Over the past decade, the incidence of pancreatic
adenocarcinoma (PAC) has been increasing, and it accounts for 2.8%
of new malignancies and 6.2% of all cancer mortalities, and is the
fourth leading cause of cancer-associated mortality in the United
States, according to the American Cancer Society (1,2). The
5-year relative overall survival (OS) rate is 6%, with a low level
of improvement over the previous 3 decades compared to other types
of tumors (1,2). Surgical resection is the only potential
curative treatment. Poor prognosis is mainly associated with late
diagnosis, low resection rate and aggressiveness of neoplasia. PAC
presents as locally advanced (LAPAC) or metastatic in the majority
of patients; thus, only 10–20% of patients are eligible for
curative surgery (2).
For many years, physicians have largely ignored the
tumor node metastasis staging system (3) to categorize patients into three
different groups: resectable, LA and metastatic PAC. At present,
with improved imaging techniques and a renewed focus on surgical
expertise, a fourth category has been proposed: borderline
resectable (BRPAC). These patients must be identified a
priori, as the goals of treatment differ from those in patients
with truly unresectable PAC (4).
Numerous anatomic definitions of BRPAC have been proposed, with the
greatest points of contention revolving around the extent of
involvement of the superior mesenteric vein (SMV) (5–7).
More effective systemic regimens, compared with
single agent gemcitabine treatment, have emerged in the treatment
of PAC. Large randomized trials of FOLFIRINOX (folinic acid,
fluorouracil, irinotecan and oxaliplatin), or gemcitabine with
nanoparticle albumin-bound (nab)-paclitaxel have revealed
significantly improved overall survival (OS) and response rates
(RRs) compared with those associated with single-agent gemcitabine
(8,9).
An increasing level of evidence supports the use of neoadjuvant
strategies aiming to control micrometastatic disease, induce tumor
shrinkage and achieve resection for cure/complete remission
(R0).
In September 2014, a 58-year-old female patient was
presented at the Lugano Regional Hospital (Lugano, Switzerland)
with long-lasting diabetes, abdominal pain and weight loss. The
Eastern Cooperative Oncology Group performance status was 1, and
laboratory parameters were normal, with the exception of
carbohydrate antigen (CA) 19–9, which was 149.2 U/ml (normal range:
<35.4 U/ml), and carcinoembryonic antigen, which was 59.1 ng/ml
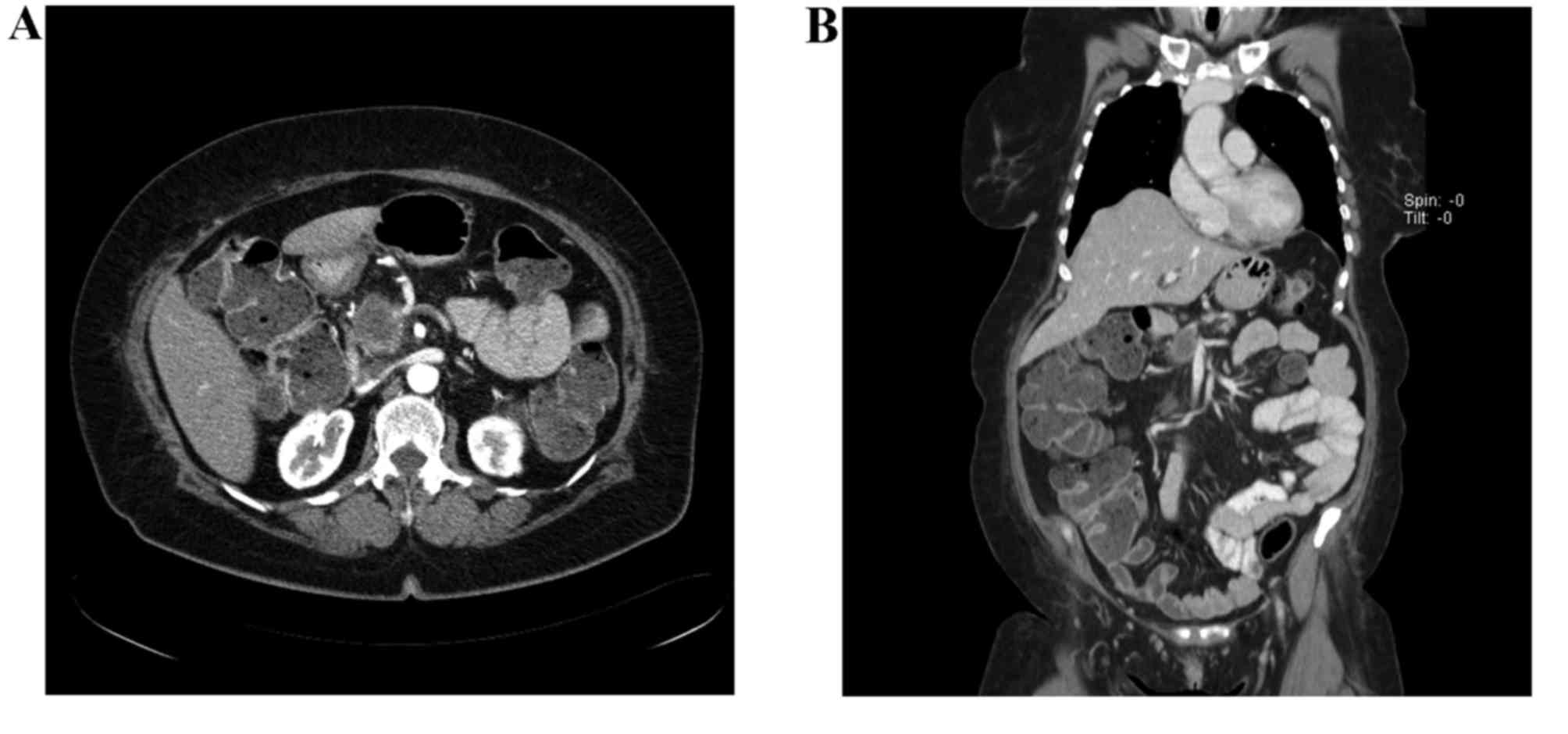
(normal range <3.0 ng/ml). A computed tomography (CT) scan
revealed a 3.7-cm mass in the pancreatic head, encasing the
porto-mesenteric axis ≥180°, with narrowing and almost complete
short segment occlusion of the SMV, and superior mesenteric artery
(SMA) abutment (Fig. 1A and B). There
was no evidence of lymph node involvement or visceral metastatic
spread. The result of an endoscopic ultrasound-guided fine needle
biopsy was concordant with adenocarcinoma, and the patient was
diagnosed with BRPAC.
