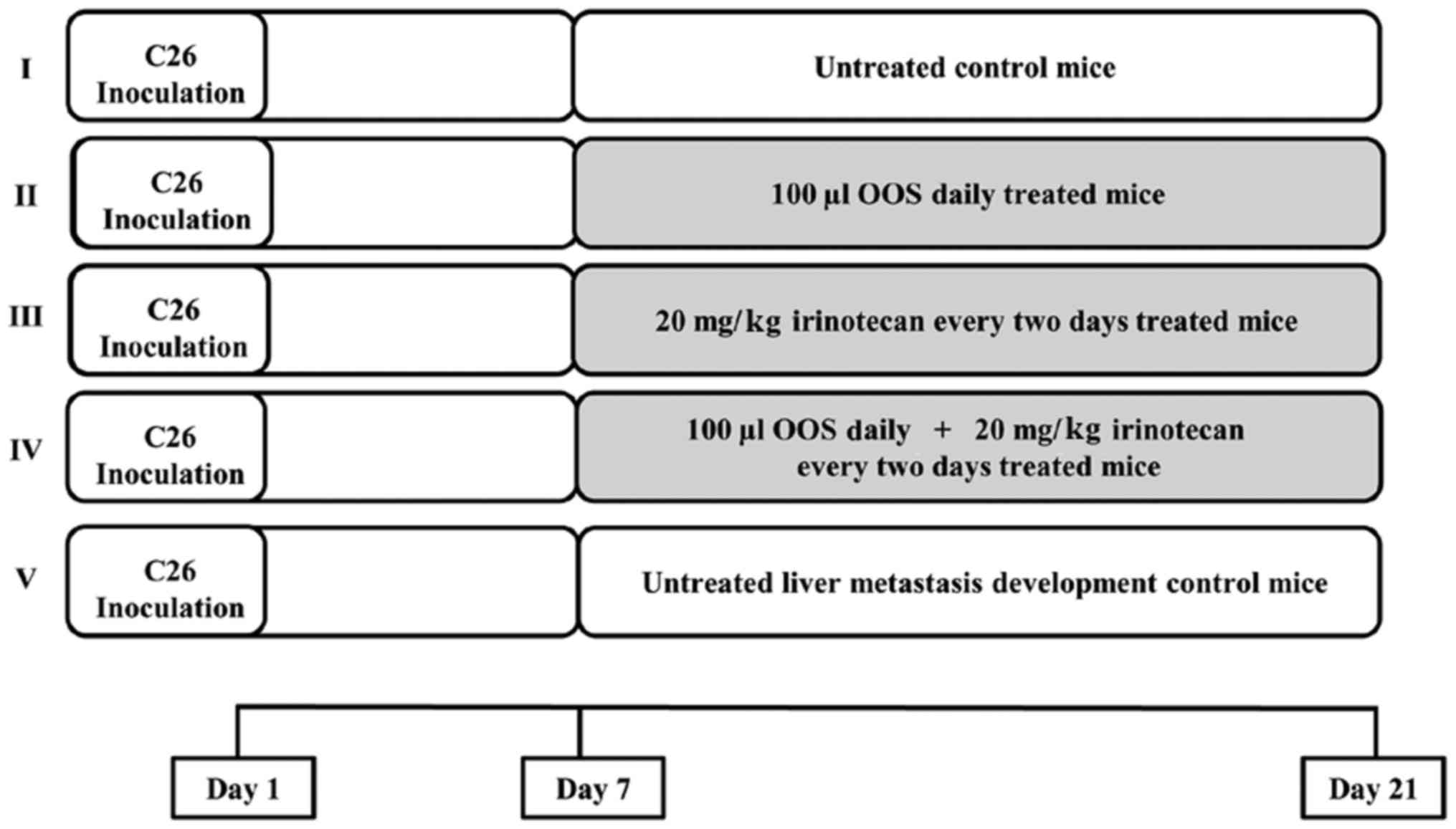
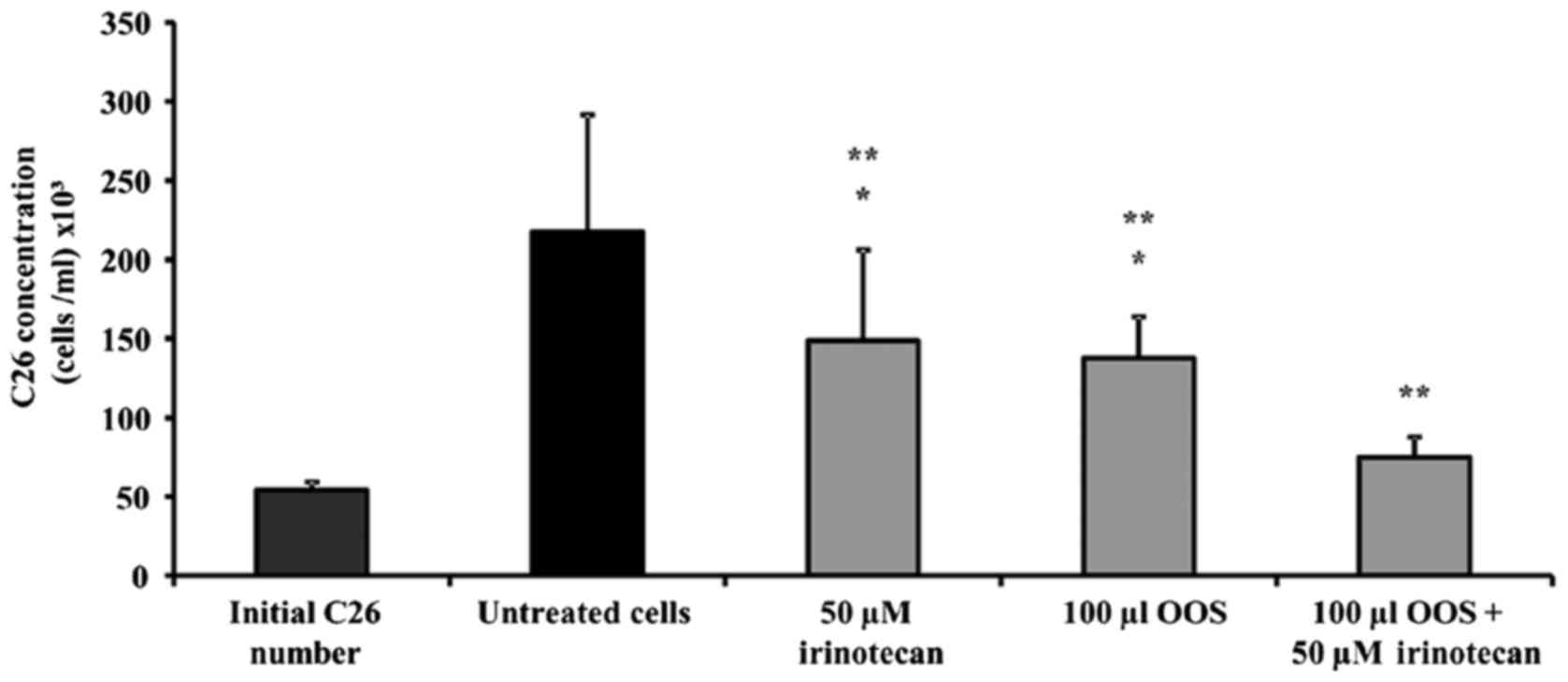
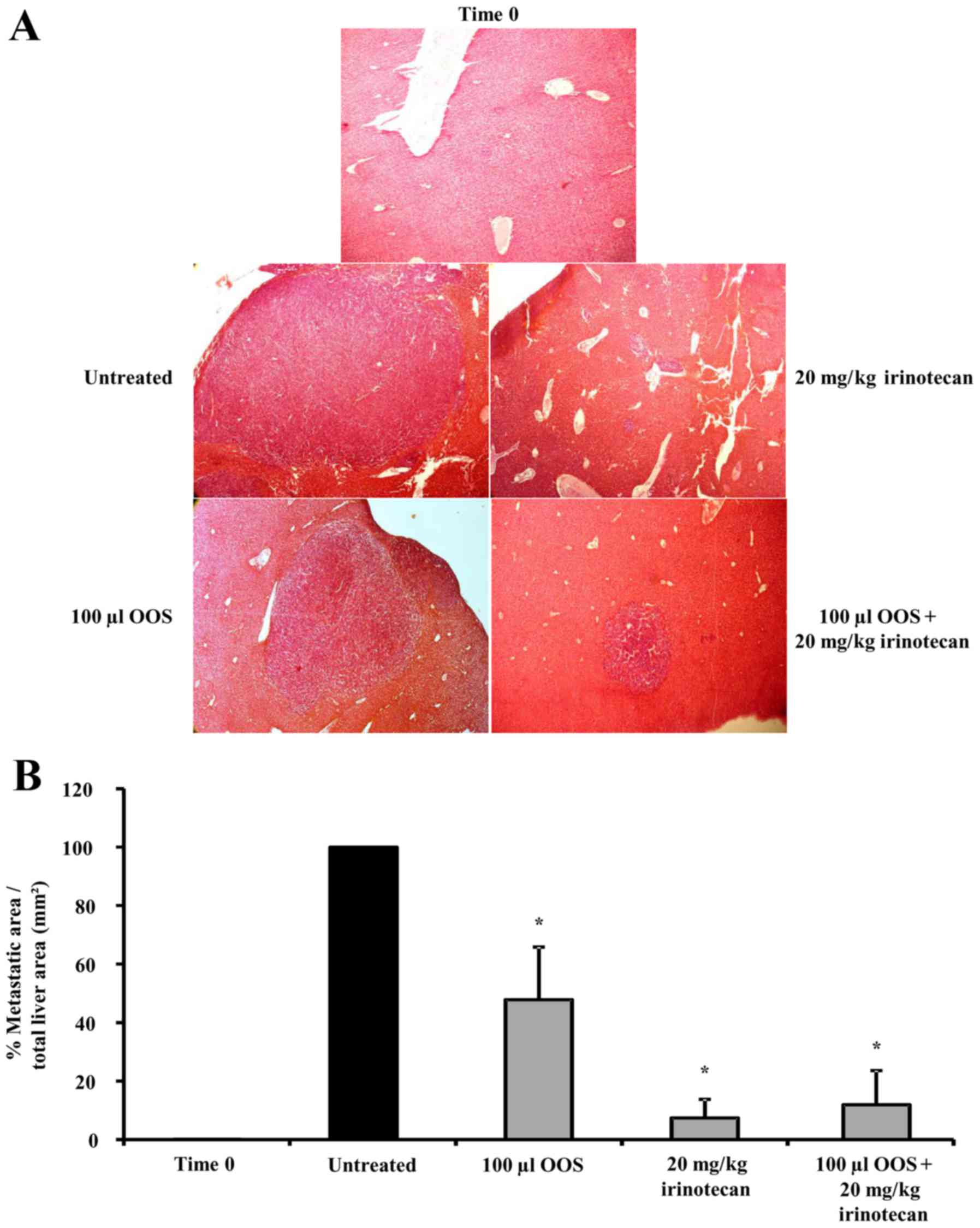
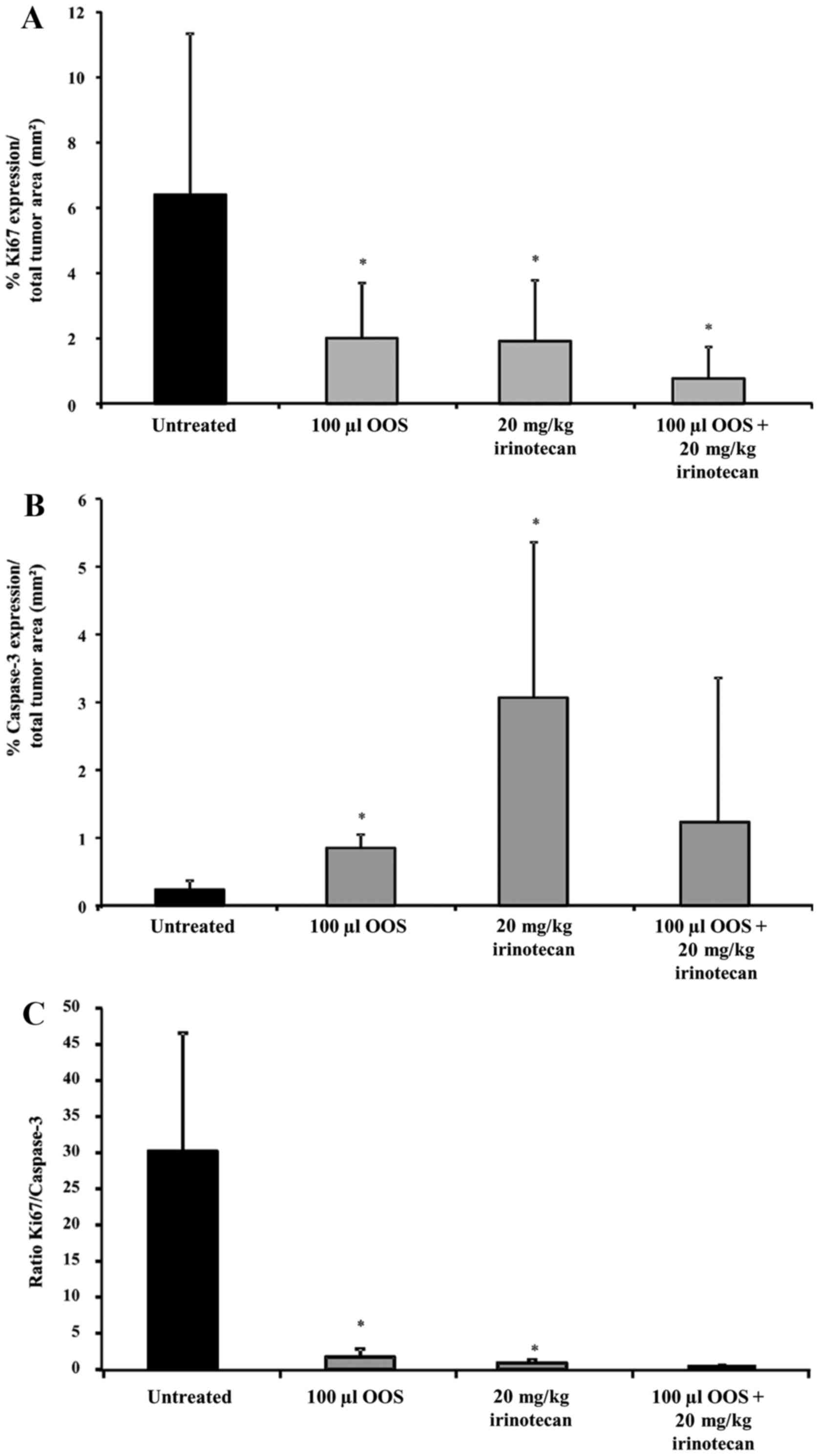
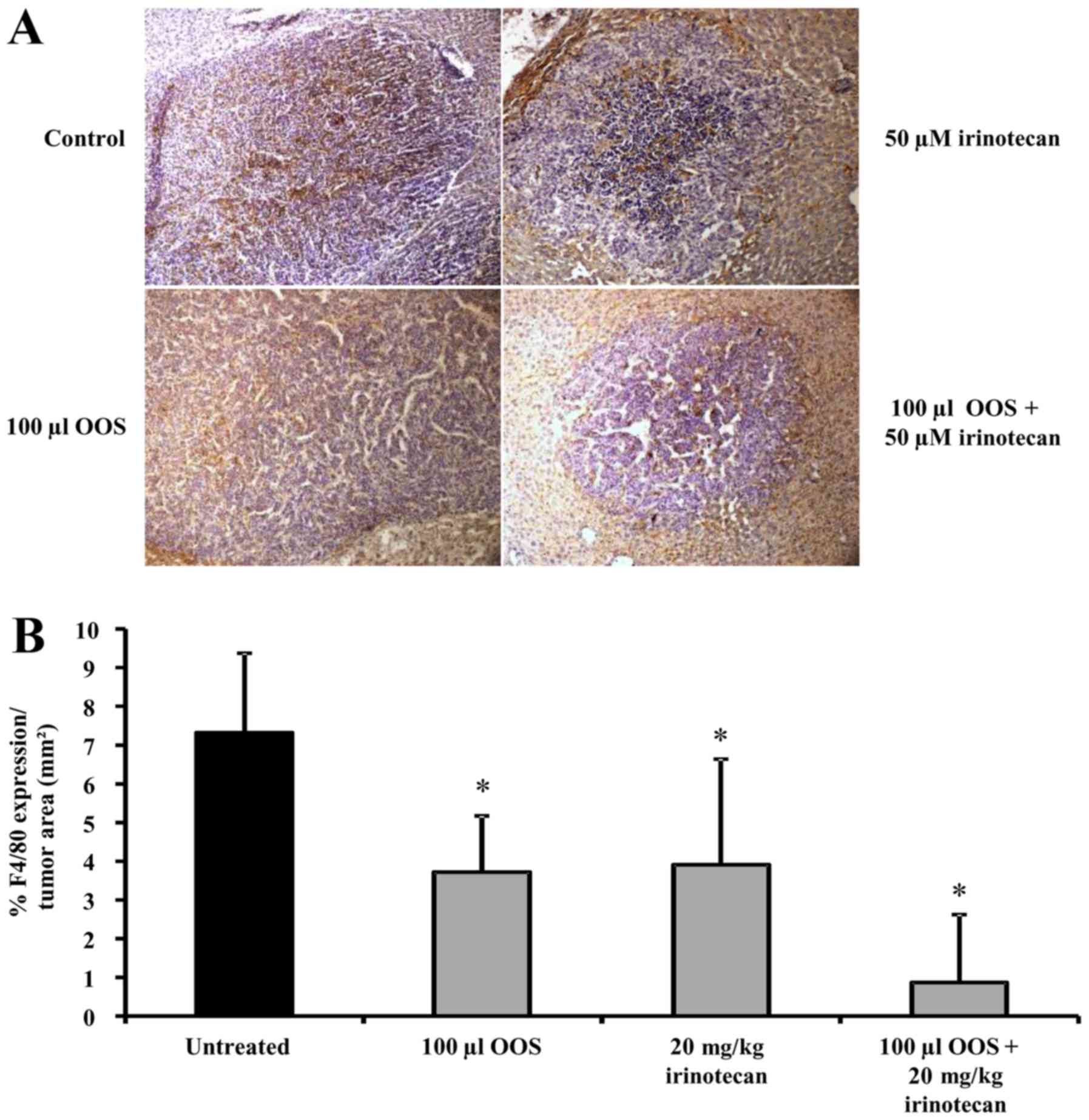
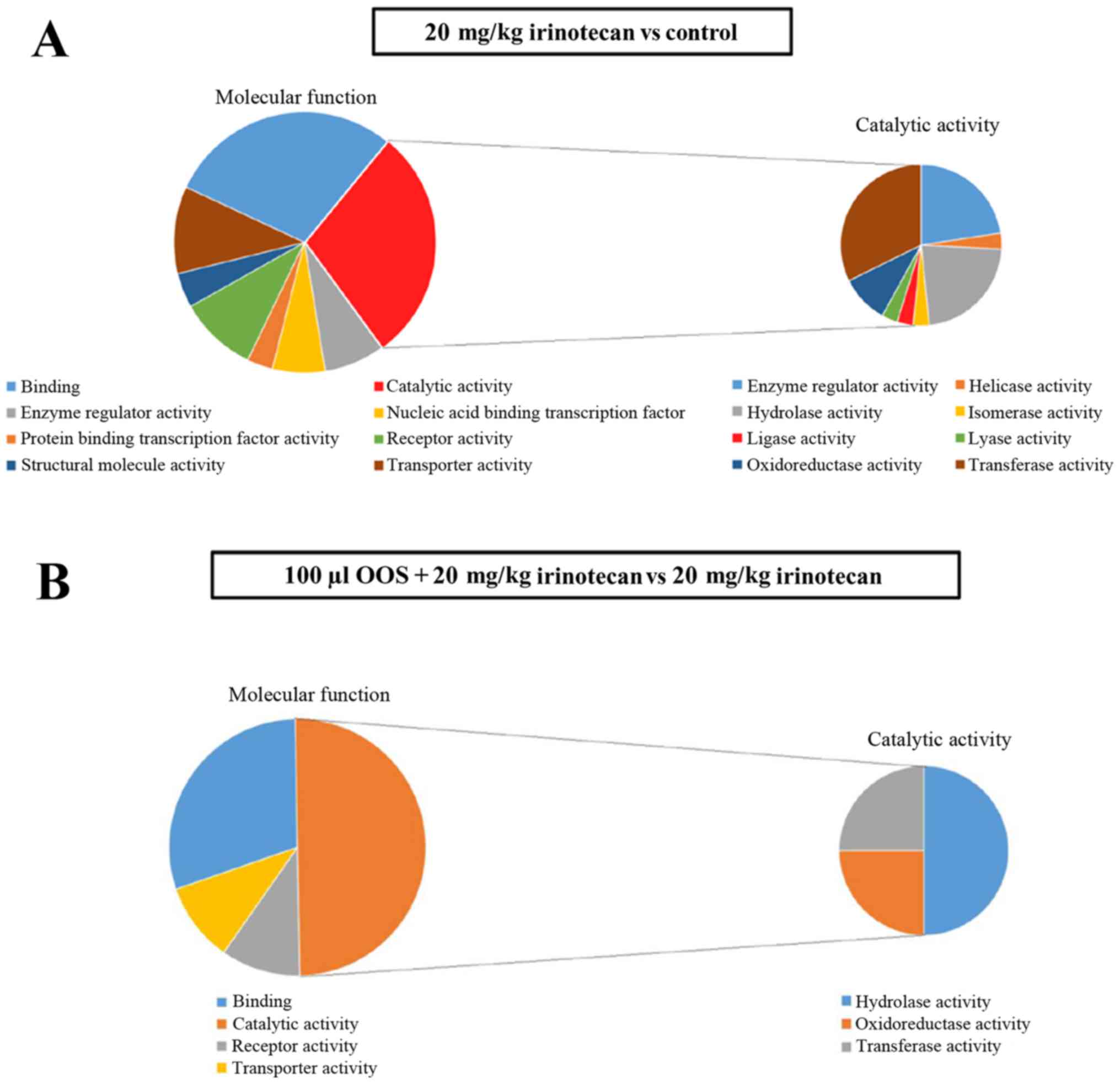
|
1
|
Fujita K, Kubota Y, Ishida H and Sasaki Y:
Irinotecan, a key chemotherapeutic drug for metastatic colorectal
cancer. World J Gastroenterol. 21:12234–12248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van den Eynden GG, Majeed AW, Illemann M,
Vermeulen PB, Bird NC, Høyer-Hansen G, Eefsen RL, Reynolds AR and
Brodt P: The multifaceted role of the microenvironment in liver
