Introduction
Several studies have demonstrated that miR-21 has
different expression profiles in various hematopoietic cells
(1). Low levels of miR-21 were
reported in naive T cells, while in regulatory helper T cells
(Treg) this level was reported as high (2). High expression of miR-21 also occurs in
memory and effector T cells, and miR-21 expression is significantly
upregulated after being stimulated by the T cell receptor (TCR)
(3). High levels of miR-21 have been
observed in Th1 cells, but miR-21 expression in Th2 cells is
stronger. Low levels of miR-21 expression have been reported in
precursor B cells and progenitor B cells but germinal center B
(GCB) has been shown to express high levels of miR-21 (4). miR-21 can serve as a negative feedback
control factor in innate immunity (5).
In the human mononuclear cell line, when LPS
stimulates THP-1 cells miR-21 expression is quickly upregulated via
the toll-like receptor (6). Prior
reports showed that LPS, IL-1α and TNF-α can induce miR-21
expression via NF-κB (7). NF-κB
activation increases the expression of miR-21, while miR-21 reduces
the NF-κB activity by downregulating TRAF6 and IRAKl (8,9). The
negative feedback mechanism of miR-21 plays an important role in
physiological and pathological processes in innate immunity, virus
infection and tumorigenesis. miR-21 also plays an indispensable
role in acquired immunity. In activated T cells, miR-21 inhibits
cell death induced by activation of T lymphocytes and inhibits
production of IL-2 (10). miR-21 also
plays an important role in peripheral immune tolerance of Treg
(11), and miR-21 has been found to
be implicated in autoimmune diseases such as rheumatoid arthritis
(RA) (11,12). In joint synovial tissue of RA
patients, the level of miR-21 has been reported to be significantly
higher compared to synovial tissue in osteoarthritis patients.
Additionally, it was reported that in vitro stimulation of
joint synovial cell cultures obtained from RA patients with TNF-α
and IL-1α, increased miR-21 expression level (13). Autoimmune lymphoproliferative syndrome
(ALPS) also known as Canale-Smith syndrome is caused by excess
hyperplasia of lymphocytes due to apoptosis disorders (14). ALPS is a rare disease caused by
lymphocyte proliferation due to programmed death of lymphocytes or
apoptosis disorders (15).
To better study the pathological and physiological
significance of miR-21, we investigated the biological functions of
miR-21 in ALPS and revealed a mechanism different from inflammation
negative feedback. It provides a new explanation for pathogenesis
of ALPS.
Materials and methods
Experimental animals
Healthy SPF male C57b6/L mice aged 6–8 weeks
(Laboratory Animal Center, Hebei Medical University, Shijiazhuang,
China; laboratory animal license no. SCXK 2015-0004) and miR-21
gene knockout mice (Jakson Laboratory, CA, USA; laboratory animal
no. 016856), weighing 20–25 g were used in the present study. Mice
were divided into two groups: i) The wild-type group (WT) and ii)
the transgenic group (TG). Approval for the animal experiments was
received from the Animal Ethics Committee of Hebei Province General
Hospital.
Experimental methods
RT-polymerase chain reaction (PCR)
Heart, liver, lung, kidney, spleen, and lymph node
tissues were extracted from mice in both groups. TRIzol (1 ml) was
added to 5 mg of each sample and after mixing, samples were left
for 5 min at room temperature. Chloroform was added (1/5 volume) to
the sample and mixed well for 1 min and left at room temperature
for 5 min. Sample was then centrifuged for 15 min at 10,000 × g at
room temperature.
Equal volume of isopropanol was then added and mixed
gently by turning the tube upside down. After 10 min at room
temperature, sample was centrifuged for 10 min at 10,000 × g at
4°C. Supernatant was discarded and 1 ml of 75% ethyl alcohol was
added to the pellet. Appropriate amount of DEPC was dissolved in
water and precipitated. The reaction system was 25 µl including
fluorescence RT-PCR reaction liquid (20 µl), DNA polymerase-I (1
µl), reverse transcriptase (0.35 µl), and template RNA (5 µl). They
were mixed and centrifuged for 10 sec at 5,000 × g.
Real-time fluorescence RT-PCR amplification process
was as follows: Reverse transcription for 30 min at 50°C;
pre-denatured for 3 min at 95°C; denatured at 95°C for 15 sec,
annealed for 30 sec at 50°C, extended for 30 min at 72°C, 5 cycles
in total; denatured for 10 sec at 95°C; annealed for 40 sec at
55°C, 45 cycles in total.
H&E staining and immune histologic chemical
staining
Paraffin sections of the heart, liver, lung, kidney,
spleen, and lymph node tissues were routinely dewaxed. Sections
were incubated with 3% H2O2 methanol solution
for 10 min at room temperature to block the endogenous peroxidase.