In February 2015, the patient underwent a
pylorus-preserving cephalic duodenopancreatectomy without vascular
resection, since neither visceral spread nor arterial infiltration
were observed intra-operatively. No macroscopic evidence of tumor
was observed. A fibrous-like tissue was observed and the entire
specimen was sent for microscopic examination. Neither residual
tumor cells nor metastases in 14 regional lymph nodes were
detected, and the surgical margins were negative. These findings
were consistent with a pathological complete response (pCR).
Pulmonary embolism occurred postoperatively, but the patient fully
recovered. Adjuvant chemotherapy was suggested, but the patient
refused due to a general worsening of condition following surgery.
In June 2015, the patient complained of abdominal discomfort, and a
CT scan revealed multiple hepatic metastasis. Chemotherapy with
gemcitabine and nab-paclitaxel was administered for 2 months, but
the disease progressed and the patient succumbed to the disease in
October 2015. The patient gave us oral and written informed consent
prior submission of the manuscript.
BRPAC is a novel entity characterized by the limited
involvement of the superior mesenteric vessels (SMA and SMV),
celiac axis and hepatic artery. Although skill-demanding, resection
is technically feasible in BRPAC, but is associated with a high
risk of positive margins and, consequently, of early recurrence
(6). The present case describes a
patient with BRPAC who achieved pCR subsequent to neoadjuvant
FOLFIRINOX, but relapsed following surgery. The present case raises
certain issues.
Firstly, a multidisciplinary approach is required to
properly assess the resectability of the PAC. There are unresolved
issues with respect to the definition of BRPAC, including how much
of the SMV or major visceral arteries must be surrounded on CT scan
to diagnose BR or LA unresectable, or whether or not the
requirement to replace a segment of vein defines resectability.
SMV, SMA, celiac artery and hepatic artery involvement have been
identified as an anatomic point of contention, and numerous
definitions have been proposed (5–7,10). The patient in the present case was
classified as BRPAC based on porto-mesenteric involvement,
segmental SMV occlusion and SMA abutment.
Modern imaging techniques allow accurate
preoperative staging, although there is no definite consensus on
the most preferable approach. CT scans are ~80% accurate with
respect to predicting resectability and almost 90% accurate in
assessing vascular invasion (11). In
experienced hands, accuracy of endoscopic ultrasound is 75–95 and
74–87% in assessing T and N stages, respectively (12–14).
Endoscopic ultrasound is particularly useful in identifying lesions
<2 cm, and may characterize the presence of vascular invasion or
venous thrombosis (12–14). There are limited data on the role of
positron emission tomography (PET)/CT in the staging of potentially
resectable tumors. In a retrospective series, PET/CT has been shown
to change the management in 17% of patients with BRPAC and 7% with
resectable PAC (P=0.019), ultimately preventing unnecessary surgery
(15). The accuracy of imaging in
determining resectability has also been evaluated in patients with
BRPAC who underwent neoadjuvant therapy, and response evaluation
criteria in solid tumors (RECIST) criteria resulted unreliable to
select patients for surgery (16).
Although only 0.8% of patients experienced downstaging to
resectable status subsequent to receiving neoadjuvant therapy, 66%
of patients underwent pancreatectomy. The OS for 129 patients was
22 months, whilst the OS of the patients who underwent
pancreatectomy was 33 months and was not correlated with RECIST
response (P=0.78) (16). In a
different study, neoadjuvant FOLFIRINOX resulted in a significant
decrease in tumor size, however 47% of patients with BRPAC or LAPAC
remained classified as unresectable. However, 92% of the patients
underwent an R0 resection, suggesting that traditional imaging no
longer predicts unresectability (17). Thus, it may be speculated that
hyperdensity surrounding the major vessels considered to be
neoplastic becomes, or was initially, fibrotic, possibly explaining
the absence of radiological changes.
Secondly, besides overall health status, the
presence of significant comorbidities and nutritional deficiency
(18), understanding tumor biology
may assist clinicians in selecting patients for surgery. No
validated prognostic biomarkers predict recurrence following
resection. A study evaluated the association between CA 19–9 and
surgical outcomes in BRPAC (19).
Normalization of CA 19–9 following neoadjuvant therapy was
associated with longer OS in resected (38 vs. 26 months, P=0.020)
and unresected (15 vs. 11 months, P=0.022) patients. Conversely,
failure of CA 19–9 to normalize was revealed to be an independent
factor of shorter OS [hazard ratio (HR)=2.13; P=0.001].
Patients exhibiting wild-type SMAD family member 4
(SMAD4) gene were shown to display a lower propensity for
metastases than those exhibiting the loss of SMAD4 (20). However, a different study failed to
demonstrate an association between SMAD4 messenger RNA expression
and OS (21). Secreted protein acidic
and cysteine rich (SPARC) is a glycoprotein expressed by stromal
cells surrounding the tumor, and is involved in cell migration and
tissue remodeling (20). Patients who
expressed SPARC in tumor stroma exhibited a significantly worse
prognosis than those who did not (median OS, 15 vs. 30 months;
P<0.001) (22), a result that has
been supported by additional studies (23,24). SPARC
is known to bind to albumin (25). By
binding to SPARC inside the extracellular matrix, nab-paclitaxel
successfully disrupts the organization of tumor cells and induces a
marked alteration in tumor architecture, resulting in increased
tumor softening and permeability (25). In human epidermal growth factor
receptor 2-positive tumors, nab-paclitaxel was shown to be
equivalent or even superior to polysorbate-based docetaxel in
tumors with medium to high SPARC levels (26).
