Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide (1). Advances in the treatment of non-small
cell lung cancer (NCSLC) in the past decade include
third-generation platinum doublets, epidermal growth factor
receptor (EGFR) tyrosine kinase inhibitors (TKIs) in EGFR
mutation-positive lung cancer, anaplastic lymphoma kinase (ALK)
TKIs in ALK rearrangement-positive disease, maintenance systemic
therapy and second- or third-line treatment, all of which have
improved survival (2–9). Third-generation chemotherapeutic agents,
including paclitaxel, docetaxel, gemcitabine, pemetrexed and
vinorelbine, in combination with platinum compounds, are the most
frequently used treatments for recurrent and advanced NSCLC
(10). Among these agents, paclitaxel
is particularly problematic with regards to toxicity and solubility
(11). Paclitaxel is a hydrophobic
compound, and the polyoxyethylated castor oil Kolliphor®
EL (Merck KGaA, Darmstadt, Germany) and ethanol are used as
vehicles for its parenteral administration; however, this route of
administration may lead to an acute hypersensitivity reaction,
requiring premedication with antihistamines or corticosteroids
(12). Nanoparticle albumin-bound
(nab)-paclitaxel is a solvent-free albumin-bound form of
paclitaxel. This composition provides a novel approach for
increasing the intratumoral concentration of the therapeutic agent
via a receptor-mediated transport process, allowing transcytosis
across the endothelial cell wall. Albumin transcytosis is then able
to deliver the associated paclitaxel to the tumor interstitium
(13,14). Nab-paclitaxel is also able to enter
the tumor interstitium through leaky junctions in the vascular
endothelium (15). A number of
previous studies have reported the efficacy of nab-paclitaxel as a
first-line chemotherapeutic agent for NSCLC (16–18);
however, to date, the efficacy of nab-paclitaxel with carboplatin
as a second-phase or later chemotherapy for NSCLC has yet to be
established (19,20). In the present study, a retrospective
evaluation of the efficacy and feasibility of nab-paclitaxel plus
carboplatin as a second-phase or later chemotherapy in patients
with recurrent and advanced NSCLC was performed.
Materials and methods
Study population
The present retrospective study included 25 eligible
patients treated at the Division of Chest Surgery of Fukushima
Medical University (Fukushima, Japan) and Fukushima Lung Cancer
Association Group of Surgeons participating institutions, including
the Department of Thoracic Surgery, Shirakawa Kosei General
Hospital (Shirakawa, Japan), the Department of Thoracic Surgery,
Takeda General Hospital (Aizuwakamatsu, Japan), the Department of
Surgery, Fukushima Rosai Hospital (Iwaki, Japan), the Department of
Thoracic Surgery, Southern Tohoku General Hospital (Koriyama,
Japan) and the Department of Thoracic Surgery, Fukushima Red Cross
Hospital (Fukushima, Japan), between July 2013 and January 2015.
These patients were histologically or cytologically confirmed as
having NSCLC prior to receiving chemotherapy. The inclusion
criteria were as follows: Patients with recurrent NSCLC following
radical surgery and patients with unresectable stage IIIB/IV NSCLC.
The staging was performed according to the 7th Edition Lung Cancer
Tumor Node Metastasis Classification and Staging System (21). Histological evaluation was performed
according to World Health Organization classification of tumors of
the lung, pleura, thymus and heart (22). The present study included 19 male and
6 female patients aged 54–78 years, with a mean age of 66.0±6.5
years. In all patients, the presence of measurable lesions was
confirmed using computed tomography (CT) scans, and the Eastern
Cooperative Oncology Group scores (23) were 0–1. Patient clinicopathological
features are summarized in Table I.
The protocol was conducted in accordance with the Declaration of
Helsinki and Good Clinical Practice guidelines. Written consent was
obtained from each patient or their family members at the time of
enrollment in the study.
 | Table I.Patients clinicopathological
characteristics. |
Table I.
Patients clinicopathological
characteristics.
