Introduction
Giant cell tumor of the tendon sheath (GCTTS) is a
type of benign soft tissue tumor that was first described by
Chassaignac in 1852 (1). GCTTS is
also termed tenosynovial giant cell tumor, pigmented nodular
tenosynovitis, xanthogranuloma, benign synovioma and fibrous
xanthoma of synovium. The World Health Organization distinguishes
between two types of giant cell lesions originating from the tendon
and the synovium (2). GCTTS can be
classified as localized (L-) or diffuse (D-) type. L-GCTTS
primarily occurs in the tendon sheaths of the hand and foot and
exhibits clear boundaries, whereas D-GCTTS occurs in large joints
with a more aggressive growth pattern and associated high
recurrence rate (2). As magnetic
resonance imaging (MRI) can be used to characterize and estimate
the extent of soft tissue tumors, this imaging technique is
currently the method of choice for the diagnosis of GCTTS (3). Certain studies have investigated the use
of MRI for the diagnosis of L-GCTTS (3–5). However,
few studies have exclusively clarified the characteristic MRI
features of L-GCTTS and D-GCTTS. Therefore, the present study aimed
to document the MRI and clinical features of L-GCTTS and D-GCTTS by
conducting a retrospective MRI and clinical review of 38 patients
that received a diagnosis of GCTTS via surgery or biopsy,
consisting of 31 patients with L-GCTTS and 7 with D-GCTTS.
Materials and methods
Patients
The present study retrospectively reviewed the MR
images of 38 patients with GCTTS, who were treated and
histologically diagnosed at The Second Affiliated Hospital of
Zhejiang University School of Medicine (Hangzhou, China) between
January 2011 and January 2015. An institutional review board
exemption and a waiver for the requirement of written informed
consent were obtained, facilitating the present study. All the
patients underwent surgical excision. The follow-up length of the
patients ranged between 6 and 60 months.
MR examination
MRI was performed using a 3.0T GE Signa MRI scanner
(GE Healthcare Life Sciences, Chalfont, UK). The scan parameters
were as follows: the time when 63% of the longitudinal
magnetization has recovered (T1)-weighted fast spin echo sequence
[repetition time/echo time (TR/TE), 500/10 msec; slice thickness,
5.0 mm; field of view, 380–520 mm; matrix scan, 256×256]; and T2
weighted turbo-spin echo sequence (TR/TE, 3000/75 msec; slice
thickness, 3.0 mm; field of view, 300–380 mm; matrix scan,
256×256).
Between 0.1 and 0.2 mmol/kg gadolinium-diethylene
triamine pentaacetic acid (Magnevist, Bayer AG, Leverkusen,
Germany), a contrast agent, was administered intravenously to the
patients undergoing contrast-enhanced MRI.
MRI analysis
A total of 2 independent radiologists, who were
aware of the diagnosis of GCTTS but were blind to the surgical
findings, inspected the MRI features of the tumors. A discussion
between the readers would occur subsequent to a disagreement in
order to reach a consensus. The readers evaluated the following
items: margination, signal intensity, signal inhomogeneity,
enhancement, tumor extent and the involvement of adjacent
tissues.
Results
Clinical data
The GCTTS group included 12 males and 26 females
with a mean age of 40 years and a range between 16 and 82 years. In
total, 38 patients consisting of 31 with L-GCTTS and 7 with D-GCTTS
were studied. The L-GCTTS group included 10 males and 21 females
with a mean age of 37 years and a range between 16 and 65 years. Of
the 31 patients with L-GCTTS, 18 of the tumors were located in the
hand and wrist, 10 in the ankle and foot, 2 in the knee joint and 1
in the temporomandibular joint. In total, 8 patients had a history
of trauma directly prior to the appearance of the mass. The mean
duration of symptoms prior to diagnosis was 3 years, with a range
between 1 month and 7 years. A total of 27 patients exhibited
painless soft tissue masses and 4 presented with slight pain. The
masses were solitary, solid and well-defined lesions with good or
poor mobility. Tumor size ranged between 0.8 and 3.2 cm with a mean
size of 3.4±1.4 cm. All patients underwent local tumor excision. In
total, 3 patients developed recurrence subsequent to surgical
excision resulting in a recurrence rate of 10%.