PAC cells overexpress epidermal growth factor
receptor, and previous studies have demonstrated correlations
between receptor/ligand co-expression and larger tumors, advanced
stage and decreased OS (27,28). Thymidylate synthase (TS) has been
evaluated in 132 resected patients, and the median OS resulted
improved in those with low TS expression compared with patients
exhibiting high TS expression, which resulted in TS being an
independent predictor of mortality at multivariate analysis
(29). However, a similar study
investigating TS expression reported conflicting results (30). Finally, non-coding RNAs have been
observed to be deregulated in numerous types of human cancer.
Studies have shown that non-coding RNAs affect the progression of
PAC, and RNA profiling may assist the prediction of outcomes with
high accuracy (31).
Thirdly, the present case reflects the growing
recognition accorded to neoadjuvant strategies to improve OS in
pancreatic cancer, a highly fatal disease. The main potential
advantages of neoadjuvant strategies are: i) increased
resectability and likelihood of margin-negative resection, which is
relevant, as patients with BRPAC or LAPAC exhibit a similar
prognosis to those with immediately resectable PAC if R0 resection
can be achieved (10); ii) increased
likelihood of completion of multimodal treatment, which is possibly
the most effective way to improve the outcome of patients with
BRPAC or LAPAC; iii) prevention of unnecessary surgery in
aggressive, treatment-resistant disease; iv) evaluation of
chemo-sensitivity and increased patient's compliance (32); v) minimization of pancreatic leak
without increase of postoperative complications (33–37); and
vi) cost-effectiveness (38).
With regards to chemotherapy, the optimal
neoadjuvant regimen has not been established to date, as current
evidence arises from small, single-institution, non-randomized
trials. These studies are difficult to interpret, as they have used
various definitions of BRPAC, different induction and
post-resection regimens, and, if applied, incorporated varied
radiation therapy plans (38–42). The most active regimens for advanced
disease offer the best chance of achieving downstaging and systemic
disease control. FOLFIRINOX regimen resulted in a statistically
significant increase in OS (11.1 vs. 6.8 months; P<0.001) and RR
(31.6 vs. 9.4%; P<0.001) compared with the results observed with
gemcitabine in patients with metastatic disease (6). Thus, FOLFIRINOX has been incorporated in
neoadjuvant trials for BRPAC and LAPAC (Table I), either alone or in combination with
chemoradiation (43–59).
In a meta-analysis of 13 of the aforementioned
studies involving 253 patients, resection rate and R0 resection
were achieved in 43.0 and 39.4% of patients, respectively. R0
resection was possible in 63.5% of patients with BRPAC and 22.5% of
patients with LAPAC (60). Three
trials reported an OS between 13.7 and 24.2 months (60), compared with the OS of ~2 years of
patients who complete adjuvant therapy subsequent to upfront
surgery (61,62).
In the single-arm pilot study Alliance A021101, 22
patients with BRPAC were treated with 4 cycles of modified
FOLFIRINOX (FOLFIRINOX without fluorouracil bolus) followed by
chemoradiation prior to pancreatectomy, and an additional 2 cycles
of adjuvant gemcitabine. In total, 68% of patients underwent
pancreatectomy, and of those patients, R0 resection was achieved
for 93% while pCR was observed in 9% (63). The single-arm phase IIa Pancreatic
Resectability in Cancers with Known Limited Extension trial is
currently ongoing, and evaluates gemcitabine and nab-paclitaxel in
a neoadjuvant setting (64).
FOLFIRINOX exhibits a relatively complex toxicity profile, which
may be a limitation of applicability. In a neoadjuvant setting,
grade 3–4 adverse events were consistent with those reported in the
original publication (8), mainly
neutropenia (3–20%, with a low rate of febrile neutropenia using
granulocyte stimulating factors) and diarrhea (≤18%) (60).
Pancreatoduodenectomy is associated with a high
morbidity rate (30–60%), and neoadjuvant therapy may put patients
at a higher risk of complications such as wound infections,
anastomotic leaks, intra-abdominal abscesses and mortality. The
morbidity and mortality rates described in neoadjuvant studies are
similar to those reported upon pancreatoduodenectomy in high-volume
centers, suggesting that surgery subsequent to neoadjuvant
chemotherapy is safe (33,34,65). In
particular, no fistulae, a major complication of PAC resection,
were reported (17,49). Although neoadjuvant therapy may allow
resection in patients with an initially unresectable disease, the
high incidence of recurrence, as in the present case, emphasizes
the systemic behavior of the disease. This raises the question if
palliative systemic chemotherapy and/or chemoradiation may achieve
the same outcome, avoiding the morbidity and potential mortality of
surgery (66). In the future,
biomarkers may assist clinicians in decisional processes. For
example, the DCP4 gene was revealed to be highly correlated with
the presence of widespread metastasis but not with locally advanced
tumors (67). In addition, in a
series of 106 patients who underwent radical surgery, all of the 6
patients that achieved a 5-year survival exhibited intact
SMAD4/DPC4 (68).
Finally, careful evaluation of histological changes
subsequent to preoperative therapy is important to accurately
assess treatment efficacy. Several variables of tumor response to
neoadjuvant therapy have been proposed, including the number of
severe degenerative cancer cells (SDCC), percentage of viable
cells, degree of fibrosis or presence of necrosis (69,70). In a
trial using SDCC to evaluate response to preoperative therapy, no
advantage in terms of OS was observed in 13/26 patients who
achieved a major response, defined as >80% SDCC (66). In trials where the percentage of
remaining viable cells was evaluated, the patients whose tumors
demonstrated minimal pathologic response exhibited more than twice
the risk of mortality compared with patients who achieved a partial
response or pCR (HR=2.74; P=0.01); although significant, this
finding should be interpreted with caution, due to the small sample
size (37,39). In total, two trials correlated
survival with the degree of fibrosis following neoadjuvant therapy,
with conflicting results (71,72).