| Clinical
factor | Number of patients
(%) |
|---|
| Gender |
|
|
Male | 19 (76) |
|
Female | 6 (24) |
| Age, years |
|
| Median
± SD | 66.0±6.5 |
|
<70 | 18 (72) |
|
≥70 | 7 (28) |
| Indications for
chemotherapy |
|
|
Recurrence following
surgery | 9 (36) |
|
Advanced cancer | 16 (64) |
| Recurrent or
metastatic sites |
|
|
Mediastinal lymph node | 8 (32) |
| Pleural
dissemination | 6 (24) |
| Adrenal
gland | 5 (20) |
|
Brain | 4 (16) |
|
Bone | 3 (12) |
|
Liver | 2 (8) |
|
Other | 2 (8) |
| Histology |
|
|
Squamous cell carcinoma | 11 (44) |
|
Adenocarcinoma | 13 (52) |
| Large
cell carcinoma | 1 (4) |
| EGFR mutation |
|
|
Positive | 5 (20) |
| Wild
type | 16 (64) |
|
Unknown | 4 (16) |
| ALK
rearrangement |
|
|
Positive | 0 (0) |
|
Negative | 6 (24) |
|
Unknown | 19 (76) |
| Phase of
nab-paclitaxel chemotherapy |
|
| 2nd
line | 13 (52) |
| 3rd
line | 6 (24) |
| 4th
line | 3 (12) |
| 5th
line | 2 (8) |
| 6th
line or later | 1 (4) |
Detection of EGFR mutations and ALK
translocation
The cobas EGFR assay (Roche Diagnostics GmbH,
Mannheim, Germany) is an allele-specific quantitative polymerase
chain reaction system that measures the amplification of DNA to
identify 41 mutations in exon 18–21 of the EGFR gene from 50 ng of
DNA derived from human formalin-fixed paraffin-embedded NSCLC
tissues. Within each reaction mixture, exon 28 was amplified as an
internal control. DNA samples obtained from specimens were
amplified using the following site-specific primers: Exon 18
forward, 5′-TGGAGCCTCTTACACCCAGT-3′ and reverse,
5′-ACAGCTTGCAAGGACTCTGG-3′; exon 19 forward,
5′-TCTGGATCCCAGAAGGTGAG-3′ and reverse, 5′-CAGCTGCCAGACATGAGAAA-3′;
exon 20 forward, 5′-CATTCATGCGTCTTCACCTG-3′ and reverse,
5′-GTCTTTGTGTTCCCGGACAT-3′; and exon 21 forward,
5′-GATCTGTCCCTCACAGCAGGGTC-3′ and reverse,
5′-GGCTTGACCTAAAGCCACCTCC-3′. All results were automatically
performed by cobas 4800 software. The analysis of the tumors for
ALK gene rearrangement was performed by immunohistochemistry on a
Histofine ALK intercalated antibody-enhanced polymer (iAEP) kit
(Nichirei Biosience Inc., Tokyo, Japan) using the ALK antibody
clone 5A4 (Nichirei Bioscience Inc.) with the iAEP detection kit
(Nichirei Bioscience Inc.). These processes were performed as
previously described (24).
Nab-paclitaxel treatment plan
A total of 25 patients, who had received previous
chemotherapy, were treated with nab-paclitaxel
(Abraxane®; Taiho Pharmaceutical Co., Ltd., Tokyo,
Japan) at a dose of 70–100 mg/m2 administered
intravenously on days 1, 8 and 15, with a carboplatin area under
the concentration-time curve (AUC) of 4–6 on day 1, every 28 days.
Treatment was repeated every 4–6 weeks until disease progression or
unacceptable toxicity occurred. If patients experienced
hematological toxicities such as grade 3 or 4 neutropenia or
thrombocytopenia during treatment, subcutaneous injection of
granulocyte colony-stimulating factor was recommended.
Assessment of response rate and
adverse events
The response to nab-paclitaxel plus carboplatin was
evaluated using the Response Evaluation Criteria in Solid Tumors
1.1 (25). Target lesions were
assessed using CT scans, and the observation indicators included
complete response (CR), partial response (PR), stable disease (SD)
and progressive disease (PD). Furthermore, the overall response
rate (ORR) and disease control rate (DCR) were assessed. Patients
who completed >2 cycles of nab-paclitaxel treatment were
evaluated for toxicity. Treatment-associated toxicities were scored
according to the National Cancer Institute's Common Terminology
Criteria for Adverse Events version 4.0 (26).
Statistical analysis
The Fisher's exact test was used for the analysis of
categorical variables and an unpaired Student's t-test was used for
the analysis of continuous variables. Survival probabilities were
estimated using the Kaplan-Meier method. Statistical analysis was
conducted using SPSS version 21.0 (IBM SPSS, Armonk, NY, USA). All
tests were two-sided and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients characteristics
Of the 25 patients, there were 9 cases of recurrent
disease following surgery and 16 advanced cases. Of these patients,
there were 11 cases of squamous cell carcinoma, 13 cases of
adenocarcinoma, and 1 case of large cell carcinoma. EGFR mutation
status was positive in 5 (20.0%) patients, and all patients
received EGFR-TKIs, including gefitinib or erlotinib, as a
component of serial chemotherapy. There were no positive cases of
ALK translocation in the present study. All patients had undergone
previous chemotherapy, and the regimen of nab-paclitaxel plus
carboplatin was administered as a second-line chemotherapy in 13
(52.0%) patients, third-line chemotherapy in 6 (24.0%) patients,
fourth-line chemotherapy in 3 (12.0%) patients and fifth-line or
later chemotherapy in 3 (12.0%) patients. Individual patients
underwent 2–6 cycles (mean, 3.1 cycles) of this regimen. The
chemotherapy regimens administered prior to nab-paclitaxel plus
carboplatin were primarily S-1 (tegafur/gimeracil/oteracil) plus
platinum, pemetrexed plus platinum, gemcitabine plus platinum,
EGFR-TKI and docetaxel monotherapy (Table II). Furthermore, later chemotherapy
regimens, administered following nab-paclitaxel plus carboplatin,
included docetaxel monotherapy, EGFR-TKI, S-1 monotherapy,
radiation therapy and best supportive care. The later phase
chemotherapy regimens, received following nab-paclitaxel plus
carboplatin, are detailed in Table
III.
 | Table II.Treatment prior to the administration
of nanoparticle albumin-bound-PTX plus carboplatin therapy. |
Table II.
