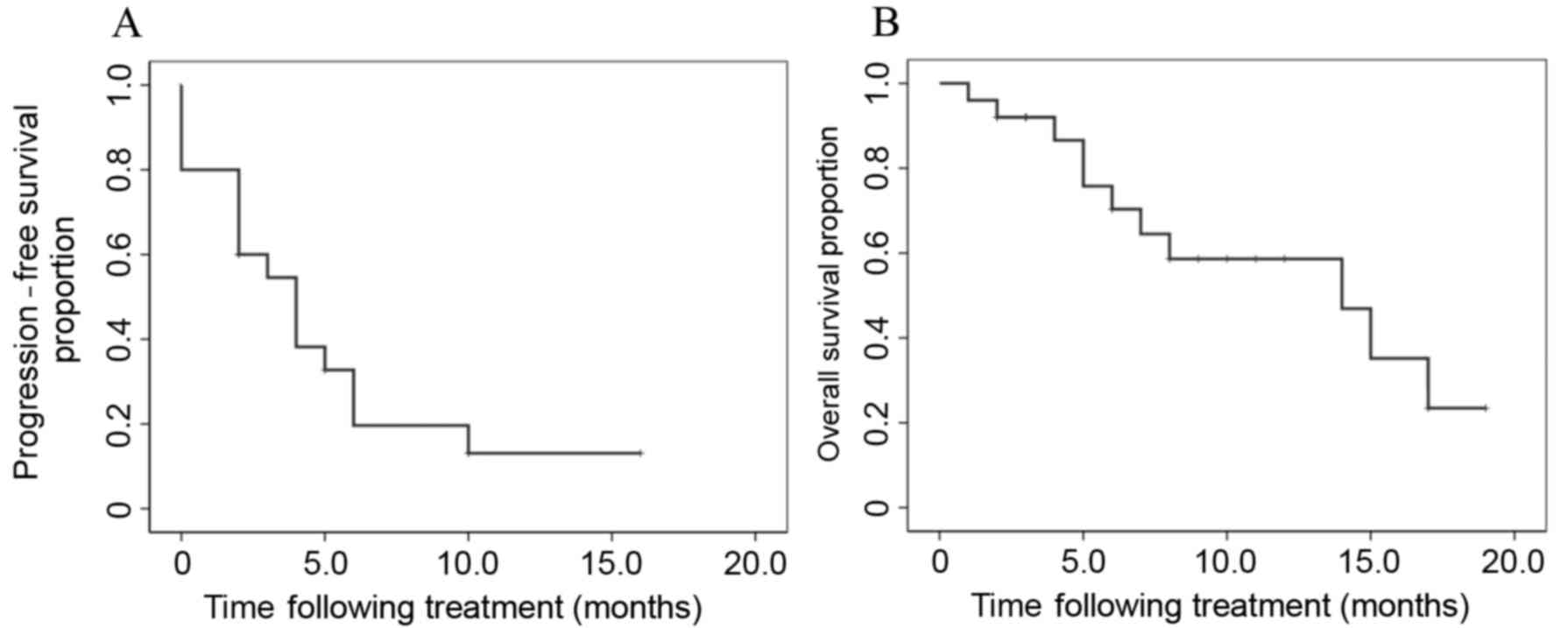
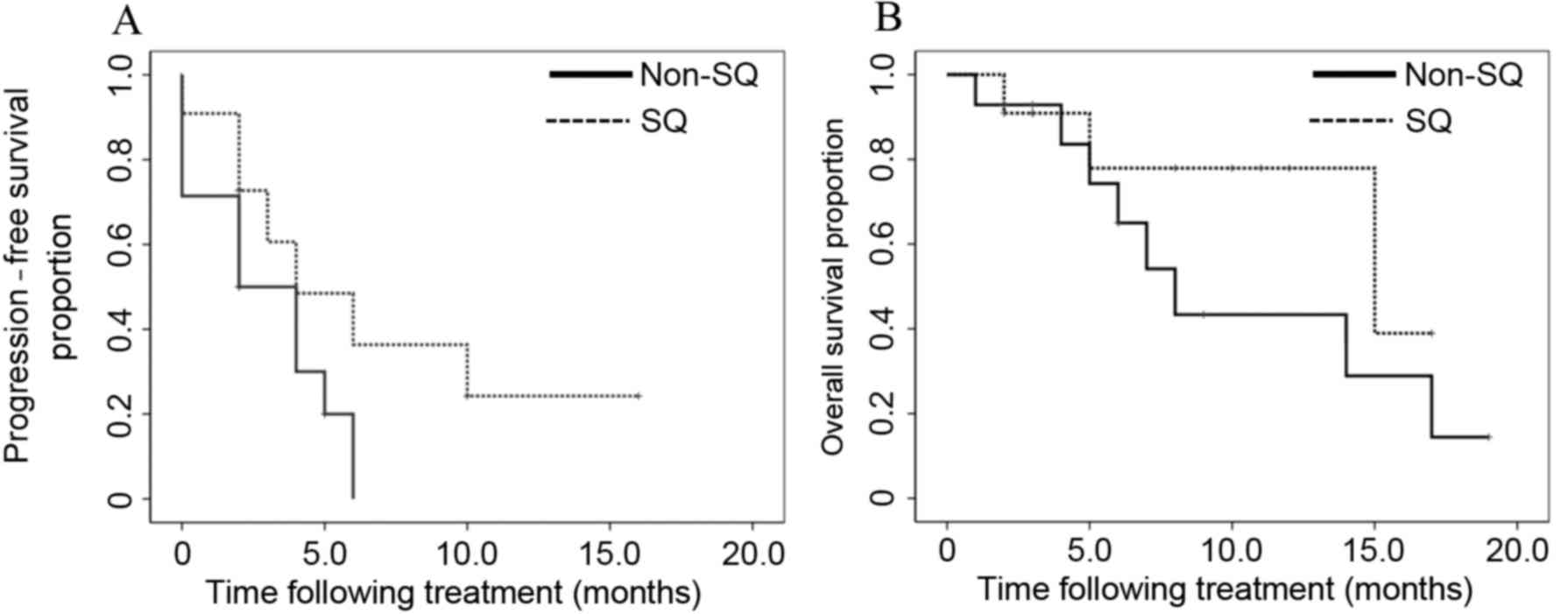
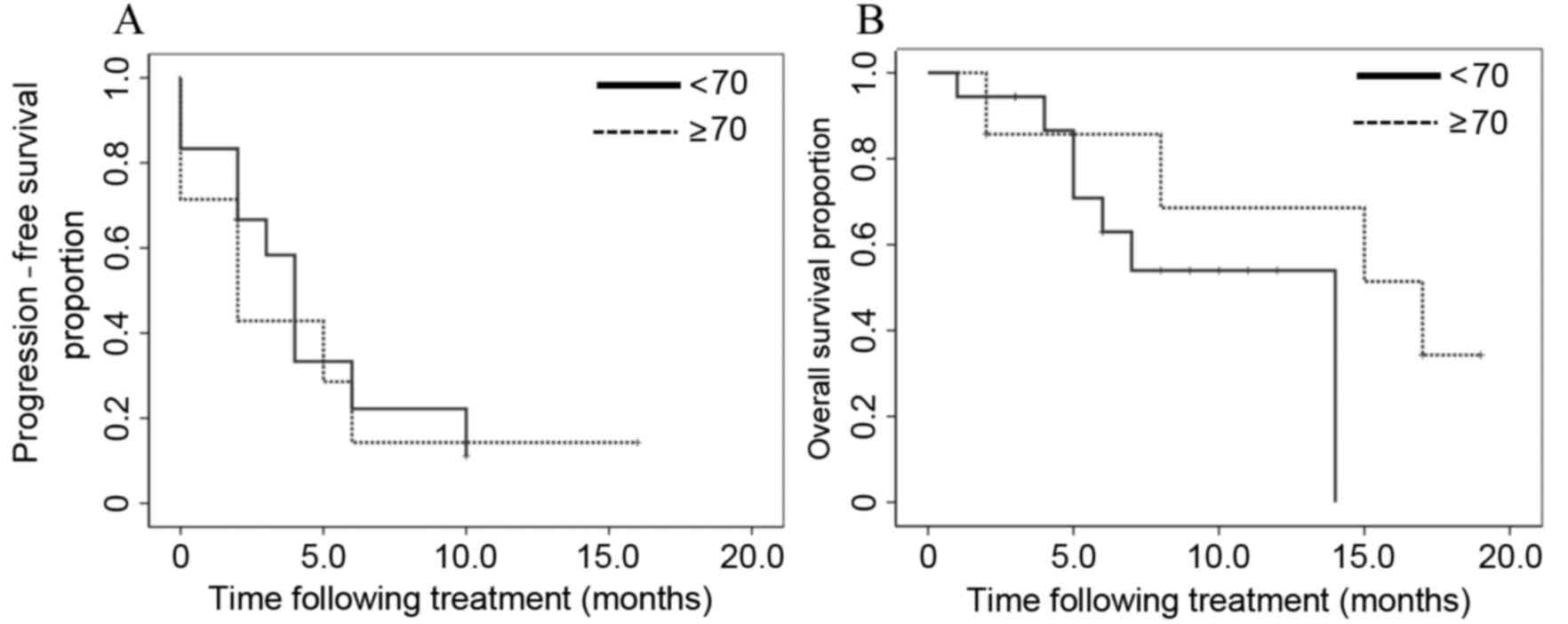
|
1
|
Surveillance, epidemiology and end results
(SEER) cancer statistics review, 1975–2010. 2013 http://seer.cancer.gov/csr/1975_201014–June;
|
|
2
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naïve patients with advanced-stage non small cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok TS, Wu Y, Thongprasert S, Yang CH, Chu
DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al:
Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N
Eng J Med. 361:947–957. 2009. View Article : Google Scholar
|
|
4
|
Ciuleanu T, Brodowicz T, Zielinski C, Kim
JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small cell lung cancer: A
randomized, double-blind, phase 3 study. Lancet. 374:1432–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fossella FV, DeVore R, Kerr RN, Crawford
J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, et al:
Randomized phase III trial of docetaxel versus vinorelbine or
ifosfamide in patients with advanced non-small cell lung cancer
previously treated with platinum-containing chemotherapy regimens.
The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol.
18:2354–2362. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shepherd FA, Dancey J, Pamlau R, Mattson
K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R,