However, when the presence of tumor necrosis and fibrosis were
analyzed, only tumor necrosis was observed to be an adverse
prognostic factor (70). The College
of American Pathologists has proposed a grading system for tumor
response subsequent to neoadjuvant therapy: 0) Complete response,
no viable cells; i) moderate response, single or small groups of
cells; ii) minimal response, residual cancer outgrown by fibrosis;
and iii) poor response, extensive residual cancer (67).
The significance and prognostic impact of pCR
subsequent to neoadjuvant therapy for PAC is unclear. In
malignancies such as rectal, esophageal and breast cancer, pCR has
been associated with improved disease-free survival and OS
(73–83). In PAC, a number of studies reported
pathologic outcomes following neoadjuvant chemotherapy with or
without fractionated radiotherapy or stereotactic body
radiotherapy, and the pCR rate ranges between 2.4 and 32.0%
(41,84–94). Two
of these studies reported a significantly improved OS for patients
who achieved pCR compared with that of patients who did not
(88,89), although this finding was not confirmed
by a different study (90). In the
aforementioned studies involving neoadjuvant FOLFIRINOX, no
specific survival data were reported in patients who achieved
pCR.
In conclusion, the present case considers certain
current issues of neoadjuvant approaches for patients with BRPAC
and LAPAC. Well-designed trials with standardized diagnostic,
surgical and pathologic procedures are required to define the
optimal treatment and the real clinical impact.
|
1
|
Siegel R, Ma JM, Zou ZH and Jemal A:
Cancer statistics, 2014. Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Joint Committee on Cancer (AJCC),
. TNM staging system. September 6–2013, American Cancer
Society;
|
|
4
|
Tamm EP, Silverman PM, Charnsangavej C and
Evans DB: Diagnosis, staging, and surveillance of pancreatic
cancer. AJR Am J Roentgenol. 180:1311–1323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Varadachary GR, Tamm EP, Abbruzzese JL,
Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB and Wolff
RA: Borderline resectable pancreatic cancer: Definitions,
management, and role of preoperative therapy. Ann Surg Oncol.
13:1035–1046. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katz MH, Marsh R, Herman JM, Shi Q,
Collison E, Venook AP, Kindler HL, Alberts SR, Philip P, Lowy AM,
et al: Borderline resectable pancreatic cancer: Need for
standardization and methods for optimal clinical trial design. Ann
Surg Oncol. 20:2787–2795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abrams RA, Lowy AM, O'Reilly EM, Wolff RA,
Picozzi VJ and Pisters PW: Combined modality treatment of
resectable and borderline resectable pancreas cancer: Expert
consensus statement. Ann Surg Oncol. 16:1751–1756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tempero MA, Malafa MP, Behrman, Benson AB
III, Casper ES, Chiorean EG, Chung V, Cohen SJ, Czito B,
Engebretson A, et al: Pancreatic adenocarcinoma, version 2.2014:
Featured updates to the NCCN guidelines. J Natl Compr Canc Netw.
12:1083–1093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valls C, Andía E, Sanchez A, Fabregat J,
Pozuelo O, Quintero JC, Serrano T, Garcia-Borobia F and Jorba R:
Dual-phase helical CT of pancreatic adenocarcinoma: Assessment of
resectability before surgery. AJR Am J Roentgenol. 178:821–826.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Legmann P, Vignaux O, Dousset B, Baraza
AJ, Palazzo L, Dumontier I, Coste J, Louvel A, Roseau G, Couturier
D and Bonnin A: Pancreatic tumors: Comparison of dual-phase helical
CT and endoscopic sonography. AJR Am J Roentgenol. 170:1315–1322.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeWitt J, Devereaux B, Chriswell M,
McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D,
Kopecky K, et al: Comparison of endoscopic ultrasonography and
multidetector computed tomography for detecting and staging
pancreatic cancer. Ann Intern Med. 141:753–763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Michl P, Pauls S and Gress TM:
Evidence-based diagnosis and staging of pancreatic cancer. Best
Pract Res Clin Gastroenterol. 20:227–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim R, Prithviraj G, Kothari N, Springett
G, Malafa M, Hodul P, Kim J, Yue B, Morse B and Mahipal A: PET/CT
fusion scan prevents futile laparotomy in early stage pancreatic
cancer. Clin Nucl Med. 40:e501–e505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katz MH, Fleming JB, Bhosale P,
Varadhachary G, Lee JE, Wolff R, Wang H, Abbruzzese J, Pisters PW,
Vauthey JN, et al: Response of borderline resectable pancreatic
cancer to neoadjuvant therapy is not reflected by radiographic
indicators. Cancer. 118:5749–5756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferrone CR, Marchegiani G, Hong TS, Ryan
DP, Deshpande V, McDonnell EI, Sabbatino F, Santos DD, Allen JN,
Blaszkowsky LS, et al: Radiological and surgical implications of
neoadjuvant treatment with FOLFIRINOX for locally advanced and
borderline resectable pancreatic cancer. Ann Surg. 261:12–17. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsu CC, Wolfgang CL, Laheru DA, Pawlik TM,
Swartz MJ, Winter JM, Robinson R, Edil BH, Narang AK, Choti MA, et
al: Early mortality risk score: Identification of poor outcomes
following upfront surgery for resectable pancreatic cancer. J
Gastrointest Surg. 16:753–761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tzeng CW, Balachandran A, Ahmad M, Lee JE,
Krishnan S, Wang H, Crane CH, Wolff RA, Varadhachary GR, Pisters
PW, et al: Serum carbohydrate antigen 19–9 represents a marker of
response to neoadjuvant therapy in patients with borderline
resectable pancreatic cancer. HPB (Oxford). 16:430–438. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iacobuzio-Donahue CA, Fu B, Yachida S, Luo
M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P,
et al: DPC4 gene status of the primary carcinoma correlates with
patterns of failure in patients with pancreatic cancer. J Clin
Oncol. 27:1806–1813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Singh P, Wig JD, Srinivasan R and Radotra
BD: A comprehensive examination of Smad4, Smad6 and Smad7 mRNA
expression in pancreatic ductal adenocarcinoma. Indian J Cancer.
