Introduction
Although colonoscopic screening and subsequent
endoscopic resection of preneoplastic lesions, such as colorectal
adenoma, have been reported to be effective for reducing the
incidence of colorectal cancer (CRC) (1), CRC still remains one of the most common
causes of cancer-related death in developed countries (2). Recently, aberrant crypt foci (ACF) have
emerged as a putative precursor to colorectal adenoma, and have
been suggested to be a potentially useful biomarker for CRC. ACF
were initially identified as the earliest recognizable lesions on
the colonic mucosa in rodents exposed to colorectal carcinogens
(3) and their presence has been
demonstrated to be an important predictor of CRC (4–6). Shortly
after the description in animals, ACF were also identified in the
human colonic mucosa, using methylene blue staining (7,8). Although
several previous epidemiological studies have revealed significant
associations between the prevalence and/or number of ACF and the
synchronous presence of advanced neoplasms, including both adenoma
and CRC (8–16), others have reported a lack of such
correlation (17,18). Therefore, the biological significance
of human ACF still remains to be established.
These discrepancies among previous reports may be
explained, at least in part, by differences in the participant
characteristics, such as race, age and behavioral factors. In
addition, it is considered that variations in the criteria for
defining the ACF counting area may also be possibly associated with
these discrepancies. In some studies, the counting of ACF was
carried out in the region from the middle Houston valve to the
dentate line (8,16,17,19). On
the other hand, in other studies, the counting was carried out in
the distal rectum up to 10–15 cm from the dentate line (9–11,13–15). Cho
et al defined the examination area as the entire rectum
(18). Since such differences affect
the interpretation of the results of previous reports, we conducted
this study to clarify whether the criteria used to define the
counting area for ACF may affect the number of ACF.
Materials and methods
Patients
The patients who underwent a total colonoscopy and
counting of ACF at the Yokohama City University Hospital from May
to August 2014 were eligible to participate in this study. All the
procedures were performed by one endoscopist proficient in ACF
counting (S.E.). The exclusion criteria were as follows; patients
with a history of familial adenomatous polyposis, hereditary
non-polyposis CRC, inflammatory bowel disease, radiation colitis,
history of previous surgical or endoscopic resection of colonic
adenomas and/or cancer, current invasive cancer, prior large-bowel
resection, except appendectomy. Of 68 patients received total
colonoscopy, 6 patients without informed consent were excluded in
addition to the aforementioned exclusion critetria. Finally a total
of 45 subjects were prospectively enrolled in this study. The
patients were divided into three groups according to the presence
of colorectal tumors; normal subjects, adenoma patients and CRC
patients. The study was conducted with the approval of the Yokohama
City University Hospital Ethics Committee. Written informed consent
was obtained from all the subjects prior to their participation in
the study.
Criteria used for endoscopic diagnosis
of ACF
ACF are identified as clusters of crypts that stain
darker than the surrounding normal mucosa. Larger sizes of the
crypts, raised appearance, thicker epithelial lining, dilated or
slit-like crypt lumina, and increased pericryptal area as compared
to the surrounding normal mucosa are the most frequently used
criteria to identify ACF (8).
Magnifying endoscopy
All subjects were asked to drink 2,000 ml of
polyethylene glycol-based solution as a bowel preparation measure
prior to the endoscopic examination. If the bowel cleaning was
insufficient, an additional polyethylene glycol-based solution was
administered. Total colonoscopy was performed before the
examination for the ACF. A Fujinon EC-490ZW5/M colonoscope was used
for the high-magnifying chromoendoscopy (Fujifilm Medical Co.,
Ltd., Tokyo, Japan). Methylene blue dye (0.2%) was applied to coat
the mucosal surface, followed by adequate washing with warm tap
water. After 2 min of staining the mucosa, the excess dye was
removed carefully by washing with water, and then observation for
ACF was commenced. We counted the number of ACF in two defined
areas; from the dentate line to the middle Houston valve
(definition 1) and the distal rectum extending 15 cm from the
dentate line (definition 2). The distribution of ACF was evaluated
in four bowel segments (middle Houston valve to the dentate line,
and the distal rectum 0–5, 5–10 and 10–15 cm). To prevent double
counting, the ACF were counted in a sequential fashion during a
single withdrawal of the endoscope.