Sections were rinsed 3 times with PBS, repaired for 8 min at high
temperature using 0.01 mol/l citrate buffer solution (pH 6.0),
cooled to the room temperature, and rinsed with PBS 3 times (5 min
each). Some sections were stained using H&E and the remaining
sections were blocked for 30 min at 37°C after dropwise addition of
goat serum operating fluid. After dropwise addition of PNA (1:200)
(Sigma-Aldrich, St. Louis, MO, USA), they were allowed to stand
overnight at 4°C and rinsed with PBS 3 times (5 min each). After
dropwise addition of biotinylated goat anti-rabbit second antibody
operating solution (cat no. P0203; Boster Inc., Wuhan, China),
sections were incubated for 20 min at 37°C and rinsed with PBS 3
times (5 min each). After addition of horseradish peroxidase-marked
streptavidin operating liquid, the sections were incubated for 20
min at 37°C and rinsed with PBS 3 times (5 min each). Sections were
then developed with DAB, re-stained with hematoxylin and
differentiated with 0.1% hydrochloric ethanol. Samples were then
immersed in tap water, dehydrated, clarified, and mounted with
neutral resin.
Analysis of serum immunoglobulin (IgG)
concentrations and types
MILLIPLEX MAP Mouse IgG Isotyping Magnetic Bead
Panel, MGAMMAG-300K (both from Biosharp, Hefei, China) were used.
We used IgG (1, 2a, 2b, and 3), IgM, and IgA (diluted at 1:25,000)
following the protocol reported by Ruan et al (16).
Flow cytometry
Mice were sacrificed and spleen, lymph node,
mesenteric lymph nodes, PP globule, and thymus gland were extracted
and placed in pre-cooled cell culture media. Two mouse thigh bones
were taken and both ends were cut out. A 1 ml syringe was used to
draw 5% FBS buffer solution (Gibco-BRL, Grand Island, NY, USA) and
the red marrow was flushed twice. In order to prepare single-cell
suspension, red blood cell lysis buffer was added to spleen and
bone marrow to remove red blood cells. Cell counts were performed
and cell concentration was adjusted to 1×106/100 µl.
Index antibody was then added and transferred to ice-bath and
incubated for ≥45 min away from light. Mouse monoclonal antibodies,
such as anti-CD95/Fas (dilution, 1:100; cat. no. 15A7), anti-CD38
(dilution, 1:100; cat. no. 90), anti-CD3 (dilution, 1:100; cat. no.
17A2), anti-CD4 (dilution, 1:100; cat. no. RM4 5), anti-CD4
(dilution, 1:100; cat. no. GK1.5), anti-CD44 (dilution, 1:100; cat.
no. IM7), anti-CD62L (dilution, 1:100; cat. no. MEL 14), anti-CD25
(dilution, 1:100; cat. no. PC61.5), anti-CD69 (dilution, 1:100;
cat. no. H1.2F3) and anti-B220/CD45R (dilution, 1:100; cat. no. RA3
6B2) were all purchased from eBioscience (San Diego, CA, USA).
FlowJo (Tree Star, Inc., Ashland, OR, USA) was used for data
analysis.
Adoptive transfer
The flow cytometer FACSAria II (BD Biosciences) was
used to sort out the non-GCB cells. The resulting cells were washed
once with cell culture medium and stabilized for 1–2 h in a culture
flask for subsequent experiments. Non-GCB cells (1×106)
and CD4+ T cells (1×106) were mixed (1:1).
Cell suspension was injected into the immunodeficiency SCID mice
via the caudal vein without any spillage (17). The percentage of the newly-generated
GCB cells from the donor and the expression of Fas in the
newly-generated GCB cells were measured at 7 days after adoptive
transfer.
Homeostatic proliferation test
Click-iT® EdU cell proliferation assay
was used. A 200 µl EdU (500 µg/ml; KeyGene Biotech, Nanjing, China)
was injected peritoneally at 24 days after adoptive transfer and
strengthened every 48 h and mice were sacrificed at day 7. To
complete the test, we followed the instructions provided by the
kit's manufacturer (18). The surface
marker was B220 or CD4 and cells were routinely stained, incubated,
fixed, and permeated. The Click-iTrM EdU test solution was then
added and flow cytometer was used to analyze the homeostatic
proliferation of the lymphocytes.
Dual-luciferase reporter gene reporting
method
The Dual-Luciferase® Reporter Assay kit
(KeyGene Biotech) was used. The vector (pZZX-Luc-Fas) of Fas
3′-non-translated region (3′-UTR), miR-146a (pEZX-miR-146), and
non-significant control miR-scramble (pEZX-miR-scramble) were
extracted and purified following the instructions provided by the
kit's manufacturer (19).