Treatment prior to the administration
of nanoparticle albumin-bound-PTX plus carboplatin therapy.
| Regimen | 1st line | 2nd line | 3rd line | 4th line | 5th line or
later |
|---|
| S-1+platinum | 8 | 0 | 0 | 0 | 0 |
|
BEV+PEM+platinum | 7 | 3 | 0 | 0 | 0 |
| PEM+platinum | 4 | 1 | 0 | 0 | 0 |
| PTX+platinum | 2 | 0 | 0 | 0 | 0 |
| GEM+platinum | 1 | 1 | 0 | 0 | 0 |
| EGFR-TKI | 2 | 4 | 1 | 0 | 4 |
| Single DOC | 0 | 4 | 1 | 0 | 0 |
| Others | 1 | 0 | 0 | 2 | 4 |
 | Table III.Later phase treatments following
nab-PTX plus carboplatin therapy. |
Table III.
Later phase treatments following
nab-PTX plus carboplatin therapy.
| Regimen | Following nab-PTX,
n | Later phase, n | Total, n |
|---|
| Continuation | 7 | 0 | 7 |
| BSC | 5 | 3 | 8 |
| Single DOC | 4 | 2 | 6 |
| Single S-1 | 3 | 3 | 5 |
| EGFR-TKI | 2 | 1 | 3 |
| RTx | 0 | 2 | 2 |
| Others | 4 | 2 | 6 |
Response to treatment and survival
analysis
CR was observed in 1 patient, PR in 7 patients, SD
in 10 patients and PD in 7 patients. The mean overall survival (OS)
time following first-line chemotherapy was 30.0 months. The ORR and
DCR obtained following treatment with nab-paclitaxel with
carboplatin were 32.0 and 72.0%, respectively (Table IV). The median progression-free
survival (PFS) time and median survival time (MST) following
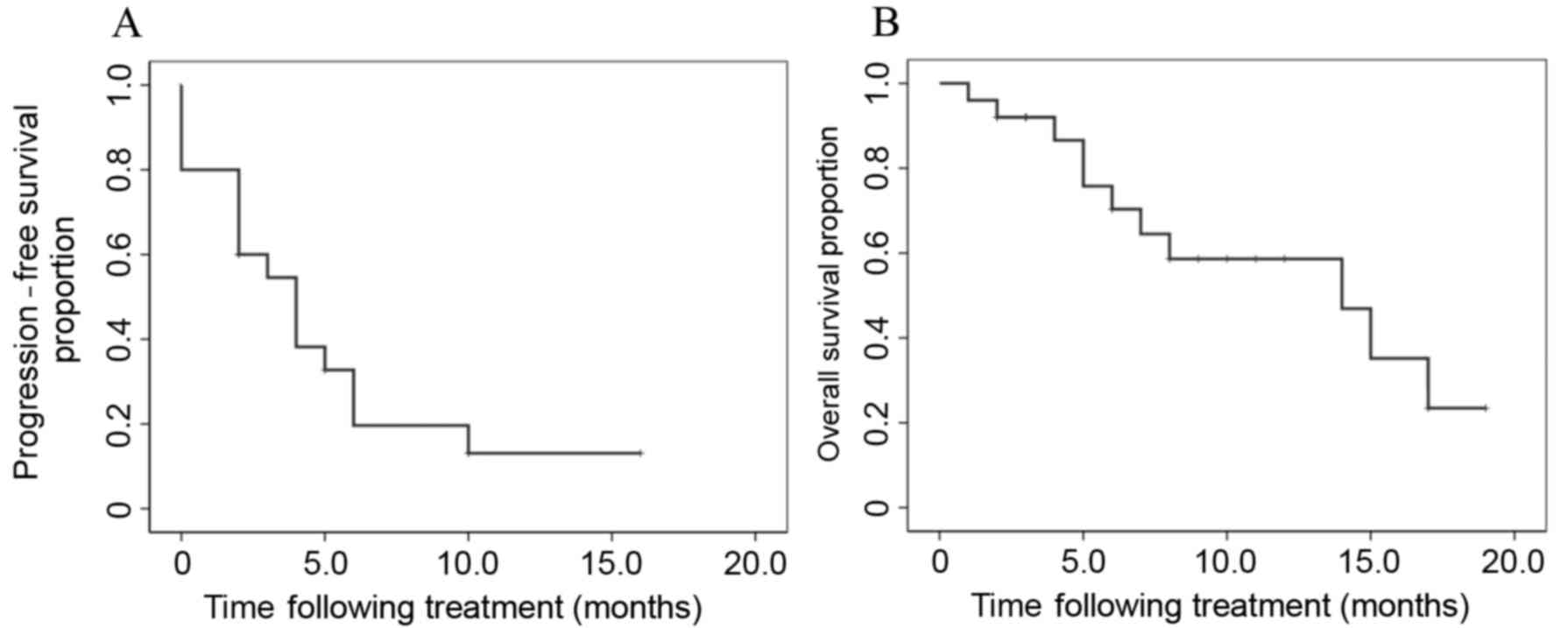
nab-paclitaxel plus carboplatin treatment were 4.0 and 14.0 months,
respectively (Fig. 1). Subgroup
analysis involved the evaluation of the ORR and DCR according to
gender (male vs. female), age (<70 vs. ≥70 years), histology
(squamous vs. non-squamous) and chemotherapy phase (second vs.