48:170–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Infante JR, Matsubayashi H, Sato N,
Tonascia J, Klein AP, Riall TA, Yeo C, Iacobuzio-Donahue C and
Goggins M: Peritumoral fibroblast SPARC expression and patient
outcome with resectable pancreatic adenocarcinoma. J Clin Oncol.
25:319–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyoshi K, Sato N, Ohuchida K, Mizumoto K
and Tanaka M: SPARC mRNA expression as a prognostic marker for
pancreatic adenocarcinoma patients. Anticancer Res. 30:867–871.
2010.PubMed/NCBI
|
|
24
|
Prenzel KL, Warnecke-Eberz U, Xi H,
Brabender J, Baldus SE, Bollschweiler E, Gutschow CA, Hölscher AH
and Schneider PM: Significant overexpression of SPARC/osteonectin
mRNA in pancreatic cancer compared to cancer of the papilla of
Vater. Oncol Rep. 15:1397–1401. 2006.PubMed/NCBI
|
|
25
|
Nagaraju GP, Dontula R, El-Rayes BF and
Lakka SS: Molecular mechanisms underlying the divergent roles of
SPARC in human carcinogenesis. Carcinogenesis. 35:967–973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Desai NP, Trieu V, Hwang LY, Wu R,
Soon-Shiong P and Gradishar WJ: Improved effectiveness of
nanoparticle albumin-bound (nab) paclitaxel versus
polysorbate-based docetaxel in multiple xenografts as a function of
HER2 and SPARC status. Anticancer Drugs. 19:899–909. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong HQ and Abbruzzese JL: Epidermal
growth factor receptor-targeted therapy for pancreatic cancer.
Semin Oncol. 29 5 Suppl 14:S31–S37. 2002. View Article : Google Scholar
|
|
28
|
Yamanaka Y, Friess H, Kobrin MS, Buchler
M, Beger HG and Korc M: Coexpression of epidermal growth factor
receptor and ligands in human pancreatic cancer is associated with
enhanced tumor aggressiveness. Anticancer Res. 13:565–569.
1993.PubMed/NCBI
|
|
29
|
Hu YC, Komorowski RA, Graewin S, Hostetter
G, Kallioniemi OP, Pitt HA and Ahrendt SA: Thymidylate synthase
expression predicts the response to 5-fluorouracil-based adjuvant
therapy in pancreatic cancer. Clin Cancer Res. 9:4165–4171.
2003.PubMed/NCBI
|
|
30
|
van der Zee JA, van Eijck CH, Hop WC, van
Dekken H, Dicheva BM, Seynhaeve AL, Koning GA, Eggermont AM and Ten
Hagen TL: Expression and prognostic significance of thymidylate
synthase (TS) in pancreatic head and periampullary cancer. Eur J
Surg Oncol. 38:1058–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin K, Luo G, Xiao Z, Liu Z, Liu C, Ji S,
Xu J, Liu L, Long J, Ni Q and Yu X: Noncoding RNAs as potential
biomarkers to predict the outcome in pancreatic cancer. Drug Des
Devel Ther. 9:1247–1255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ansari D, Gustafsson A and Andersson R:
Update on the management of pancreatic cancer: Surgery is not
enough. World J Gastroenterol. 21:3157–3165. 2015.PubMed/NCBI
|
|
33
|
Evans DB, Varadhachary GR, Crane CH, Sun
CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA,
et al: Preoperative gemcitabine-based chemoradiation for patients
with resectable adenocarcinoma of the pancreatic head. J Clin
Oncol. 26:3496–3502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Varadhachary GR, Wolff RA, Crane CH, Sun
CC, Lee JE, Pisters PW, Vauthey JN, Abdalla E, Wang H, Staerkel GA,
et al: Preoperative gemcitabine and cisplatin followed by
gemcitabine-based chemoradiation for resectable adenocarcinoma of
the pancreatic head. J Clin Oncol. 26:3487–3495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho SW, Tzeng CW, Johnston WC, Cassera MA,
Newell PH, Hammill CW, Wolf RF, Aloia TA and Hansen PD: Neoadjuvant
radiation therapy and its impact on complications after
pancreaticoduodenectomy for pancreatic cancer: Analysis of the
American College of Surgeons National Surgical Quality Improvement
Program (ACS-NSQIP). HPB (Oxford). 16:350–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lind PA, Isaksson B, Almström M, Johnsson
A, Albiin N, Byström P and Permert J: Efficacy of preoperative
radiochemotherapy in patients with locally advanced pancreatic
carcinoma. Acta Oncol. 47:413–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Evans DB, Rich TA, Byrd DR, Cleary KR,
Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ and Ames FC:
Preoperative chemoradiation and pancreaticoduodenectomy for
adenocarcinoma of the pancreas. Arch Surg. 127:1335–1339. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abbott DE, Tzeng CW, Merkow RP, Cantor SB,
Chang GJ, Katz MH, Bentrem DJ, Bilimoria KY, Crane CH, Varadhachary
GR, et al: The cost-effectiveness of neoadjuvant chemoradiation is
superior to a surgery-first approach in the treatment of pancreatic
head adenocarcinoma. Ann Surg Oncol. 20 Suppl 3:S500–S5008. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katz MH, Pisters PW, Evans DB, Sun CC, Lee
JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA, et al:
Borderline resectable pancreatic cancer: The importance of this
emerging stage of disease. J Am Coll Surg. 206:833–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chun YS, Milestone BN, Watson JC, Cohen
SJ, Burtness B, Engstrom PF, Haluszka O, Tokar JL, Hall MJ,
Denlinger CS, et al: Defining venous involvement in borderline
resectable pancreatic cancer. Ann Surg Oncol. 17:2832–2838. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rose JB, Rocha FG, Alseidi A, Biehl T,
Moonka R, Ryan JA, Lin B, Picozzi V and Helton S: Extended
neoadjuvant chemotherapy for borderline resectable pancreatic
cancer demonstrates promising postoperative outcomes and survival.