Statistical analysis
The numbers of ACF was compared among the three
groups (normal subjects, adenoma patients and CRC patients) using
the Kruskal Wallis test. The correlation of the number of ACF with
the criteria for defining the ACF counting area was evaluated by
Pearson's correlation coefficient. The relationship between the
distance from the middle Houston valve to the dentate line and the
patient physique (gender, height and BMI) was also evaluated by
Pearson's correlation coefficient. Unless otherwise specified,
P-values <0.05 were considered to denote statistical
significance. All the analyses were performed using the SPSS
statistical package (version 11.0; SPSS, Inc., Chicago, IL,
USA).
Results
Patients characteristics
The patients characteristics are shown in Table I. The prevalence of ACF was 84% and
the number of ACF was 4.7±5.7. Consistent with previous studies
(11–14), the number of ACF in the distal rectum
extending 15 cm from the dentate line was significantly higher in
the CRC patients than in the normal subjects and/or adenoma
patients (the number in normal subjects, adenoma patients and CRC
patients, 2.1±1.9, 5.7±2.5 and 9.8±10.1, respectively,
P<0.01).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Normal subjects | Adenoma patients | CRC patients | P-value |
|---|
| Number of
subjects | 20 | 19 | 6 |
|
| Age, mean ± SD | 62.4±10.5 | 65.6±9.8 | 69.2±6.9 | N.S. |
| Sex, male/Female | 11/8 | 13/7 | 4/2 | N.S. |
| Presence of ACF |
|
|
|
|
| Total
number | 42 | 109 | 59 |
|
|
Prevalence, (%) | 15/20 (75) | 17/19 (89) | 6/6 (100) | N.S. |
| Mean ±
SD | 2.1±1.9 |
5.7±2.5 |
9.8±10.1 | <0.01 |
The distribution of ACF and number of
ACF depending on the criteria used for defining the ACF counting
area
The distribution of the ACF is shown in Table II. In all enrolled patients, the
middle Houston valve was located within 15 cm from the dentate
line. The average distance from the middle Houston valve to the
dentate line was 8.5±1.3 cm. Most of the ACF (170/210, 81%) were