third or later phase; Table IV). In
these subgroup analyses, the only parameter that differed
significantly was the ORR, which was 16.7 vs. 71.4% in patients
aged <70 and ≥70 years, respectively (P=0.008). However, no
significant differences in DCR were identified between these age
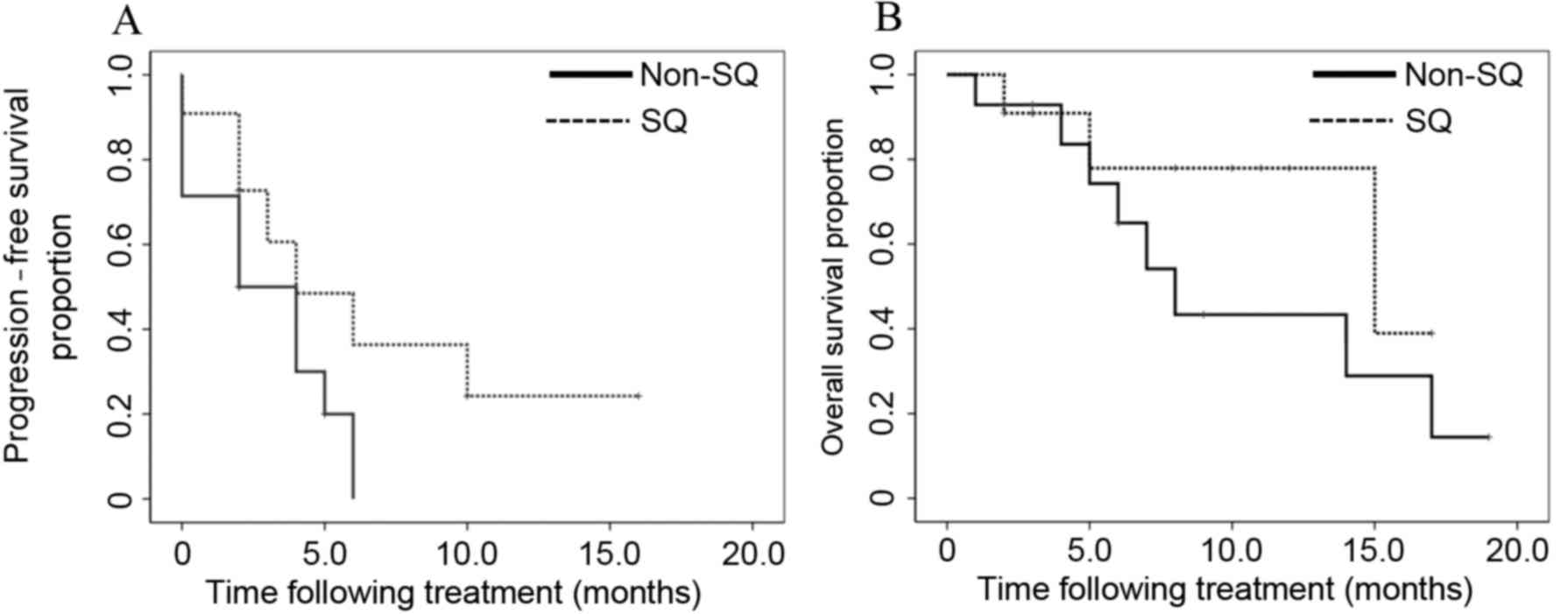
groups (P=0.968). In the groups subdivided according to histology
(squamous vs. non-squamous), no significant differences were
observed in the PFS and OS between the two groups (P=0.110 and
P=0.245, respectively; Fig. 2). In
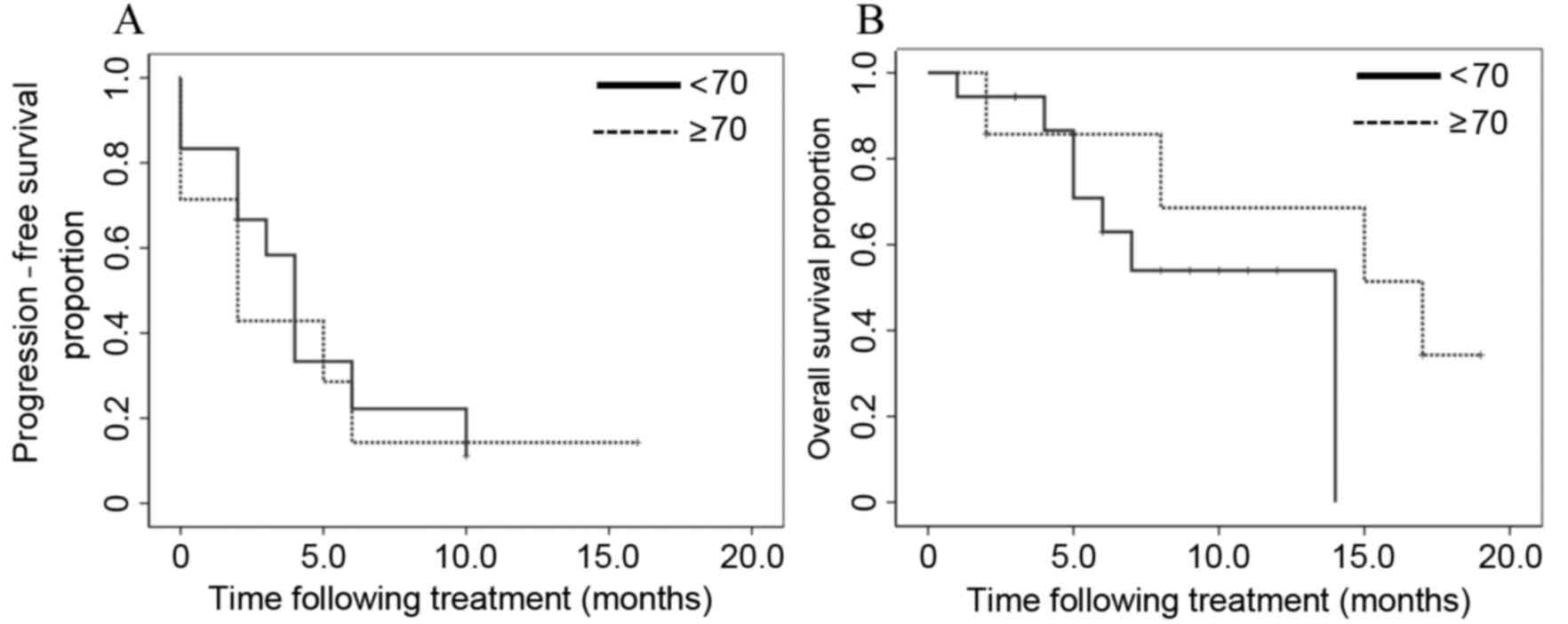
the subgroups divided according to age (<70 vs. ≥70 years), no
significant differences were identified in the PFS and OS between
the two groups (P=0.727 and P=0.270, respectively; Fig. 3).
 | Table IV.Response rates following nanoparticle
albumin-bound-paclitaxel plus carboplatin treatment. |
Table IV.
Response rates following nanoparticle
albumin-bound-paclitaxel plus carboplatin treatment.
| Clinical
factor | Number of
patients | CR | PR | SD | PD | ORR, % | P-value | DCR, % | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
|
|
|
Male | 19 | 1 | 6 | 7 | 5 | 36.8 | 0.356 | 73.7 | 0.739 |
|
Female | 6 | 0 | 1 | 3 | 2 | 16.7 |