et al: Prospective randomized trial of docetaxel versus best
supportive care in patients with non-small cell lung cancer
previously treated with platinum-based chemotherapy. J Clin Oncol.
18:2095–2103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shepherd FA, Pereira J Rodrigues, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small cell lung cancer. N Eng J Med. 353:123–132. 2005.
View Article : Google Scholar
|
|
8
|
Hanna N, Shepherd FA, Fossella FV, Pereira
JR, de Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M,
Muller T, et al: Randomized phase III trial of pemetrexed versus
docetaxel in patients with non-small cell lung cancer previously
treated with chemotherapy. J Clin Oncol. 22:1589–1597. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, de Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Eng J Med. 368:2385–2394. 2013. View Article : Google Scholar
|
|
10
|
Baggstrom MQ, Stinchcombe TE, Fried DB,
Poole C, Hensing TA and Socinski MA: Third-generation chemotherapy
agents in the treatment of advanced non-small cell lung cancer. A
meta-analysis. J Thorac Oncol. 2:845–853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang Y, Wang L, Xia GH and Shi MQ:
Clinical investigation of efficacy of albumin bound paclitaxel plus
platinum compounds as first-line chemotherapy for stage III/IV
squamous non-small cell lung cancer. Asian Pac J Cancer Prev.
15:7453–7457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly K, Crowley J, Bunn PA Jr, Presant
CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR,
Moore DF, et al: Randomized phase III trial of paclitaxel plus
carboplatin versus sinorelbine plus cisplatin in the treatment of
patients with advanced non-small cell lung cancer: A Southwest
Oncology Group trial. J Clin Oncol. 19:3210–3218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
John TA, Vogel SM, Tiruppathi C, Malik AB
and Minshall RD: Quantitative analysis of albumin uptake and
transport in the rat microvessel endothelial monolayer. Am J
Physiol Lung Cell Mol Physiol. 284:L187–L196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schnitzer JE and Oh P: Albondin-mediated
capillary permeability to albumin. Differential role of receptors
in endothelial transcytosis and endocytosis of native and modified
albumins. J Biol Chem. 269:6072–6082. 1994.PubMed/NCBI
|
|
15
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
16
|
Socinski MA, Bondarenko I, Karaseva NA,
Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P,
Zhang H, et al: Weekly nab-paclitaxel in combination with
carboplatin versus solvent-based paclitaxel plus carboplatin as
first-line therapy in patients with advanced non-small cell lung
cancer: Final results of a phase III trial. J Clin Oncol.