Ann Surg Oncol. 21:1530–1537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takahashi H, Ohigashi H, Gotoh K,
Marubashi S, Yamada T, Murata M, Ioka T, Uehara H, Yano M and
Ishikawa O: Preoperative gemcitabine-based chemoradiation therapy
for resectable and borderline resectable pancreatic cancer. Ann
Surg. 258:1040–1050. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mahaseth H, Brutcher E, Kauh J, Hawk N,
Kim S, Chen Z, Kooby DA, Maithel SK, Landry J and El-Rayes BF:
Modified FOLFIRINOX regimen with improved safety and maintained
efficacy in pancreatic adenocarcinoma. Pancreas. 42:1311–1315.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Boone BA, Steve J, Krasinskas AM, Zureikat
AH, Lembersky BC, Gibson MK, Stoller RG, Zeh HJ and Bahary N:
Outcomes with FOLFIRINOX for borderline resectable and locally
unresectable pancreatic cancer. J Surg Oncol. 108:236–241. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gunturu KS, Yao X, Cong X, Thumar JR,
Hochster HS, Stein SM and Lacy J: FOLFIRINOX for locally advanced
and metastatic pancreatic cancer: Single institution retrospective
review of efficacy and toxicity. Med Oncol. 30:3612013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Peddi PF, Lubner S, McWilliams R, Tan BR,
Picus J, Sorscher SM, Suresh R, Lockhart AC, Wang J, Menias C, et
al: Multi-institutional experience with FOLFIRINOX in pancreatic
adenocarcinoma. JOP. 13:497–501. 2012.PubMed/NCBI
|
|
47
|
Hosein PJ, Macintyre J, Kawamura C,
Maldonado JC, Ernani V, Loaiza-Bonilla A, Narayanan G, Ribeiro A,
Portelance L, Merchan JR, et al: A retrospective study of
neoadjuvant FOLFIRINOX in unresectable or borderline-resectable
locally advanced pancreatic adenocarcinoma. BMC Cancer. 12:1992012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tinchon C, Hubmann E, Pichler A, Keil F,
Pichler M, Rabl H, Uggowitzer M, Jilek K, Leitner G and Bauernhofer
T: Safety and efficacy of neoadjuvant FOLFIRINOX treatment in a
series of patients with borderline resectable pancreatic ductal
adenocarcinoma. Acta Oncol. 52:1231–1233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Christians KK, Tsai S, Mahmoud A, Ritch P,
Thomas JP, Wiebe L, Kelly T, Erickson B, Wang H, Evans DB and
George B: Neoadjuvant FOLFIRINOX for borderline resectable pancreas
cancer: A new treatment paradigm? Oncologist. 19:266–274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Faris JE, Blaszkowsky LS, McDermott S,
Guimaraes AR, Szymonifka J, Huynh MA, Ferrone CR, Wargo JA, Allen
JN, Dias LE, et al: FOLFIRINOX in locally advanced pancreatic
cancer: The Massachusetts General Hospital Cancer Center
experience. Oncologist. 18:543–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
James ES, Yao X, Cong X, Li J, Hahn C,
Kaley K, Kortmansky JS, Fischbach NA, Chang BW, Salem RR, et al:
Interim analysis of a phase II study of dose-modified FOLFIRINOX
(mFOLFIRINOX) in locally advanced (LAPC) and metastatic pancreatic
cancer (MPC). J Clin Oncol. 32 Suppl:e152262014. View Article : Google Scholar
|
|
52
|
Hazariwala R, Landry J, El-Reyes B, et al:
Neoadjuvant FOLFIRINOX and radiation therapy improves resectability
of pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2 Suppl
1:872013.
|
|
53
|
Vasile E, de Lio N, Cappelli C, et al:
Phase II study of neoadjuvant chemotherapy with modified FOLFOXIRI
in borderline resectable or unresectable stage III pancreatic
cancer. J Clin Oncol. 15(Suppl 1): 312013.
|
|
54
|
Kharofa J, Kelly TR, Ritch PS, George B,
Wiebe LA, Thomas JP, Christians KK, Evans DP and Erickson B:
5-FU/leucovorin, irinotecan, oxaliplatin (FOLFIRINOX) induction
followed by chemoXRT in borderline resectable pancreatic cancer. J
Clin Oncol. 30 Suppl:e146132012.
|
|
55
|
Lowery MA, Yu KH, Adel NG, Apollo AJ,
Boyar MS, Caron P, Ilson D, Segal NH, Janjigian YY, Janjigian DR,
et al: Activity of front-line FOLFIRINOX (FFX) in stage III/IV
pancreatic adenocarcinoma (PC) at Memorial Sloan-Kettering Cancer
Center (MSKCC). J Clin Oncol. 30 Suppl:40572012.