located in the bowel segment from the middle Houston valve to the
dentate line. There were few dispersion of the ACF number
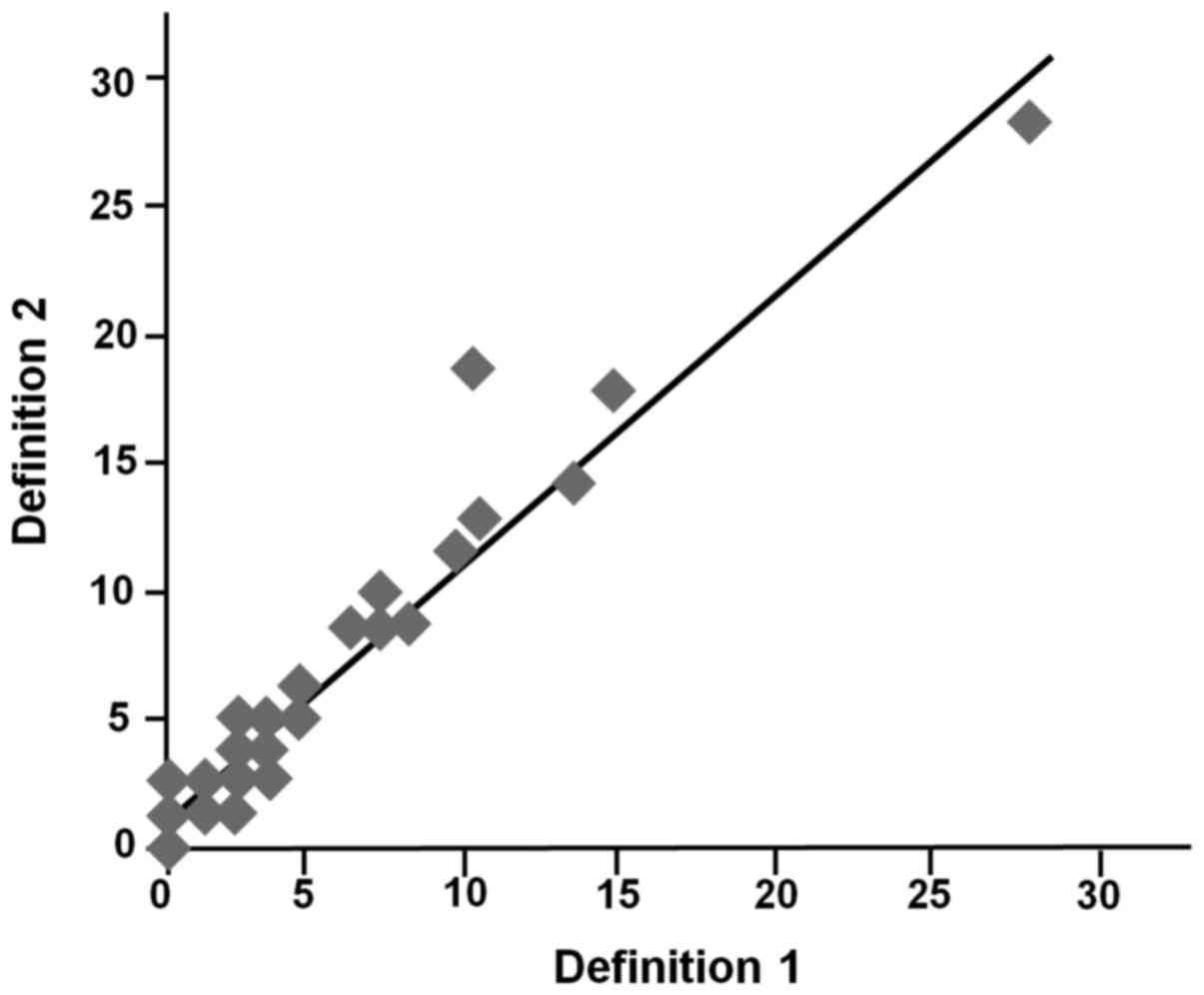
regardless of the different definition of counting area. (r=0.94,
P<0.001) (Fig. 1), suggesting that
such descrepancy may not affect the interpretation of the results
of previous studies (8–19).
 | Table II.Distribution of ACF. |
Table II.
Distribution of ACF.
|
| Bowel segment |
|---|
|
|
|
|---|
|
| Definition 1 | Definition 2 |
|---|
|
|
|
|
|---|
| Variable | Dentate line-middle
Houston valve | 0-5 cm | 5–10 cm | 10–15 cm |
|---|
| ACF number | 170 | 77 | 105 | 28 |
Correlation between the patient physiques and the
length of the bowel segment from the dentate line to the middle
Houston valve. The height of the participants was 165.8±7.2 cm
(male, 167.5±6.0; female, 155.0±4.2, P<0.01) and the BMI was
23.4±2.7 kg/m2 (male, 23.3±2.5; female, 24.3±4.0,
P=0.77). Gender was not associated with the length of the bowel
segment from the middle Houston valve to the dentate line (male,