| 66.7 |
|
| Age, years |
|
|
|
|
|
|
|
|
|
|
<70 | 18 | 0 | 3 | 10 | 5 | 16.7 | 0.008a | 72.2 | 0.968 |
|
≥70 | 7 | 1 | 4 | 0 | 2 | 71.4 |
| 71.4 |
|
| Histology |
|
|
|
|
|
|
|
|
|
| Sq | 11 | 0 | 4 | 5 | 2 | 36.4 | 0.678 | 81.8 | 0.332 |
|
Non-Sq | 14 | 1 | 3 | 5 | 5 | 28.6 |
| 64.3 |
|
| Phase |
|
|
|
|
|
|
|
|
|
| 2nd
line | 13 | 0 | 6 | 4 | 3 | 46.2 | 0.114 | 76.9 | 0.568 |
| 3rd
line or later | 12 | 1 | 1 | 6 | 4 | 16.7 |
| 66.7 |
|
| Total | 25 | 1 | 7 | 10 | 7 | 32.0 | N.A. | 72.0 | N.A. |
Adverse events
The treatment-associated adverse events that
occurred most frequently were myelosuppression, sensory neuropathy,
gastrointestinal symptoms and baldness, the majority of which were
grade 1–2 (Table V), whereas grade
3–4 neutropenia was present in 7 (28.0%) patients, thrombocytopenia
in 3 (12.0%) patients and anemia in 2 (8.0%) patients. No grade 3–4
sensory neuropathies were observed. Dose reduction was required in
28.0% of patients due to toxicity, but no grade 5 adverse effects
were observed. In addition, 5 (20.0%) patients did not experience
any adverse events.
 | Table V.Grade of adverse events following
treatment with nanoparticle albumin-bound-paclitaxel plus
carboplatin. |
Table V.