|
|
56
|
Blazer M, Wu C, Goldberg RM, Phillips G,
Schmidt C, Muscarella P, Wuthrick E, Williams TM, Reardon J,
Ellison EC, et al: Neoadjuvant modified (m) FOLFIRINOX for locally
advanced unresectable (LAPC) and borderline resectable (BRPC)
adenocarcinoma of the pancreas. Ann Surg Oncol. 22:1153–1159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nanda RH, El-Rayes B, Maithel SK and
Landry J: Neoadjuvant modified FOLFIRINOX and chemoradiation
therapy for locally advanced pancreatic cancer improves
resectability. J Surg Oncol. 111:1028–1034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Paniccia A, Edil BH, Schulick RD, Byers
JT, Meguid C, Gajdos C and McCarter MD: Neoadjuvant FOLFIRINOX
application in borderline resectable pancreatic adenocarcinoma: A
retrospective cohort study. Medicine (Baltimore). 93:e1982014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mellon EA, Hoffe SE, Springett GM, Frakes
JM, Strom TJ, Hodul PJ, Malafa MP, Chuong MD and Shridhar R:
Long-term outcomes of induction chemotherapy and neoadjuvant
stereotactic body radiotherapy for borderline resectable and
locally advanced pancreatic adenocarcinoma. Acta Oncol. 54:979–985.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Petrelli F, Coinu A, Borgonovo K, Cabiddu
M, Ghilardi M, Lonati V, Aitini E and Barni S: Gruppo Italiano per
lo Studio dei Carcinomi dell'Apparato Digerente (GISCAD):
FOLFIRINOX-based neoadjuvant therapy in borderline resectable or
unresectable pancreatic cancer: A meta-analytical review of
published studies. Pancreas. 44:515–521. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative-intent resection of pancreatic cancer:
A randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Neoptolemos JP, Stocken DD, Bassi C,
Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger
S, Mariette C, et al: Adjuvant chemotherapy with fluorouracil plus
folinic acid vs gemcitabine following pancreatic cancer resection:
A randomized controlled trial. JAMA. 304:1073–1081. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Katz MH, Shi Q, Ahmad SA, Herman JM, de
Marsh RW, Collisson EA, Schwartz LH, Martin RC, Conway WC, Truty M,
et al: Preoperative modified FOLFIRINOX (mFOLFIRINOX) followed by
chemoradiation (CRT) for borderline resectable (BLR) pancreatic
cancer (PDAC): Initial results from Alliance Trial A021101. J Clin
Oncol. 33(Suppl): abstr 4008. 2015.
|
|
64
|
Pancreatic Resectability in Cancers With
Known Limited Extension (PRICKLE)-A Single-centre Phase 2a Study of
Gemcitabine Plus Nab-paclitaxel for Borderline Unresectable Locally
Advanced Pancreatic Cancer. ClinicalTrials.gov Identifier:
NCT02124369. 2017.
|
|
65
|
Breslin TM, Hess KR, Harbison DB, Jean ME,
Cleary KR, Dackiw AP, Wolff RA, Abbruzzese JL, Janjan NA, Crane CH,
et al: Neoadjuvant chemoradiotherapy for adenocarcinoma of the
pancreas: Treatment variables and survival duration. Ann Surg
Oncol. 8:123–132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ishikawa O, Ohhigashi H, Teshima T,
Chatani M, Inoue T, Tanaka S, Kitamura T, Wada A, Sasaki Y, Imaoka
S, et al: Clinical and histopathological appraisal of preoperative
irradiation for adenocarcinoma of the pancreatoduodenal region. J
Surg Oncol. 40:143–151. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Washington K, Berlin J and Branton P:
Protocol for the examination of specimens from patients with
carcinoma of the exocrine pancreas: Protocol applies to all
epithelial tumors of the exocrine pancreas. Endocrine tumors and
tumors of the ampulla of Vater are not included Northfield, IL:
College of American Pathologists; 2010
|
|
68
|
Oshima M, Okano K, Muraki S, Haba R, Maeba
T, Suzuki Y and Yachida S: Immunohistochemically detected
expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4)
strongly predicts survival in patients with resectable pancreatic
cancer. Ann Surg. 258:336–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Pendurthi TK, Cooper HS, Young NA, et al:
Histopathologic effects of preoperative chemoradiotheraoy on
pancreatic carcinoma. Gastroenterology. 110:A1410. 1996.
|
|
70
|
White RR, Xie HB, Gottfried MR, Czito BG,
Hurwitz HI, Morse MA, Blobe GC, Paulson EK, Baillie J, Branch MS,
et al: Significance of histological response to preoperative
chemoradiotherapy for pancreatic cancer. Ann Surg Oncol.
12:214–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sasson AR, Wetherington RW, Hoffman JP,
Ross EA, Cooper H, Meropol NJ, Freedman G, Pingpank JF and
Eisenberg BL: Neoadjuvant chemoradiotherapy for adenocarcinoma of
the pancreas: Analysis of histopathology and outcome. Int J
Gastrointest Cancer. 34:121–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hoffman JP, Weese JL, Solin LJ, Engstrom
P, Agarwal P, Barber LW, Guttmann MC, Litwin S, Salazar H and
Eisenberg BL: A pilot study of preoperative chemoradiation for
patients with localized adenocarcinoma of the pancreas. Am J Surg.
169:71–78. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Aschele C, Cionini L, Lonardi S, Pinto C,
Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti
P, et al: Primary tumor response to preoperative chemoradiation
with or without oxaliplatin in locally advanced rectal cancer:
Pathologic results of the STAR-01 randomized phase III trial. J
Clin Oncol. 29:2773–2780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
García-Aguilar J, de Anda E Hernandez,
Sirivongs P, Lee SH, Madoff RD and Rothenberger DA: A pathologic
complete response to preoperative chemoradiation is associated with
lower local recurrence and improved survival in rectal cancer
patients treated by mesorectal excision. Dis Colon Rectum.
46:298–304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Martin ST, Heneghan HM and Winter DC:
Systematic review and meta-analysis of outcomes following
pathological complete response to neoadjuvant chemoradiotherapy for
rectal cancer. Br J Surg. 99:918–928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Maas M, Nelemans PJ, Valentini V, Das P,
Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R,
Haustermans K, et al: Long-term outcome in patients with a
pathological complete response after chemoradiation for rectal
cancer: A pooled analysis of individual patient data. Lancet Oncol.