The D-GCTTS group included 2 males and 5 females
with a mean age of 57 years and a range between 36 and 82 years. Of
the 7 patients with D-GCTTS, 6 of the tumors were located in the
ankle and foot and 1 was in the hand and wrist. A total of 2
patients had experienced a trauma directly preceding the appearance
of the mass. The mean duration of symptoms prior to diagnosis in
the D-GCTTS group was 1.5 years, with a range between 1 month and 2
years. Of the 7 patients, 3 exhibited a painless soft tissue mass
and 4 presented with varying degrees of pain. Tumor size ranged
between 1.4 and 8.5 cm with a mean size of 5.8±1.9 cm. All patients
underwent surgical excision. In total, 4 patients developed
recurrence subsequent to surgical excision, resulting in a
recurrence rate of 57.1%.
MRI findings
All 31 patients with L-GCTTS were examined using MRI
and 14/31 patients received contrast medium-enhanced MRI scanning
with a fat suppression sequence. All 31 lesions were located in
association with or partially/completely enveloping a tendon and
were well marginated. On the T1-weighted image (WI), the signal
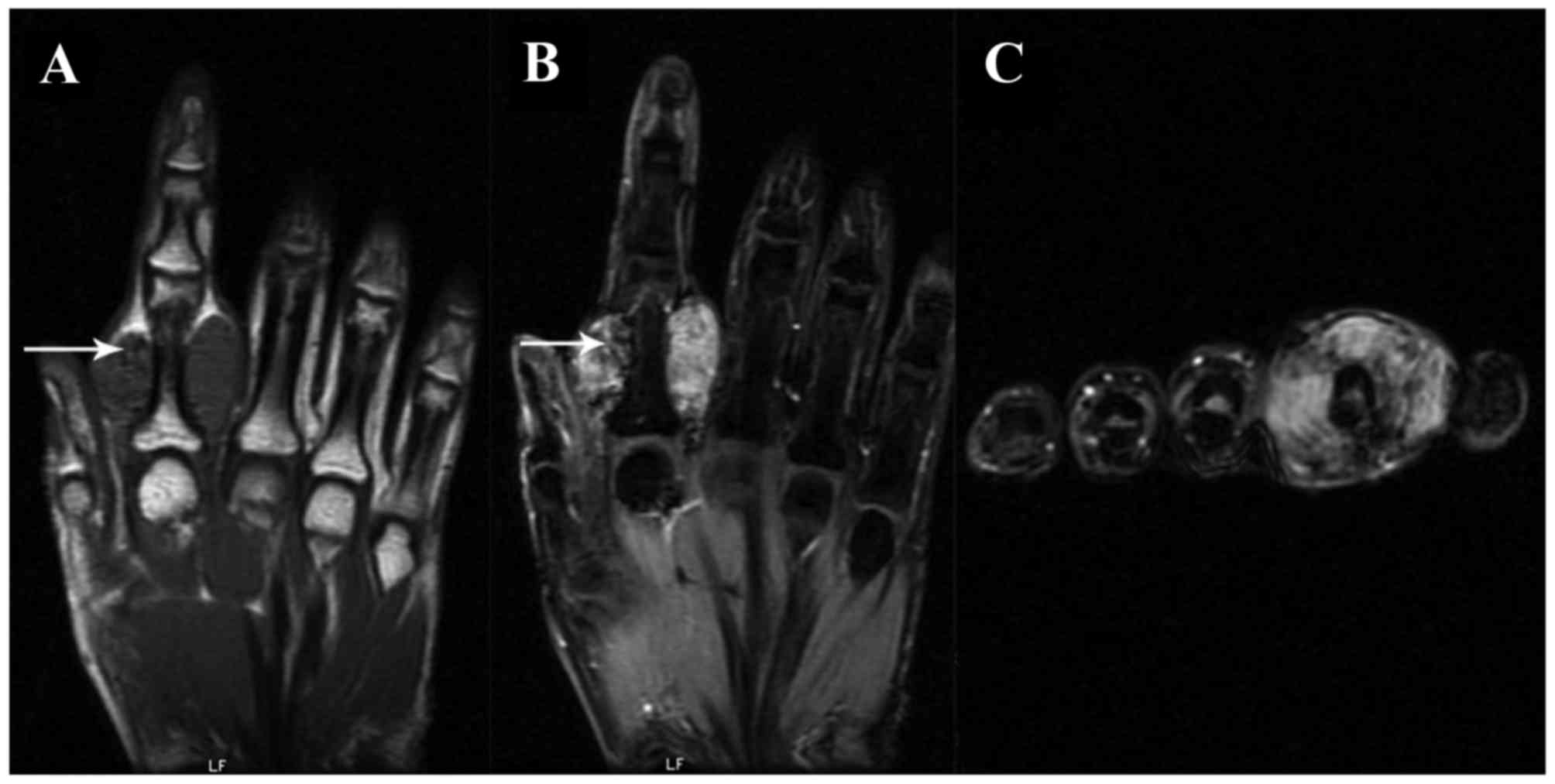
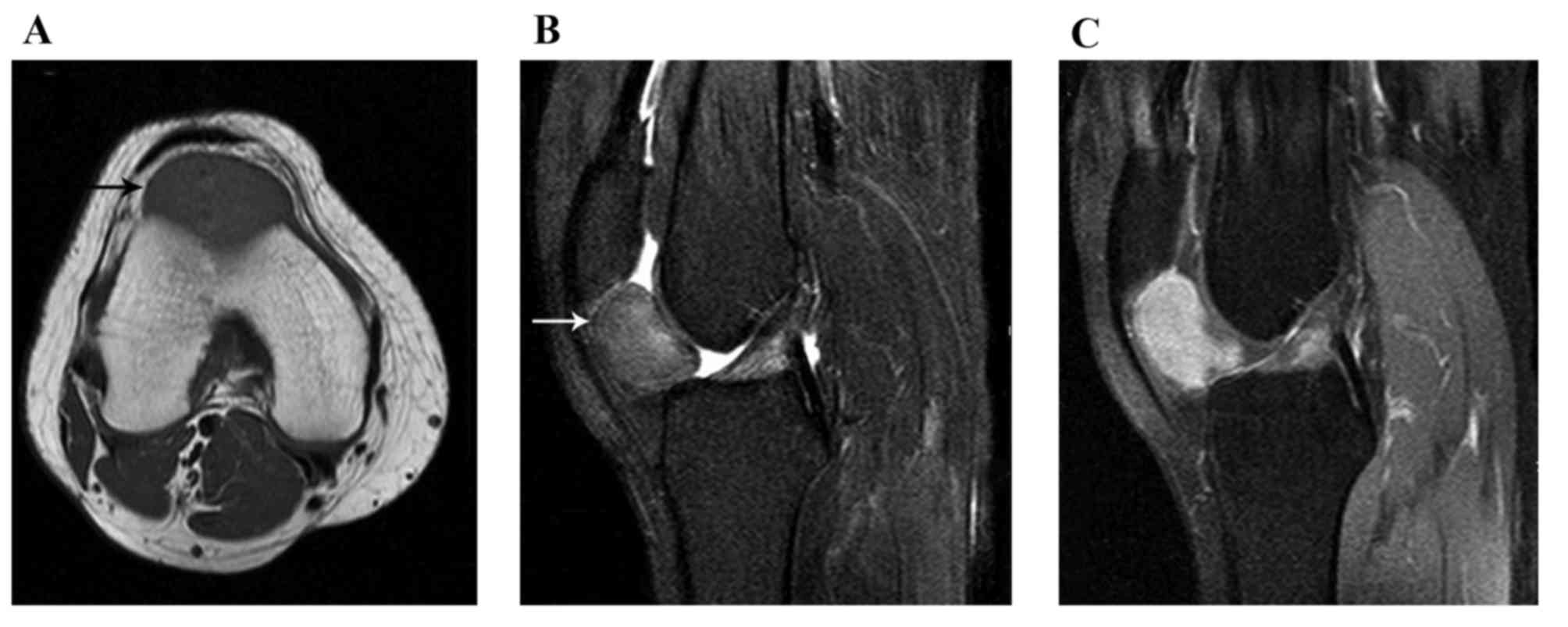
intensities of the L-GCTTS were almost isointense in 26 patients
(Figs. 1 and 2) and were slightly hypointense in 5
patients. On the T2WI, the signal intensities were hyperintense in
27 patients (Figs. 1 and 2) and isointense in 4 patients. Small
scattered foci (Fig. 1) and/or
capsules (Fig. 2) of hypointensity
were observed in all 31 lesions on T1WIs and T2WIs. On the
contrast-enhanced T1WI, heterogeneous enhancement was present in
10/14 patients (Fig. 1) and
homogeneous enhancement in 4 patients (Fig. 2).