Grade of adverse events following
treatment with nanoparticle albumin-bound-paclitaxel plus
carboplatin.
|
| CTCAE grade |
|---|
|
|
|
|---|
| Adverse events | I/II (%) | III (%) | IV (%) |
|---|
| Leukopenia | 5 (20) | 6 (24) | 1 (4) |
|
Thrombocytopenia | 4 (16) | 3 (12) | 0 (0) |
| Anemia | 0 (0) | 2 (8) | 0 (0) |
| Neurotoxicity | 7 (28) | 0 (0) | 0 (0) |
| Gastrointestinal
symptoms | 5 (20) | 1 (4) | 0 (0) |
| Baldness | 3 (12) | 0 (0) | 0 (0) |
| Edema | 1 (4) | 0 (0) | 0 (0) |
| Vertigo | 1 (4) | 0 (0) | 0 (0) |
Discussion
In patients with recurrent or advanced NSCLC who
relapsed following previous platinum-based chemotherapy or EGFR-TKI
treatment, docetaxel monotherapy is considered to be the current
standard treatment (5,7). During a previous phase III study, in
which docetaxel was administered to NSCLC patients who had
previously been treated with platinum-based chemotherapy, the time
to progression and MST following docetaxel monotherapy was 2.4 and
7.0 months, respectively (6). In the
current retrospective study, the median PFS and MST following
nab-paclitaxel plus carboplatin treatment were 4.0 and 14.0 months,
respectively. These results reveal that nab-paclitaxel plus
carboplatin treatment, administered as a second or later-line
chemotherapy, may be a promising therapeutic approach; however,
careful consideration is required, as the present study was
retrospective with a small sample size. In addition, the dose
setting for each agent varied as the condition of the patients
depended on their previous treatments and disease status.
The use of nab-paclitaxel in combination with
carboplatin in chemotherapy-naïve patients with stage III/IV NSCLC
has exhibited a promising efficacy (16). A previous phase III trial revealed
that the ORR of nab-paclitaxel with carboplatin was significantly
higher, compared with that obtained following traditional
solvent-based paclitaxel treatment, and that the PFS and OS were
similar in the two groups (PFS, 6.3 vs. 5.8 months; and OS, 12.1
vs. 11.2 months; P=0.214 and P=0.271, respectively) (16).
In the present study, 11/25 (44%) patients had
squamous cell carcinoma. The prognosis of squamous cell carcinoma
is poor, as compared with that of other non-squamous subtypes
(27). The treatment options for
patients with squamous cell carcinoma are also currently limited
(National Comprehensive Cancer Network guidelines) (28), and EGFR mutations and ALK
rearrangements, which are targetable by TKIs, are rare (29). In addition, bevacizumab and pemetrexed
are not indicated for use in these patients (2,30). A
subset analysis of previous phase III trials (14), based on predefined stratification
factors, revealed that patients with squamous histology treated
with nab-paclitaxel in combination with carboplatin had a
significantly higher ORR, compared with those patients who received
solvent-based paclitaxel plus carboplatin (41 vs. 24%; P<0.001).
In the current study, the ORR and DCR of the patients with squamous
cell carcinoma treated with nab-paclitaxel plus carboplatin were
observed to be 36.4 and 81.8%, respectively. These results
demonstrate a higher efficacy, compared with that of traditional
solvent-based paclitaxel, and may be useful as a second-phase or
later chemotherapy for the treatment of squamous cell
carcinoma.
In the present study, the most common adverse
effects observed during nab-paclitaxel treatment were
myelosuppression, sensory neuropathy, gastrointestinal reactions
and alopecia. The majority of patients experienced grade 1 or 2
adverse events and there were no treatment-associated mortalities
during or following treatment with nab-paclitaxel. In a previous
phase III trial, grade ≥3 neutropenia, thrombocytopenia, anemia and
sensory neuropathy were reported at a rate of 41, 18, 27 and 3%,
respectively, in the nab-paclitaxel with carboplatin treatment
group (16). The present study
observed rates of 28, 12, 8 and 0%, respectively, which were
comparable with the prospective data, despite the inclusion of
patients who had undergone previous chemotherapy that may have
adversely affected their physical condition. Therefore,
nab-paclitaxel in combination with platinum appears to be an
optimal late-phase treatment option, due to its efficacy and
favorable safety profile.
The number of elderly patients diagnosed with lung
cancer has increased worldwide (31,32).
Comorbid diseases and adverse medical conditions, including chronic
lung disease, insufficient cardiac function, renal impairment and
other age-associated conditions, have become major concerns in the
treatment of elderly NSCLC patients, who occasionally experience
difficulties in undergoing cytotoxic chemotherapy (33). Therefore, regimens that may be used to
treat elderly patients with comorbidities are restricted. In the
current study, the ORR of patients aged ≥70 years were superior to
that of patients aged <70 years, and the DCR of patients aged
≥70 years were similar to that of patients aged <70 years. In
addition, the adverse events observed in patients aged ≥70 years
were similar to those observed in patients aged <70 years. These
findings demonstrate that this regimen may also be promising for
use in elderly patients with NSCLC.