8.4±1.3; female, 9.2±1.1, P=0.90). In addition, the height
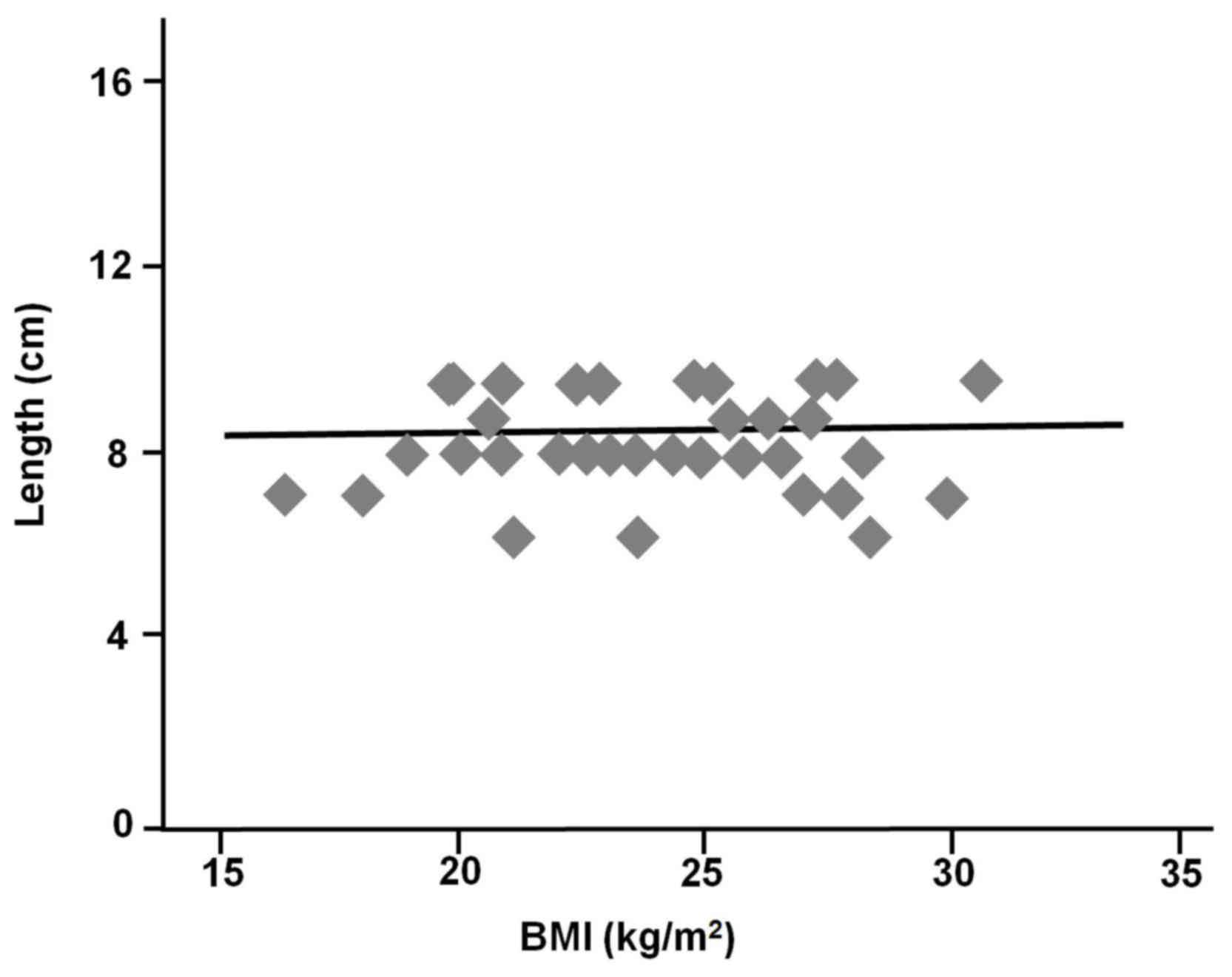
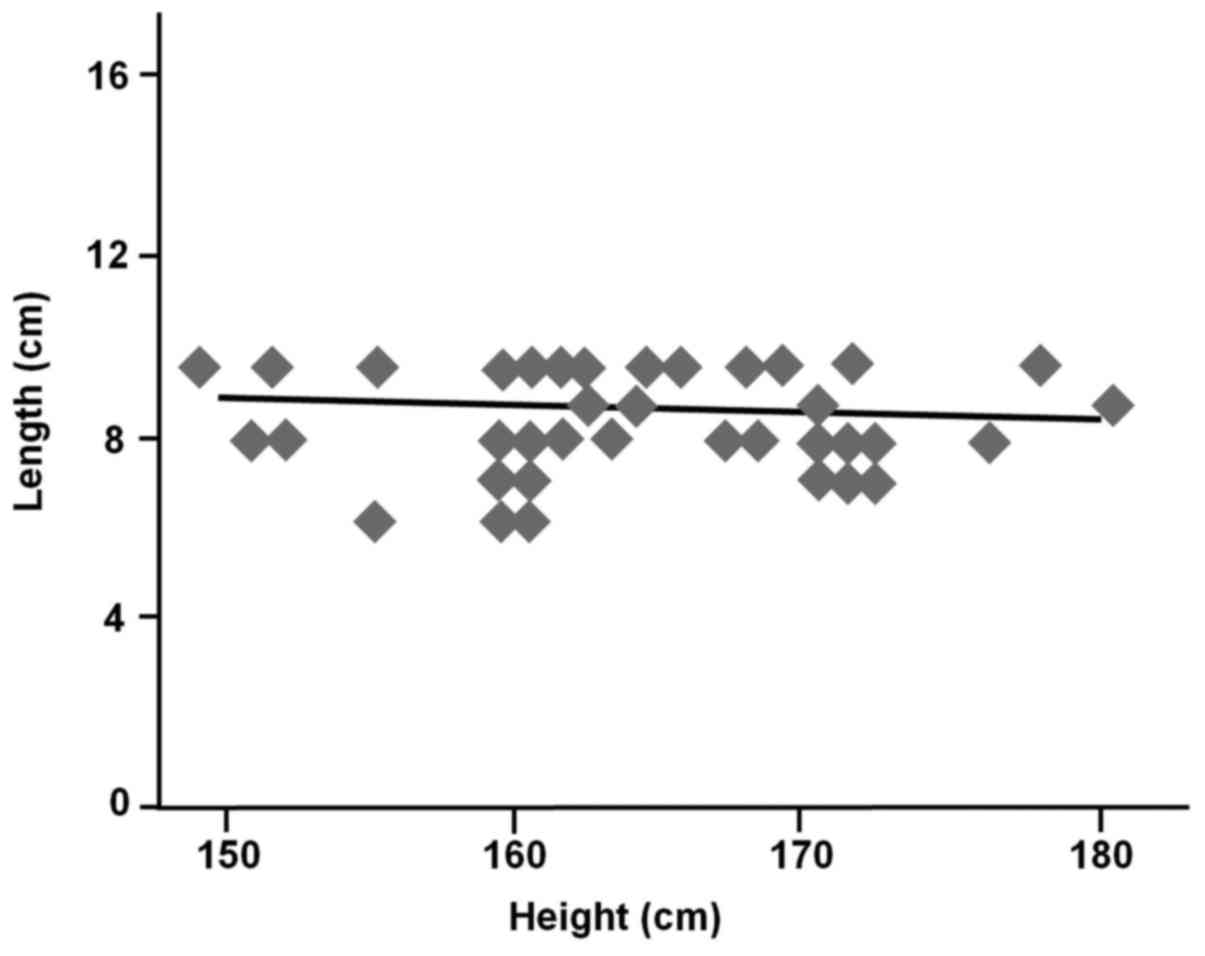
(r=−0.18, P=0.28) and BMI (r=0.002, P=0.99) were also not
correlated significantly with the length of the bowel segment from
the middle Houston valve to the dentate line (Figs. 2 and 3).
Discussion
To evaluate the efficacy of chemopreventive agents
in CRC chemoprevention trials, reliable surrogate biomarkers are
required. Recently, several trials have been conducted using the
presence of ACF as the endpoint (18–21). Our
recent large scale epidemiological study demonstrated that ACF may
serve as a reliable surrogate biomarker for CRC in humans (18). On the other hand, a multicenter study
conducted in the US by Mutch et al raised serious questions
about whether ACF can be used as a surrogate biomarker for CRC
(17). To establish the clinical
significance of ACF, it is important to determine the cause of this
aforementioned discrepancy in interpretation among previous
studies.