Statistical analysis
We used SPSS 19.0 software (IBM, Armonk, NY, USA)
for data analysis. Measurement data were presented as mean ±
standard deviation. Dependent t-test was used for intergroup
significance comparison. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-21 gene knockout
characteristics
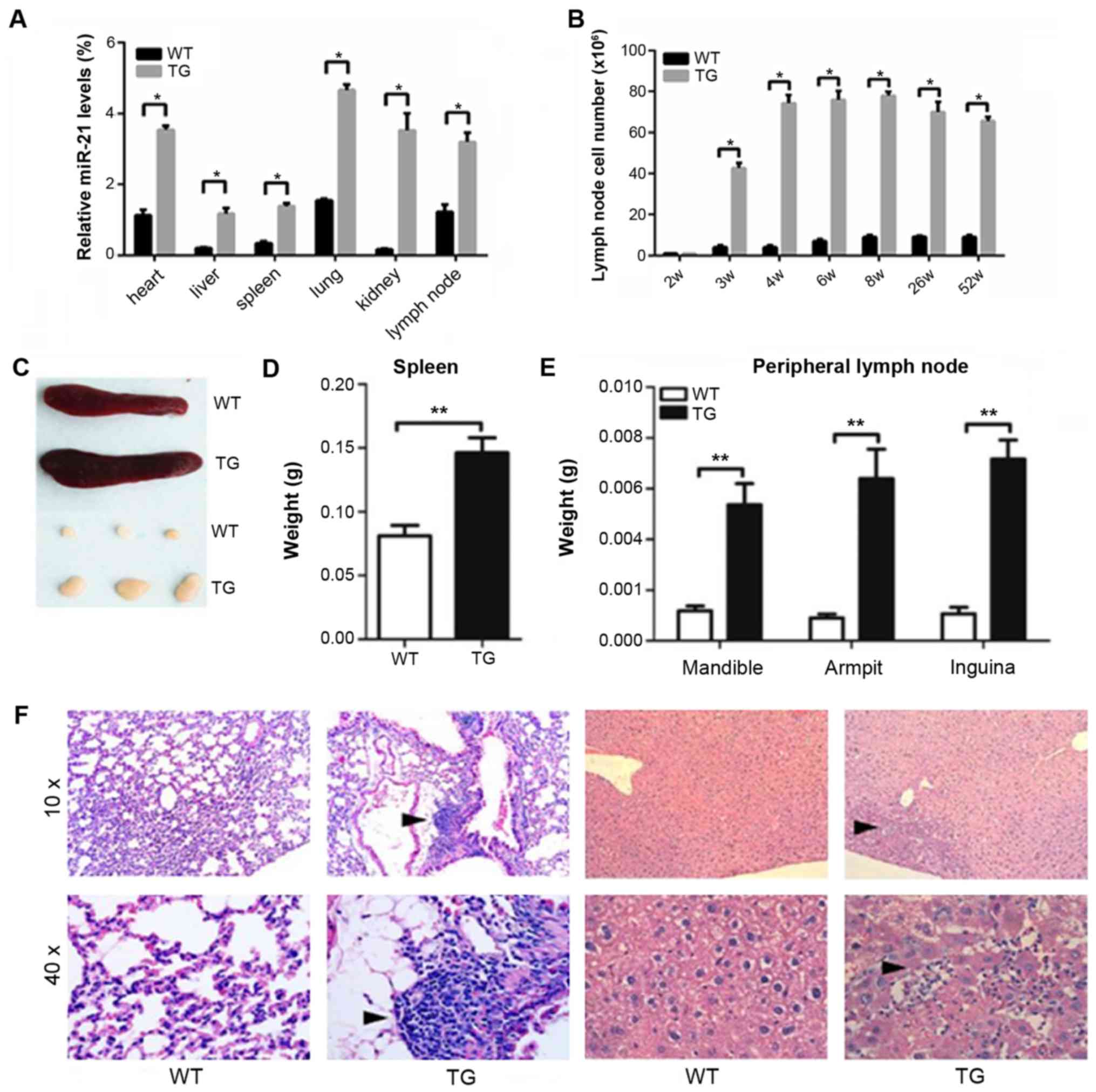
miR-21 expression levels in various visceral organs
were measured using quantitative PCR (Fig. 1A). In the TG, miR-21 expression levels
in spleen and lymph node were significantly higher than those in
the WT at 3 weeks after birth (Fig.