In the present study, the previous chemotherapy
regimens administered prior to nab-paclitaxel primarily consisted
of platinum-doublets or EGFR-TKI (Table
II). In addition, later chemotherapy regimens, which were
administered following nab-paclitaxel plus carboplatin treatment,
included docetaxel monotherapy, EGFR-TKI, S-1 monotherapy,
radiation therapy and best supportive care. In later chemotherapy
phases, platinum-doublet chemotherapy is rarely selected, as the
physical condition of patients who require later-phase chemotherapy
is typically poor. Two retrospective studies evaluated the efficacy
of nab-paclitaxel monotherapy following previous chemotherapy, and
reported that the ORR was 30.0 (34)
and 28.6% (35), and the median PFS
was 5.0 (34) and 4.0 months
(35), respectively. In the current
study, the median PFS was 4.0 months, which was comparable to the
PFS reported in previous studies; the PFS was also high, as
compared with the PFS obtained using docetaxel monotherapy as a
second-line chemotherapy.
To the best of our knowledge, the results of the
present study are the first to demonstrate that nab-paclitaxel plus
carboplatin is a promising and tolerable late-phase chemotherapy
for NSCLC. However, the current study had two limitations. Firstly,
only a small sample size of 25 patients was evaluated, and,
secondly, there was a risk of selection bias due to the
retrospective nature of the study. In conclusion, nab-paclitaxel
plus carboplatin administered as a second-phase or later-phase
chemotherapy offers a small but significant survival benefit for
patients with recurrent and advanced-stage IIIB/IV NSCLC, with
tolerable adverse effects. However, further prospective studies of
this regimen as a late-phase chemotherapeutic agent are
required.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
EGFR
|
epidermal growth factor receptor
|
|
TKIs
|
tyrosine kinase inhibitors
|
|
ALK
|
anaplastic lymphoma kinase
|
|
nab-paclitaxel
|
nanoparticle albumin-bound
paclitaxel
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
AUC
|
area under the curve
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
ORR
|
overall response rate
|
|
DCR
|
disease control rate
|
References
|
1
|
Surveillance, epidemiology and end results
(SEER) cancer statistics review, 1975–2010. 2013 http://seer.cancer.gov/csr/1975_201014–June;
|
|
2
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naïve patients with advanced-stage non small cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok TS, Wu Y, Thongprasert S, Yang CH, Chu
DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Eng J Med. 361:947–957. 2009. View Article : Google Scholar
|
|
4
|
Ciuleanu T, Brodowicz T, Zielinski C, Kim
JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small cell lung cancer: A
randomized, double-blind, phase 3 study. Lancet. 374:1432–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fossella FV, DeVore R, Kerr RN, Crawford
J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, et al:
Randomized phase III trial of docetaxel versus vinorelbine or
ifosfamide in patients with advanced non-small cell lung cancer
previously treated with platinum-containing chemotherapy regimens.
The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol.
18:2354–2362. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shepherd FA, Dancey J, Pamlau R, Mattson
K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R,
et al: Prospective randomized trial of docetaxel versus best
supportive care in patients with non-small cell lung cancer
previously treated with platinum-based chemotherapy. J Clin Oncol.
18:2095–2103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shepherd FA, Pereira J Rodrigues, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small cell lung cancer. N Eng J Med. 353:123–132. 2005.
View Article : Google Scholar
|
|
8
|
Hanna N, Shepherd FA, Fossella FV, Pereira
JR, de Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M,
Muller T, et al: Randomized phase III trial of pemetrexed versus
docetaxel in patients with non-small cell lung cancer previously
treated with chemotherapy. J Clin Oncol. 22:1589–1597. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, de Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Eng J Med. 368:2385–2394. 2013. View Article : Google Scholar
|
|
10
|
Baggstrom MQ, Stinchcombe TE, Fried DB,
Poole C, Hensing TA and Socinski MA: Third-generation chemotherapy
agents in the treatment of advanced non-small cell lung cancer. A
meta-analysis. J Thorac Oncol. 2:845–853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang Y, Wang L, Xia GH and Shi MQ:
Clinical investigation of efficacy of albumin bound paclitaxel plus
platinum compounds as first-line chemotherapy for stage III/IV
squamous non-small cell lung cancer. Asian Pac J Cancer Prev.
15:7453–7457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly K, Crowley J, Bunn PA Jr, Presant
CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR,
Moore DF, et al: Randomized phase III trial of paclitaxel plus
carboplatin versus sinorelbine plus cisplatin in the treatment of
patients with advanced non-small cell lung cancer: A Southwest
Oncology Group trial. J Clin Oncol. 19:3210–3218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
John TA, Vogel SM, Tiruppathi C, Malik AB
and Minshall RD: Quantitative analysis of albumin uptake and
transport in the rat microvessel endothelial monolayer. Am J
Physiol Lung Cell Mol Physiol. 284:L187–L196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schnitzer JE and Oh P: Albondin-mediated
capillary permeability to albumin. Differential role of receptors
in endothelial transcytosis and endocytosis of native and modified
albumins. J Biol Chem. 269:6072–6082. 1994.PubMed/NCBI
|
|
15
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
16
|
Socinski MA, Bondarenko I, Karaseva NA,
Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P,
Zhang H, et al: Weekly nab-paclitaxel in combination with
carboplatin versus solvent-based paclitaxel plus carboplatin as
first-line therapy in patients with advanced non-small cell lung
cancer: Final results of a phase III trial. J Clin Oncol.