In most of previous studies, while the ACF were
identified by high-magnification chromoscopic colonoscopy using the
same dye (methylene blue) and same criteria for endoscopic
detection, the criteria used to define ACF counting area during
colonoscopy differed among studies. Therefore, we hypothesized that
differences in the ACF counting area may be the reason for the
discrepancies in the interpretation of the clinical significance of
ACF among previous studies. Our results revealed that differences
in the counting area did not affect the results of ACF counting,
because most ACF were located in the bowel segment from the middle
Houston valve to the dentate line. As we confirmed that the results
of ACF counting did not differ significantly between the two
different ACF counting areas most frequently used in previous
studies: The region from the middle Houston valve to the dentate
line (8,16,19,22) and
the distal rectum up to 15 cm from the dentate line (11,14),
additional studies would be needed to explain the discrepancies in
the interpretation of the clinical significance of ACF among
previous studies.
Our results demonstrated that the patient physique
was not associated with the length of the bowel segment from the
middle Houston valve to the dentate line. For reasonably comparing
the results from different studies, common criteria are required
for defining the ACF counting area. Although additional studies are
needed to confirm that the same results could be applied to western
populations, we propose that the most appropriate counting area for
ACFs during colonoscopy is from the middle Houston valve to the
dentate line, because the middle Houston valve is an easily
recognized landmark.
In conclusion, ACF were predominantly identified in
the bowel segment from the middle Houston valve to the dentate
line, and result of counting of the number of ACF was not affected
depending on which of the two counting areas frequently used in
previous ACF studies, were used. These results indicated that the
discrepancies in the reports about the biological significance of
ACF among previous studies may not be explained by differences in
the criteria used for defining the ACF counting area, but other
factors (e.g., race and/or behavioral characteristics), and warrant
further investigation involving a larger group of patients of
different races and behavioural features.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for research on the Third-Term Comprehensive Control Research for
Cancer from the Ministry of Health, Labour and Welfare, Japan, to
A. N.
Glossary
Abbriviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
ACF
|
aberrant crypt foci
|
|
BMI
|
body mass index
|
References
|
1
|
Winawer SJ, Zauber AG, Ho MN, O'Brien MJ,
Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond J, Panish JF,
et al: Prevention of colorectal cancer by colonoscopic polypectomy.
The National Polyp Study Workgroup. N Engl J Med. 329:1977–1981.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bird RP: Observation and quantification of
aberrant crypts in the murine colon treated with a colon
carcinogen: Preliminary findings. Cancer Lett. 37:147–151. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McLellan EA and Bird RP: Aberrant crypts:
Potential preneoplastic lesions in the murine colon. Cancer Res.
48:6187–6192. 1988.PubMed/NCBI
|
|
5
|
McLellan EA, Medline A and Bird RP: Dose
response and proliferative characteristics of aberrant crypt foci:
Putative preneoplastic lesions in rat colon. Carcinogenesis.
12:2093–2098. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McLellan EA, Medline A and Bird RP:
Sequential analyses of the growth and morphological characteristics
of aberrant crypt foci: Putative preneoplastic lesions. Cancer Res.
51:5270–5274. 1991.PubMed/NCBI
|
|
7
|
Pretlow TP, Barrow BJ, Ashton WS,
O'Riordan MA, Pretlow TG, Jurcisek JA and Stellato TA: Aberrant
crypts: Putative preneoplastic foci in human colonic mucosa. Cancer
Res. 51:1564–1567. 1991.PubMed/NCBI
|
|
8
|
Takayama T, Katsuki S, Takahashi Y, Ohi M,
Nojiri S, Sakamaki S, Kato J, Kogawa K, Miyake H and Niitsu Y:
Aberrant crypt foci of the colon as precursors of adenoma and
cancer. N Engl J Med. 339:1277–1284. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adler DG, Gostout CJ, Sorbi D, Burgart LJ,
Wang L and Harmsen WA: Endoscopic identification and quantification
of aberrant crypt foci in the human colon. Gastrointest Endosc.
