2013. According to Pubmed
search results, ~40 cases of synchronous ipsilateral RCC and renal
pelvic UC have been reported in the literature to date (2,3,14,15). The
average age of the reviewed patients was 65±11 years; the
male/female ratio was 1.6; and the left-to-right-side ratio was
1.9. In total, 73% of the cases presented with hematuria, 37% with
flank pain and 10% without obvious symptoms, and no identifiable
past medical history could be observed (2,3,14–16).
Accurate preoperative diagnosis of RCC with
synchronous ipsilateral UC of the renal pelvis is important to
guide the selection of a surgical operation method. The rareness of
such disease causes a high misdiagnosis rate (3). Preoperative examination should be
comprehensive to obtain as much information as possible and to
ensure the identification of suspicious masses. Intraoperative
frozen section of the suspicious solid mass may confirm the
diagnosis during operation, so that ureterectomy can be performed
(3).
The standard surgical procedure of RCC is radical
nephrectomy or partial nephrectomy, according to the
characteristics of the tumor (17).
For UC of the renal pelvis, the recurrence rate is 30–70%, and
nephroureterectomy represents the main line of treatment (18). The 5-year survival rate of high-grade
pT3 UC of the upper tract, such as the one described in the present
case, is only 25% (19). In summary,
radical nephroureterectomy should be performed in cases with
synchronous ipsilateral RCC and UC, and transperitoneal
laparoscopic nephroureterectomy is a less invasive method for
suspicious UC of the renal pelvis.
Multiple primary malignancies tend to exhibit poor
prognosis. In total, 24% of such cases had tumor metastases at
initial examination, and 34% of the patients had bladder neoplasms
(15). In the present report, routine
follow-up demonstrated recurrence of RCC and UC, despite the fact
that the patient received chemotherapy and the lesion was resected
completely according to the pathological results. To the best of
our knowledge, the current study discusses the first reported
patient who has suffered recurrence of both RCC and UC during
follow-up.
CSCs are considered to possess resistance to
chemotherapy, and there is a direct link between the expression of
CSC markers and patient survival (5,20). The
abnormal detection of CSC markers in primary or recurrent lesions
prior and subsequent to chemotherapy may partly explain the high
rate of metastatic recurrences and short survival, which is clearly
reflected in this case. However, the roles of CD44 and ALDH1A1 in
UC require further investigation.
In conclusion, adjuvant therapy should be
administered according to the staging and pathological grading of
RCC with synchronous ipsilateral UC of the renal pelvis, and new
treatments against the cancer stem cells fraction should be used in
combination with chemotherapy to improve the outcome of such
patients.
Glossary
Abbreviations
Abbreviations:
|
RCC
|
renal cell carcinoma
|
|
UC
|
urothelial carcinoma
|
|
CSCs
|
cancer stem-like cells
|
|
ALDH1A1
|
aldehyde dehydrogenase 1 A1
|
|
CD44
|
cluster of differentiation 44
|
|
CT
|
computed tomography
|
References
|
1
|
Rabbani F, Grimaldi G and Russo P:
Multiple primary malignancies in renal cell carcinoma. J Urol.
160:1255–1259. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atilgan D, Uluocak N and Parlaktas BS:
Renal cell carcinoma of the kidney with synchronous ipsilateral
transitional cell carcinoma of the renal pelvis. Case Rep Urol.
2013:1941272013.PubMed/NCBI
|
|
3
|
Hirohashi Y, Torigoe T, Inoda S, Takahashi
A, Morita R, Nishizawa S, Tamura Y, Suzuki H, Toyota M and Sato N:
Immune response against tumor antigens expressed on human cancer
stem-like cells/tumor-initiating cells. Immunotherapy. 2:201–211.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vermeulen L, de Sousa e Melo F, Richel DJ
and Medema JP: The developing cancer stem-cell model: clinical
challenges and opportunities. Lancet Oncol. 13:e83–e89. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keymoosi H, Gheytanchi E, Asgari M,
Shariftabrizi A and Madjd Z: ALDH1 in combination with CD44 as
putative cancer stem cell markers are correlated with poor
prognosis in urothelial carcinoma of the urinary bladder. Asian Pac
J Cancer Prev. 15:2013–2020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mikami S, Mizuno R, Kosaka T, Saya H, Oya
M and Okada Y: Expression of TNF-α and CD44 is implicated in poor
prognosis, cancer cell invasion, metastasis and resistance to the
sunitinib treatment in clear cell renal cell carcinomas. Int J
Cancer. 136:1504–1514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueda K, Ogasawara S, Akiba J, Nakayama M,
Todoroki K, Ueda K, Sanada S, Suekane S, Noguchi M, Matsuoka K and
Yano H: Aldehyde dehydrogenase 1 identifies cells with cancer stem
cell-like properties in a human renal cell carcinoma cell line.
PLoS One. 8:e754632013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
American Cancer Society, . Cancer Facts
& Figures 2013. American Cancer Society, Inc.; Atlanta, GA:
2013
|
|
9
|
Chan KS, Volkmer JP and Weissman I: Cancer
stem cells in bladder cancer: A revisited and evolving concept.
Curr Opin Urol. 20:393–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kirkali Z and Tuzel E: Transitional cell
carcinoma of the ureter and renal pelvis. Crit Rev Oncol Hematol.
47:155–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Graves RC and Templeton ER: Combined
tumors of the kidney. J Urol. 5:517–537. 1921.
|
|
13
|
Leveridge M, Isotalo PA, Boag AH and
Kawakami J: Synchronous ipsilateral renal cell carcinoma and
urothelial carcinoma of the renal pelvis. Can Urol Assoc J.
3:64–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Z, Min J, Yu D, Shi H and Xie D:
Renal collision tumour of papillary cell carcinoma and chromophobe
cell carcinoma with sarcomatoid transformation: A case report and
review of the literature. Can Urol Assoc J. 8:E536–E539. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Demir A, Onol FF, Bozkurt S and Türkeri L:
Synchronous ipsilateral conventional renal cell and transitional
cell carcinoma. Int Urol Nephrol. 36:499–502. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han P, Wei Q, Shi M and Yang YR:
Ipsilateral synchronous renal pelvic transitional cell carcinoma,
squamous cell carcinoma and adenocarcinoma. Chin Med J (Engl).
117:1590–1591. 2004.PubMed/NCBI
|
|
17
|
Antonelli A, Cozzoli A, Nicolai M, Zani D,
Zanotelli T, Perucchini L, Cunico SC and Simeone C: Nephron-sparing
surgery versus radical nephrectomy in the treatment of
intracapsular renal cell carcinoma up to 7cm. Eur Urol. 53:803–809.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krabbe LM, Bagrodia A, Westerman ME and
Margulis V: Diagnosis and management of upper tract urothelial
carcinoma. Minerva Urol Nefrol. 66:37–48. 2014.PubMed/NCBI
|
|
19
|
Abouassaly R, Alibhai SM, Shah N,
Timilshina N, Fleshner N and Finelli A: Troubling outcomes from
population-level analysis of surgery for upper tract urothelial
carcinoma. Urology. 76:895–901. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Creighton CJ, Li X, Landis M, Dixon JM,
Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A,
Herschkowitz JI, et al: Residual breast cancers after conventional
therapy display mesenchymal as well as tumor-initiating features.
Proc Natl Acad Sci USA. 106:13820–13825. 2009. View Article : Google Scholar : PubMed/NCBI
|