30:2055–2062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang JJ, Huang C, Chen GY, Song Y, Cheng
Y, Yan HH, Zhou Q and Wu YL: A randomized phase II clinical trial
of nab-paclitaxel and carboplatin compared with gemcitabine and
carboplatin as first-line therapy in locally advanced or metastatic
squamous cell carcinoma of lung. BMC Cancer. 14:6842014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langer CJ, Hirsh V, Ko A, Renschler MF and
Socinski MA: Weekly nab-paclitaxel in combination with carboplatin
as first-line therapy in patients with advanced non-small cell lung
cancer: Analysis of safety and efficacy in patients with renal
impairment. Clin Lung Cancer. 16:112–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Huang X, Wang S, Zhen X, Lin J, Li
P and Lin L: Nab-paclitaxel (abraxane)-based chemotherapy to treat
elderly patients with advanced non-small cell lung cancer: A single
center, randomized and open-label clinical trial. Chin J Cancer
Res. 27:190–196. 2015.PubMed/NCBI
|
|
20
|
Ishihara M, Igawa S, Maki S, Harada S,
Kusuhara S, Niwa H, Otani S, Sasaki J, Jiang SX and Masuda N:
Successful chemotherapy with nab-paclitaxel in a heavily treated
non-small cell lung cancer patient: A case report. Case Rep Oncol.
7:401–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: Introduction to The 2015 World Health
Organization Classification of Tumors of the Lung, Pleura, Thymus,
and Heart. J Thorac Oncol. 10:1240–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conklin CM, Craddock KJ, Have C, Laskin J,
Couture C and Ionescu DN: Innmunohistochemistry is a reliable
screening tool for identification of ALK rearrangement in non-small
cell lung carcinoma and is antibody dependent. J Thorac Oncol.
8:45–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, National cancer
institute of the United states, National cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
26
|
National Cancer Institute, . Cancer
Therapy Evaluation Program. Common Terminology Criteria for Adverse
Events. Version 4.0. http://ctep/.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmAugust
2–2012.
|
|
27
|
Kawase A, Yoshida J, Ishii G, Nakao M,
Aokage K, Hishida T, Nishimura M and Nagai K: Differences between
squamous cell carcinoma and adenocarcinoma of the lung: Are
adenocarcinoma and squamous cell carcinoma prognostically equal?
Jpn J Clin Oncol. 42:189–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdfJanuary
17–2013
|
|
29
|
Lindeman NI, Cagle PT, Beasley MB, Chitale
DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS,
Squire J, et al: Molecular testing guideline for selection of lung
cancer patients for EGFR and ALK tyrosine kinase inhibitors:
Guideline from the college of American pathologists, international
association for the study of lung cancer, and association for
molecular pathology. J Thorac Oncol. 8:823–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sandler AB, Schiller JH, Gray R, Dimery I,
Brahmer J, Samant M, Wang LI and Johnson DH: Retrospective
evaluation of the clinical and radiographic risk factors associated
with severe pulmonary hemorrhage in first-line advanced,
unresectable non-small-cell lung cancer treated with carboplatin
and paclitaxel plus bevacizumab. J Clin Oncol. 27:1405–1412. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsuda A, Matsuda T, Shibata A, Katanoda
K, Sobue T and Nishimoto H; Japan Cancer Surveillance Research
Group, : Cancer incidence and incidence rates in Japan in 2008: A
study of 25 population-based cancer registries for the Monitoring
of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol.
44:388–396. 2013. View Article : Google Scholar
|
|
32
|
Katanoda K, Hori M, Matsuda T, Shibata A,
Nishino Y, Hattori M, Soda M, Ioka A, Sobue T and Nishimoto H: An
updated report on the trends in cancer incidence and mortality in
Japan, 1958–2013. Jpn J Clin Oncol. 45:390–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oshita F, Kurata T, Kasai T, Fukuda M,
Yamamoto N, Ohe Y, Tamura T, Eguchi K, Shinkai T and Saijo N:
Prospective evaluation of the feasibility of cisplatin-based
chemotherapy for elderly lung cancer patients with normal organ
functions. Jpn J Cancer Res. 86:1198–1202. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng Q, Yao Y and Nan K: Weekly
intravenous nanoparticle albumin-bound paclitaxel for elderly
patients with stage IV non-small cell lung cancer: A series of 20
cases. J Biomed Res. 26:159–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xing PY, Li JL, Wang Y, Hao XZ, Wang B,
Yang L, Shi YK and Zhang XR: Efficacy and safety of albumin-bound
paclitaxel in treating recurrent advanced non-small cell lung
cancer. Chin J Cancer Res. 25:200–205. 2013.PubMed/NCBI
|