All 7 patients with D-GCTTS were examined using MRI
and 5/7 patients received contrast medium-enhanced MRI scanning
with a fat suppression sequence. All 7 lesions presented as an
aggressive soft tissue mass infiltrating the tendon sheath and
adipose tissue around the affected joint. In addition, all 7
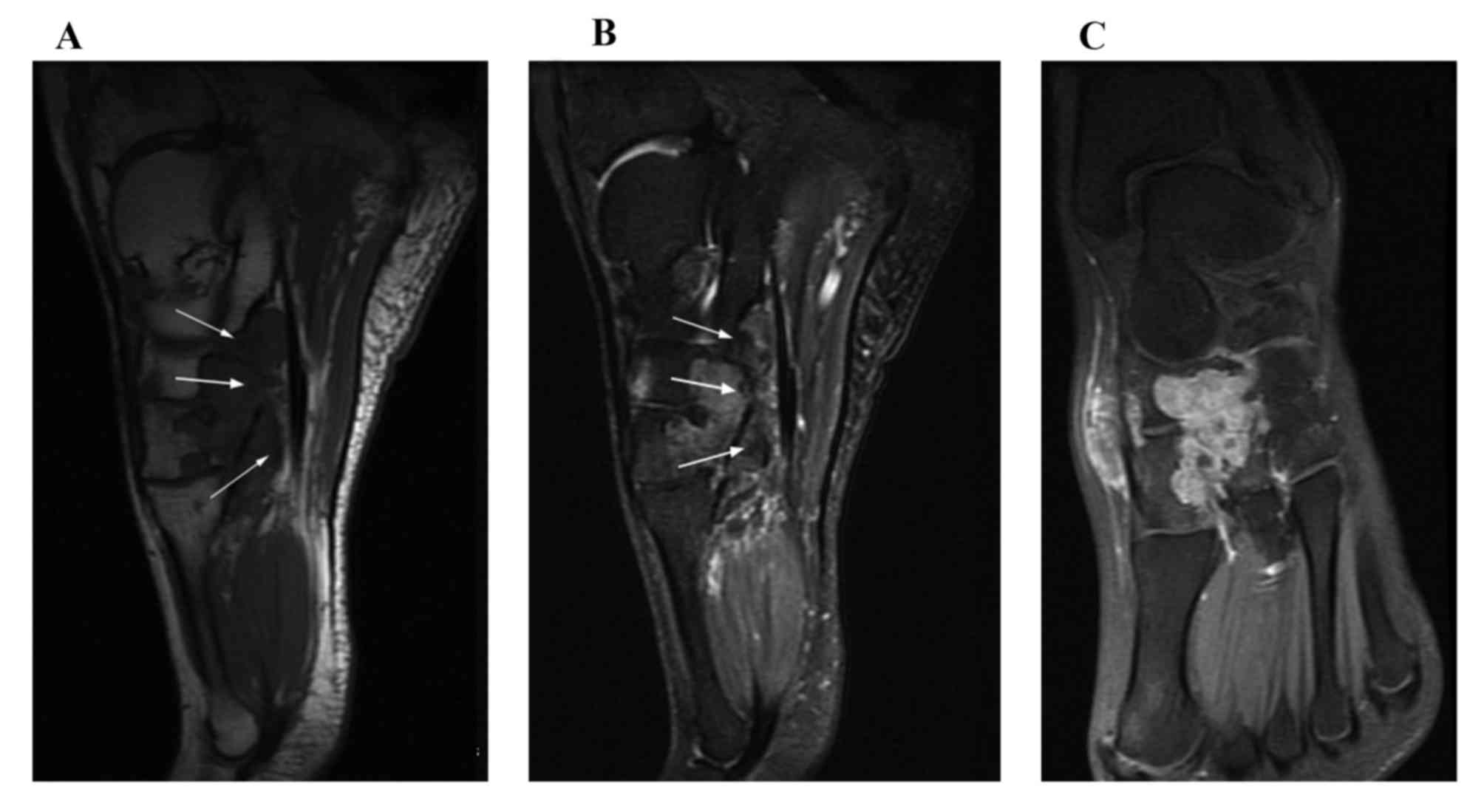
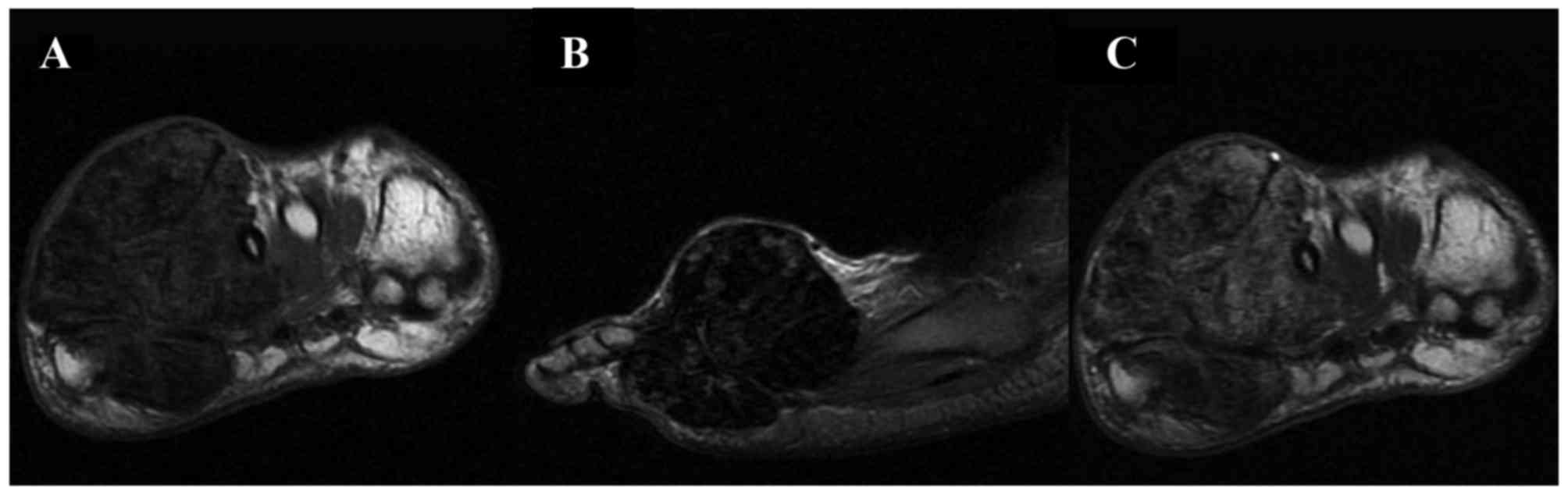
lesions were accompanied by adjacent bone destruction. On the T1WI,
the signal intensities of the D-GCTTS were almost isointense in 6
cases (Fig. 3) and were almost
hypointense in 1 case (Fig. 4). On
the T2WI, the signal intensities were heterogeneously mixed, with
hyperintensity with hypointense areas in 4 cases (Fig. 3), almost hyperintensive levels in 2
cases and almost hypointensive levels in 1 case (Fig. 4). On contrast-enhanced T1WIs, marked
heterogeneous enhancement was present in 4/5 cases (Fig. 3) and intermediate heterogeneous
enhancement was present in 1 case (Fig.
4).
Discussion
GCTTS frequently presents as a firm, slow-growing,
multilobular, non-tender mass located adjacent to the tendon sheath
synovium. The tumor usually affects individuals aged 30–50 years
and females exhibit slight predominance (6,7). GCTTS
occurs in two different clinical presentations: L-GCTTS on fingers
and toes and D-GCTTS that primarily occurs around large joints. The
etiology of D-GCTTS remains to be established, and was previously
termed extra-articular pigmented villonodular synovitis (PVNS) as
it shares similar histological characteristics with PVNS (8). L-GCTTS is the second most common tumor
of the hand following ganglion cysts (9). L-GCTTS primarily occurs in hands
(10), feet and knees (11–13)
whereas D-GCTTS occurs in large load-bearing joints including
knees, hips, ankles, shoulders and elbows (14). Consistent with previous studies
(6,7),
GCTTS mainly affected females and young adults (mean age=41 years)
in the present study. In addition, the present study demonstrated
that L-GCTTS primarily affected young adults (mean age=37 years),
whereas D-GCTTS was more frequently identified in elderly patients
(mean age=57 years). Consistent with previous studies (10–13), the
L-GCTTS tumors in the present study were mainly located in the
hands and feet. However, it may be noted that L-GCTTS of the knee
and temporomandibular joint were rare in the present study. The
difference between the present study and a previous study (14) was that D-GCTTS tumors were primarily
located in ankle and foot (6 of 7 cases) in the present study. The
slight differences between the present study and the previous
study, with respect to the locations of D-GCTTS, may be attributed
to the small sample size of the present study.