56:657–662. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hurlstone DP, Karajeh M, Sanders DS, Drew
SK and Cross SS: Rectal aberrant crypt foci identified using
high-magnification-chromoscopic colonoscopy: Biomarkers for flat
and depressed neoplasia. Am J Gastroentrol. 100:1283–1289. 2005.
View Article : Google Scholar
|
|
11
|
Seike K, Koda K, Oda K, Kosugi C, Shimizu
K, Nishimura M, Shioiri M, Takano S, Ishikura H and Miyazaki M:
Assessment of rectal aberrant crypt foci by standard chromoscopy
and its predictive value for colonic advanced neoplasms. Am J
Gastroenterol. 101:1362–1369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim J, Ng J, Arozulllah A, Ewing R, Llor
X, Carroll RE and Benya RV: Aberrant crypt focus size predicts
distal polyp histopathology. Cancer Epidemiol Biomarkers Prev.
17:1155–1162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rudolph RE, Dominitz JA, Lampe JW, Levy L,
Qu P, Li SS, Lampe PD, Bronner MP and Potter JD: Risk factors for
colorectal cancer in relation to number and size of aberrant crypt
foci in humans. Cancer Epidemiol Biomarkers Prev. 14:605–608. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moxon D, Raza M, Kenney R, Ewing R,
Arozullah A, Mason JB and Carroll RE: Relationship of aging and
tobacco use with the development of aberrant crypt foci in a
predominantly African-American population. Clin Gastroenterol
Hepatol. 3:271–278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stevens RG, Swede H, Heinen CD, Jablonski
M, Grupka M, Ross B, Parente M, Tirnauer JS, Giardina C, Rajan TV,
et al: Aberrant crypt foci in patients with a positive family
history of sporadic colorectal cancer. Cancer Lett. 248:262–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakai E, Takahashi H, Kato S, Uchiyama T,
Hosono K, Endo H, Maeda S, Yoneda M, Taguri M and Nakajima A:
Investigation of the prevalence and number of aberrant crypt foci
associated with human colorectal neoplasm. Cancer Epidemiol
Biomaekers Prev Res. 20:1918–1924. 2011. View Article : Google Scholar
|
|
17
|
Mutch MG, Schoen RE, Fleshman JW, Rall CJ,
Dry S, Seligson D, Charabaty A, Chia D, Umar A, Viner J, et al: A
multicenter study of prevalence and risk factors for aberrant crypt
foci. Clin Gastroenterol Hepatol. 7:568–574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho NL, Redston M, Zauber AG, Carothers
AM, Hornick J, Wilton A, Sontag S, Nishioka N, Giardiello FM,
Saltzman JR, et al: Aberrant crypt foci in the adenoma prevention
with celecoxib trial. Cancer Prev Res. 1:21–31. 2008. View Article : Google Scholar
|
|
19
|
Hosono K, Endo H, Takahashi H, Sugiyama M,
Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, et al:
Metformin suppresses colorectal aberrant crypt foci in a short-term
clinical trial. Cancer Prev Res. 3:1077–1083. 2010. View Article : Google Scholar
|
|
20
|
Takayama T, Nagashima H, Maeda M, Nojiri
S, Hirayama M, Nakano Y, Takahashi Y, Sato Y, Sekikawa H, Mori M,
et al: Randomized double-blind trial of sulindac and etodolac to
eradicate aberrant crypt foci and to prevent sporadic colorectal
polyps. Clin Cancer Res. 17:3803–3811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ezuka A, Sakai E, Kawana K, Nagase H,
Kakuta Y, Uchiyama S, Ohkubo H, Higurashi T, Nonaka T, Endo H, et
al: Association between factors associated with colorectal cancer
and rectal aberrant crypt foci in humans. Oncol Lett. 10:3689–3695.
2015.PubMed/NCBI
|
|
22
|
Ohkubo H, Takahashi H, Yamada E, Sakai E,
Higurashi T, Uchiyama T, Hosono K, Endo H, Taguri M and Nakajima A:
Natural history of human aberrant crypt foci and correlation with
risk factors for colorectal cancer. Oncol Rep. 27:1475–1480.
2012.PubMed/NCBI
|