metastasis: Biology and clinical implications. Cancer Res.
73:2031–2043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gol'dberg ED, Amosova EN, Zueva EP, Razina
TG, Krylova SG and Zorikov PS: Licorice preparations improve
efficiency of chemotherapy and surgical treatment of transplanted
tumors. Bull Exp Biol Med. 145:252–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kontek R, Drozda R, Sliwiński M and
Grzegorczyk K: Genotoxicity of irinotecan and its modulation by
vitamins AC and E in human lymphocytes from healthy individuals and
cancer patients. Toxicol In Vitro. 24:417–424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arabski M, Kazmierczak P,
Wisniewska-Jarosinska M, Poplawski T, Klupinska G, Chojnacki J,
Drzewoski J and Blasiak J: Interaction of amoxicillin with DNA in
human lymphocytes and H. pylori-infected and non-infected gastric
mucosa cells. Chem Biol Interact. 152:13–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hernández-Garcia S, González V, Sanz E and
Pandiella A: Effect of oncoxin oral solution in HER2-overexpressing
breast cancer. Nutr Cancer. 67:1159–1169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Márquez J, Mena J, Hernandez-Unzueta I,
Benedicto A, Sanz E, Arteta B and Olaso E: Ocoxin® oral
solution slows down tumor growth in an experimental model of
colorectal cancer metastasis to the liver in balb/c mice. Oncol
Rep. 35:1265–1272. 2016.PubMed/NCBI
|
|
8
|
Diaz-Rodriguez E, Hernández-Garcia S, Sanz
E and Pandiella A: Antitumoral effect of ocoxin on acute myeloid
leukemia. Oncotarget. 7:6231–6242. 2016.PubMed/NCBI
|
|
9
|
Mi H, Muruganujan A, Casagrande JT and
Thomas PD: Large-scale gene function analysis with the PANTHER
classification system. Nat Protoc. 8:1551–1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mi H, Poudel S, Muruganujan A, Casagrande
JT and Thomas PD: PANTHER version 10: Expanded protein families and
functions, and analysis tools. Nucleic Acids Res. 44(D1):
D336–D342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langford DJ, Bailey AL, Chanda ML, Clarke
SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T,
Lacroix-Fralish ML, et al: Coding of facial expressions of pain in
the laboratory mouse. Nat Methods. 7:447–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mego M, Chovanec J, Vochyanova-Andrezalova
I, Konkolovsky P, Mikulova M, Reckova M, Miskovska V, Bystricky B,
Beniak J, Medvecova L, et al: Prevention of irinotecan induced
diarrhea by probiotics: A randomized double blind, placebo
controlled pilot study. Complement Ther Med. 23:356–362. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mikalauskas S, Mikalauskiene L, Bruns H,
Nickkholgh A, Hoffmann K, Longerich T, Strupas K, Büchler MW and
Schemmer P: Dietary glycine protects from chemotherapy-induced
hepatotoxicity. Amino Acids. 40:1139–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dayem-Uddin M, Islam M, Mahmood I, Gosh A,
Khatun R and Kundu S: Findings of the 3-month supportive treatment
with oncoxin solution beside the standard modalities of patients
with different neoplastic diseases. TAJ. 22:172–175. 2009.