30:2055–2062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang JJ, Huang C, Chen GY, Song Y, Cheng
Y, Yan HH, Zhou Q and Wu YL: A randomized phase II clinical trial
of nab-paclitaxel and carboplatin compared with gemcitabine and
carboplatin as first-line therapy in locally advanced or metastatic
squamous cell carcinoma of lung. BMC Cancer. 14:6842014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langer CJ, Hirsh V, Ko A, Renschler MF and
Socinski MA: Weekly nab-paclitaxel in combination with carboplatin
as first-line therapy in patients with advanced non-small cell lung
cancer: Analysis of safety and efficacy in patients with renal
impairment. Clin Lung Cancer. 16:112–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Huang X, Wang S, Zhen X, Lin J, Li
P and Lin L: Nab-paclitaxel (abraxane)-based chemotherapy to treat
elderly patients with advanced non-small cell lung cancer: A single
center, randomized and open-label clinical trial. Chin J Cancer
Res. 27:190–196. 2015.PubMed/NCBI
|
|
20
|
Ishihara M, Igawa S, Maki S, Harada S,
Kusuhara S, Niwa H, Otani S, Sasaki J, Jiang SX and Masuda N:
Successful chemotherapy with nab-paclitaxel in a heavily treated
non-small cell lung cancer patient: A case report. Case Rep Oncol.
7:401–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: Introduction to The 2015 World Health
Organization Classification of Tumors of the Lung, Pleura, Thymus,
and Heart. J Thorac Oncol. 10:1240–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conklin CM, Craddock KJ, Have C, Laskin J,
Couture C and Ionescu DN: Innmunohistochemistry is a reliable
screening tool for identification of ALK rearrangement in non-small
cell lung carcinoma and is antibody dependent. J Thorac Oncol.
8:45–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, National cancer
institute of the United states, National cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
26
|
National Cancer Institute, . Cancer
Therapy Evaluation Program. Common Terminology Criteria for Adverse
Events. Version 4.0. http://ctep/.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmAugust
2–2012.
|
|
27
|
Kawase A, Yoshida J, Ishii G, Nakao M,
Aokage K, Hishida T, Nishimura M and Nagai K: Differences between
squamous cell carcinoma and adenocarcinoma of the lung: Are
adenocarcinoma and squamous cell carcinoma prognostically equal?
Jpn J Clin Oncol. 42:189–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdfJanuary
17–2013
|
|
29
|
Lindeman NI, Cagle PT, Beasley MB, Chitale
DA, Dacic S, Giaccone G, Jenkins RB, Kwiatkowski DJ, Saldivar JS,
Squire J, et al: Molecular testing guideline for selection of lung
cancer patients for EGFR and ALK tyrosine kinase inhibitors:
Guideline from the college of American pathologists, international
association for the study of lung cancer, and association for
molecular pathology. J Thorac Oncol. 8:823–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sandler AB, Schiller JH, Gray R, Dimery I,
Brahmer J, Samant M, Wang LI and Johnson DH: Retrospective
evaluation of the clinical and radiographic risk factors associated
with severe pulmonary hemorrhage in first-line advanced,
unresectable non-small-cell lung cancer treated with carboplatin
and paclitaxel plus bevacizumab. J Clin Oncol. 27:1405–1412. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsuda A, Matsuda T, Shibata A, Katanoda
K, Sobue T and Nishimoto H; Japan Cancer Surveillance Research
Group, : Cancer incidence and incidence rates in Japan in 2008: A
study of 25 population-based cancer registries for the Monitoring
of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol.
44:388–396. 2013. View Article : Google Scholar
|
|
32
|
Katanoda K, Hori M, Matsuda T, Shibata A,
Nishino Y, Hattori M, Soda M, Ioka A, Sobue T and Nishimoto H: An
updated report on the trends in cancer incidence and mortality in
Japan, 1958–2013. Jpn J Clin Oncol. 45:390–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oshita F, Kurata T, Kasai T, Fukuda M,
Yamamoto N, Ohe Y, Tamura T, Eguchi K, Shinkai T and Saijo N:
Prospective evaluation of the feasibility of cisplatin-based
chemotherapy for elderly lung cancer patients with normal organ
functions. Jpn J Cancer Res. 86:1198–1202. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng Q, Yao Y and Nan K: Weekly
intravenous nanoparticle albumin-bound paclitaxel for elderly
patients with stage IV non-small cell lung cancer: A series of 20
cases. J Biomed Res. 26:159–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xing PY, Li JL, Wang Y, Hao XZ, Wang B,
Yang L, Shi YK and Zhang XR: Efficacy and safety of albumin-bound
paclitaxel in treating recurrent advanced non-small cell lung
cancer. Chin J Cancer Res. 25:200–205. 2013.PubMed/NCBI
|