11:835–844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Meredith KL, Weber JM, Turaga KK, Siegel
EM, McLoughlin J, Hoffe S, Marcovalerio M, Shah N, Kelley S and
Karl R: Pathologic response after neoadjuvant therapy is the major
determinant of survival in patients with esophageal cancer. Ann
Surg Oncol. 17:1159–1167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pasini F, de Manzoni G, Zanoni A,
Grandinetti A, Capirci C, Pavarana M, Tomezzoli A, Rubello D and
Cordiano C: Neoadjuvant therapy with weekly docetaxel and
cisplatin, 5-fluorouracil continuous infusion, and concurrent
radiotherapy in patients with locally advanced esophageal cancer
produced a high percentage of long-lasting pathological complete
response: A phase 2 study. Cancer. 119:939–945. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Rohatgi P, Swisher SG, Correa AM, Wu TT,
Liao Z, Komaki R, Walsh GL, Vaporciyan AA, Rice DC, Roth JA and
Ajani JA: Characterization of pathologic complete response after
preoperative chemoradiotherapy in carcinoma of the esophagus and
outcome after pathologic complete response. Cancer. 104:2365–2372.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Berger AC, Farma J, Scott WJ, Freedman G,
Weiner L, Cheng JD, Wang H and Goldberg M: Complete response to
neoadjuvant chemoradiotherapy in esophageal carcinoma is associated
with significantly improved survival. J Clin Oncol. 23:4330–4337.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vallböhmer D, Hölscher AH, DeMeester S,
DeMeester T, Salo J, Peters J, Lerut T, Swisher SG, Schröder W,
Bollschweiler E and Hofstetter W: A multicenter study of survival
after neoadjuvant radiotherapy/chemotherapy and esophagectomy for
ypT0N0M0R0 esophageal cancer. Ann Surg. 252:744–749. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Symmans WF, Peintinger F, Hatzis C, Rajan
R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, et
al: Measurement of residual breast cancer burden to predict
survival after neoadjuvant chemotherapy. J Clin Oncol.
25:4414–4422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Adams S, Chakravarthy AB, Donach M, Spicer
D, Lymberis S, Singh B, Bauer JA, Hochman T, Goldberg JD, Muggia F,
et al: Preoperative concurrent paclitaxel-radiation in locally
advanced breast cancer: Pathologic response correlates with
five-year overall survival. Breast Cancer Res Treat. 124:723–732.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sherman WH, Chu K, Chabot J, Allendorf J,
Schrope BA, Hecht E, Jin B, Leung D, Remotti H, Addeo G, et al:
Neoadjuvant gemcitabine, docetaxel, and capecitabine followed by
gemcitabine and capecitabine/radiation therapy and surgery in
locally advanced, unresectable pancreatic adenocarcinoma. Cancer.
121:673–680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Boone BA, Steve J, Zenati MS, Hogg ME,
Singhi AD, Bartlett DL, Zureikat AH, Bahary N and Zeh HJ III: Serum
CA 19–9 response to neoadjuvant therapy is associated with outcome
in pancreatic adenocarcinoma. Ann Surg Oncol. 21:4351–4358. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rajagopalan MS, Heron DE, Wegner RE, Zeh
HJ, Bahary N, Krasinskas AM, Lembersky B, Brand R, Moser AJ, Quinn
AE and Burton SA: Pathologic response with neoadjuvant chemotherapy
and stereotactic body radiotherapy for borderline resectable and
locally-advanced pancreatic cancer. Radiat Oncol. 8:2542013.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lee JL, Kim SC, Kim JH, Lee SS, Kim TW,
Park DH, Seo DW, Lee SK, Kim MH, Kim JH, et al: Prospective
efficacy and safety study of neoadjuvant gemcitabine with
capecitabine combination chemotherapy for borderline-resectable or
unresectable locally advanced pancreatic adenocarcinoma. Surgery.
152:851–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhao Q, Rashid A, Gong Y, Katz MH, Lee JE,
Wolf R, Balachandran A, Varadhachary GR, Pisters PW, Wang H, et al:
Pathologic complete response to neoadjuvant therapy in patients
with pancreatic ductal adenocarcinoma is associated with a better
prognosis. Ann Diagn Pathol. 16:29–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chatterjee D, Katz MH, Rashid A,
Varadhachary GR, Wolff RA, Wang H, Lee JE, Pisters PW, Vauthey JN,
Crane C, et al: Histologic grading of the extent of residual
carcinoma following neoadjuvant chemoradiation in pancreatic ductal
adenocarcinoma: A predictor for patient outcome. Cancer.
118:3182–3190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Barugola G, Partelli S, Crippa S, Capelli
P, D'Onofrio M, Pederzoli P and Falconi M: Outcomes after resection
of locally advanced or borderline resectable pancreatic cancer
after neoadjuvant therapy. Am J Surg. 203:132–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Le Scodan R, Mornex F, Partensky C,
Mercier C, Valette PJ, Ychou M, Bibeau F and Scoazec JY: Histologic
assessment of treatment effect of preoperative chemoradiation in
patients presenting with resectable pancreatic adenocarcinoma.
Cancer Radiother. 15:97–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Turrini O, Ychou M, Moureau-Zabotto L,
Rouanet P, Giovannini M, Moutardier V, Azria D, Delpero JR and
Viret F: Neoadjuvant docetaxel-based chemoradiation for resectable
adenocarcinoma of the pancreas: New neoadjuvant regimen was safe
and provided an interesting pathologic response. Eur J Surg Oncol.
36:987–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Magnin V, Moutardier V, Giovannini MH,
Lelong B, Giovannini M, Viret F, Monges G, Bardou VJ, Alzieu C and
Delpero JR: Neoadjuvant preoperative chemoradiation in patients
with pancreatic cancer. Int J Radiat Oncol Biol Phys. 55:1300–1304.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wanebo HJ, Glicksman AS, Vezeridis MP,
Clark J, Tibbetts L, Koness RJ and Levy A: Preoperative
chemotherapy, radiotherapy, and surgical resection of locally
advanced pancreatic cancer. Arch Surg. 135:81–88. 2000. View Article : Google Scholar : PubMed/NCBI
|