|
|
15
|
Kim JA, Kim DH, Hossain MA, Kim MY, Sung
B, Yoon JH, Suh H, Jeong TC, Chung HY and Kim ND: HS-1793, a
resveratrol analogue, induces cell cycle arrest and apoptotic cell
death in human breast cancer cells. Int J Oncol. 44:473–480.
2014.PubMed/NCBI
|
|
16
|
Gullett NP, Amin AR Ruhul, Bayraktar S,
Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ and Kucuk O:
Cancer prevention with natural compounds. Semin Oncol. 37:258–281.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mehta RG, Murillo G, Naithani R and Peng
X: Cancer chemoprevention by natural products: How far have we
come? Pharm Res. 27:950–961. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho YH, Yoon SY and Kim SN: Irinotecan
monotherapy versus irinotecan-based combination as second-line
chemotherapy in advanced gastric cancer: A meta-analysis. Cancer
Res Treat. 49:255–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kachalaki S, Ebrahimi M, Khosroshahi L
Mohamed, Mohammadinejad S and Baradaran B: Cancer chemoresistance;
biochemical and molecular aspects: A brief overview. Eur J Pharm
Sci. 89:20–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stringer AM, Gibson RJ, Logan RM, Bowen
JM, Yeoh AS, Laurence J and Keefe DM: Irinotecan-induced mucositis
is associated with changes in intestinal mucins. Cancer Chemother
Pharmacol. 64:123–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stringer AM, Gibson RJ, Bowen JM, Logan
RM, Ashton K, Yeoh AS, Al-Dasooqi N and Keefe DM:
Irinotecan-induced mucositis manifesting as diarrhoea corresponds
with an amended intestinal flora and mucin profile. Int J Exp
Pathol. 90:489–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kung HN, Weng TY, Liu YL, Lu KS and Chau
YP: Sulindac compounds facilitate the cytotoxicity of β-lapachone
by up-regulation of NAD(P)H quinone oxidoreductase in human lung
cancer cells. PLoS One. 9:e881222014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei D, Zhang X, Zou H, Wang L, Fu B, Wu X,
Luo Z, Li X, Ge J, Li Y, et al: WW domain containing oxidoreductase
induces apoptosis in gallbladder-derived malignant cell by
upregulating expression of P73 and PUMA. Tumour Biol. 35:1539–1550.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinho SS and Reis CA: Glycosylation in
cancer: Mechanisms and clinical implications. Nat Rev Cancer.
15:540–555. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sheta R, Woo CM, Roux-Dalvai F, Fournier
F, Bourassa S, Droit A, Bertozzi CR and Bachvarov D: A metabolic
labeling approach for glycoproteomic analysis reveals altered
glycoprotein expression upon GALNT3 knockdown in ovarian cancer
cells. J Proteomics. 145:91–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feferman L, Bhattacharyya S, Deaton R,
Gann P, Guzman G, Kajdacsy-Balla A and Tobacman JK: Arylsulfatase B
(N-acetylgalactosamine-4-sulfatase): Potential role as a biomarker
in prostate cancer. Prostate Cancer Prostatic Dis. 16:277–284.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brinkmann K, Zigrino P, Witt A, Schell M,
Ackermann L, Broxtermann P, Schüll S, Andree M, Coutelle O,
Yazdanpanah B, et al: Ubiquitin C-terminal hydrolase-L1 potentiates
cancer chemosensitivity by stabilizing NOXA. Cell Rep. 3:881–891.
2013. View Article : Google Scholar : PubMed/NCBI
|