L-GCTTS typically exhibits small, scattered foci of
low signal on T1WIs and T2WIs due to the presence of hemosiderin
(15). The lesion may also be
characterized by a low signal intensity capsule as a result of
fibrosis or hemosiderin deposition. L-GCTTS is well delineated and
lobulated with an incomplete fibrous capsule; however, the tumor
may exhibit variability in signal intensity on MR images. De
Beuckeleer et al (4) observed
that the majority of signal intensities of L-GCTTS were isointense
to the signal intensities of muscle on T1WI and T2WI. Jelinek et
al (16) investigated the MRI
features of 9 L-GCTTS. All 9 lesions were hypointense on the T1WI.
On the T2WI, the signal intensities were equal to skeletal muscle
in 2 patients, lower in 3 patients, slightly higher in 2 patients
and more heterogeneous in 2 patients. Kitagawa et al
(5) described the MRI features of 25
cases of L-GCTTS. The signal intensities of L-GCTTS that the
authors observed were isointense to that of skeletal muscle or
hyperintense on the T1WI; on the T2WI, the majority of signal
intensities were hyperintense; and L-GCTTS enhanced following
gadolinium administration. De Beuckeleer et al (4) identified that 10/13 cases of L-GCTTS
exhibited highly homogeneous enhancement due to the presence of
numerous proliferative capillaries in the collagenous stroma.
Kitagawa et al (5) observed
that 13/18 lesions were not homogeneously enhanced whereas 5
lesions exhibited homogeneous enhancement. In the present study
involving 31 patients with L-GCTTS, on the T1WI the signal
intensities of L-GCTTS were isointense in 26 patients and
hypointense in 5 patients. On the T2WI, the signal intensities were
hyperintense in 27 patients and isointense in 4 patients. On
contrast-enhanced T1WI, the majority of signal intensities were
heterogeneously enhanced. These findings were consistent with the
findings of Kitagawa et al (5).
D-GCTTS is less well-defined than L-GCTTS and
generally develops outside the joint, growing in a multinodular
manner that is more irregular than that of L-GCTTS (17). MRI often reveals equal or higher
signals than muscle on T1WI, whereas the features on T2WI vary and
may be characterized by hypointense, isointense or hyperintense
signals (18). Low signal intensity
on the T1WI and T2WI is an indication of the hemosiderin content
typical for this type of tumor (19).
The findings of the present study with respect to D-GCTTS were
consistent with the findings of the aforementioned studies.
Additionally, the present study observed that D-GCTTS is
heterogeneous with larger areas of hypointensity on T1WI and T2WI
compared with L-GCTTS, and has enhanced heterogeneity on
contrast-enhanced T1WIs compared with L-GCTTS. In the present
study, the tumors exhibited predominantly low signal intensities on
T1WI and T2WI. The present study speculates that D-GCTTS possesses
more hemosiderin deposits, which reduce the T2-relaxation time due
to a magnetic susceptibility effect, compared with L-GCTTS. In the
present study, heterogeneous enhancement was present in all the
contrast-enhanced cases, but the association between MR and
histological findings was not evaluated.
In conclusion, L-GCTTS typically presents as a
well-defined mass eccentrically located in association with or
partially/completely enveloping a tendon. D-GCTTS is less
well-defined and more aggressive than L-GCTTS, growing in a
multinodular manner that is more irregular than that of L-GCTTS.
GCTTS typically exhibits a low signal on T1WIs and T2WIs due to the
presence of hemosiderin. D-GCTTS is more heterogeneous with larger
areas of hypointensity on T1WI and T2WI, with enhanced
heterogeneity on contrast-enhanced T1WI compared with L-GCTTS. The
present study demonstrates that the characteristic internal signals
of GCTTS, including L-GCTTS and D-GCTTS, are demonstrated clearly
by MRI examination. MRI is currently the optimal modality for
preoperative assessment of tumor size, extent and invasion of
adjacent joint and tenosynovial space.
References
|
1
|
Sharon WWJ: Enzinger and Weiss's soft
tissue tumors. 4th. St Louis, Mosby: pp. 1037–1054. 2001
|
|
2
|
Fletcher CDMBJ, Hogendoorn P and Mertens
F: WHO Classification of Tumours of Soft Tissue and Bone. WHO;
2013
|
|
3
|
Ho CY and Maleki Z: Giant cell tumor of
tendon sheath: Cytomorphologic and radiologic findings in 41
patients. Diagn Cytopathol. 40:(Suppl 2). E94–E98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Beuckeleer L, De Schepper A, De Belder
F, Van Goethem J, Marques MC, Broeckx J, Verstraete K and Vermaut
F: Magnetic resonance imaging of localized giant cell tumour of the
tendon sheath (MRI of localized GCTTS). Eur Radiol. 7:198–201.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitagawa Y, Ito H, Amano Y, Sawaizumi T
and Takeuchi T: MR imaging for preoperative diagnosis and
assessment of local tumor extent on localized giant cell tumor of
tendon sheath. Skeletal Radiol. 32:633–638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suresh SS and Zaki H: Giant cell tumor of
tendon sheath: Case series and review of literature. J Hand
Microsurg. 2:67–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams EL, Yoder EM and Kasdan ML: Giant
cell tumor of the tendon sheath: Experience with 65 cases. Eplasty.
12:e502012.PubMed/NCBI
|
|
8
|
Ravi V, Wang WL and Lewis VO: Treatment of
tenosynovial giant cell tumor and pigmented villonodular synovitis.
Curr Opin Oncol. 23:361–366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Darwish FM and Haddad WH: Giant cell
tumour of tendon sheath: Experience with 52 cases. Singapore Med J.
49:879–882. 2008.PubMed/NCBI
|
|
10
|
Di Grazia S, Succi G, Fragetta F and
Perrotta RE: Giant cell tumor of tendon sheath: Study of 64 cases
and review of literature. G Chir. 34:149–152. 2013.PubMed/NCBI
|
|
11
|
Villani C, Tucci G, Di Mille M, Di Gennaro
S and Corsi A: Extra-articular localized nodular synovitis (giant
cell tumor of tendon sheath origin) attached to the subtalar joint.
Foot Ankle Int. 17:413–416. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheppard DG, Kim EE, Yasko AW and Ayala A:
Giant-cell tumor of the tendon sheath arising from the posterior
cruciate ligament of the knee: A case report and review of the
literature. Clin Imaging. 22:428–430. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thaxton L, AbuRahma AF, Chang HH and
Boland JP: Localized giant cell tumor of tendon sheath of upper
back. Surgery. 118:901–903. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Somerhausen NS and Fletcher CD:
Diffuse-type giant cell tumor: Clinicopathologic and
immunohistochemical analysis of 50 cases with extraarticular
disease. Am J Surg Pathol. 24:479–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sherry CS and Harms SE: MR evaluation of
giant cell tumors of the tendon sheath. Magn Reson Imaging.
7:195–201. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jelinek JS, Kransdorf MJ, Shmookler BM,
Aboulafia AA and Malawer MM: Giant cell tumor of the tendon sheath:
MR findings in nine cases. AJR Am J Roentgenol. 162:919–922. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wan JM, Magarelli N, Peh WC, Guglielmi G
and Shek TW: Imaging of giant cell tumour of the tendon sheath.
Radiol Med. 115:141–151. 2010.(In English, Italian). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang K, Zhu B, Yang S, Liu Z, Yu M and Liu
X: Primary diffuse-type tenosynovial giant cell tumor of the spine:
A report of 3 cases and systemic review of the literature. Turk
Neurosurg. 24:804–813. 2014.PubMed/NCBI
|
|
19
|
Bredell M, Schucknecht B and
Bode-Lesniewska B: Tenosynovial, diffuse type giant cell tumor of
the temporomandibular joint, diagnosis and management of a rare
tumor. J Clin Med Res. 7:262–266. 2015. View Article : Google Scholar : PubMed/NCBI
|