1B). The average lymph node volume in the TG was 3 times larger
than that in the WT at 3 weeks after birth. The average volume was
8 times larger at 4 weeks after birth, and ~4 times larger at 6
weeks (Fig. 1C). In the TG, we
detected inflammatory cell infiltration in lung and liver at 8
weeks after birth (Fig. 1D).
miR-21 gene knockout mice exhibiting
characteristics of autoimmune lymphoid hyperplasia syndrome
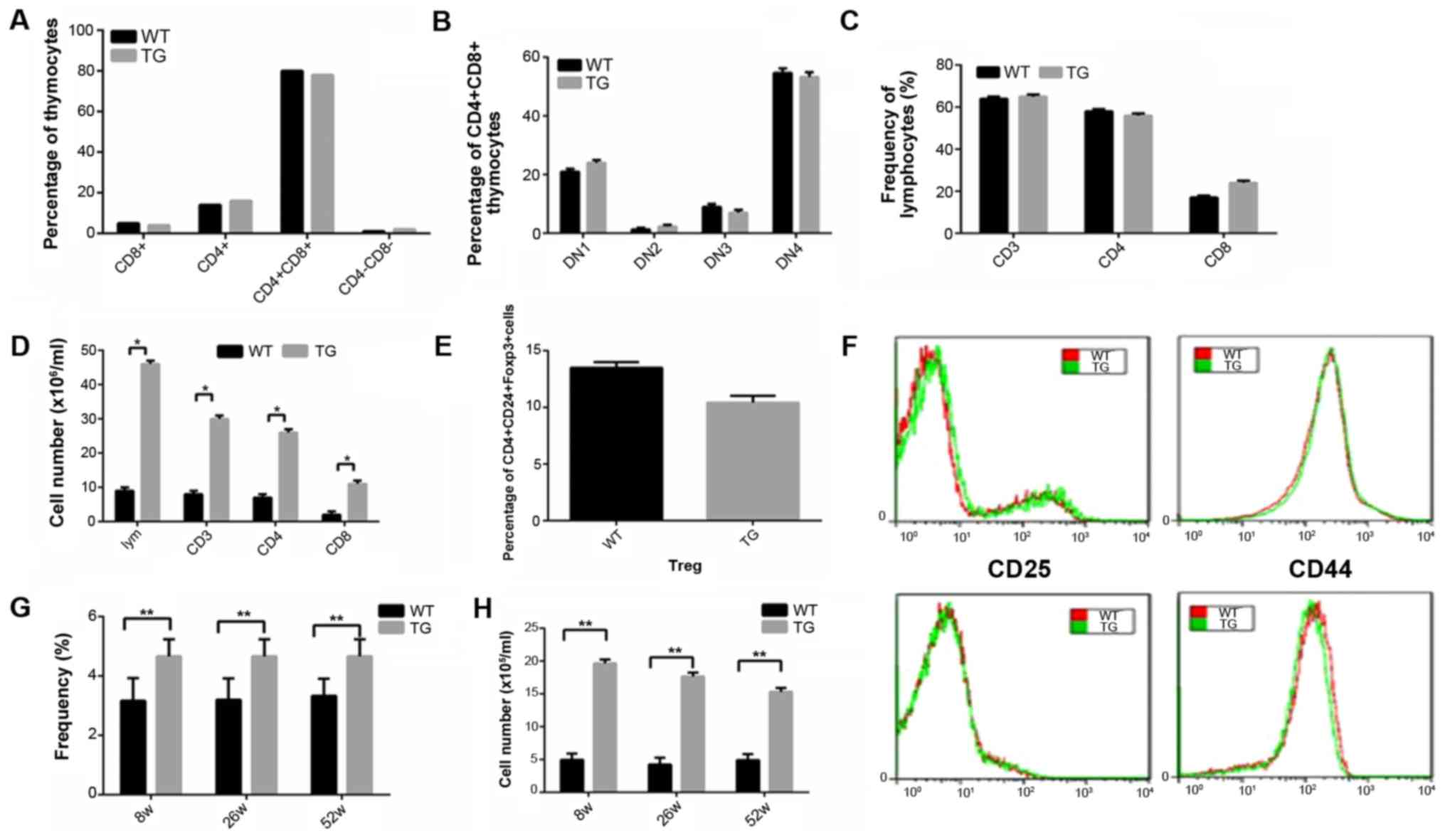
Results obtained from immunophenotyping showed that
in the TG, the number of T cells in thymus gland was at normal
level (Fig. 2A-C). However, the
number of various cell subsets increased significantly (Fig. 2D), while the number of other cell
types, such as Treg cells decreased slightly (Fig. 2E). Results revealed that the levels
CD25, CD69, CD44 and CD62L were at normal levels (Fig. 2F).
In the TG, the percentage of double negative T cells
(DNT) in the spleen increased significantly.
CD3+CD4−CD8− TCRaIB+
served as the surface markers and we detected the frequency and
absolute quantity of the DNT cells in the spleen at 8, 26 and 52
weeks. It was found that in the transgenic mice, DNT cells were
accumulated in the spleen (Fig. 2G and
H).
miR-21 gene knockout mice
spontaneously collect the germinal center B cells and increasing
IgG in serum
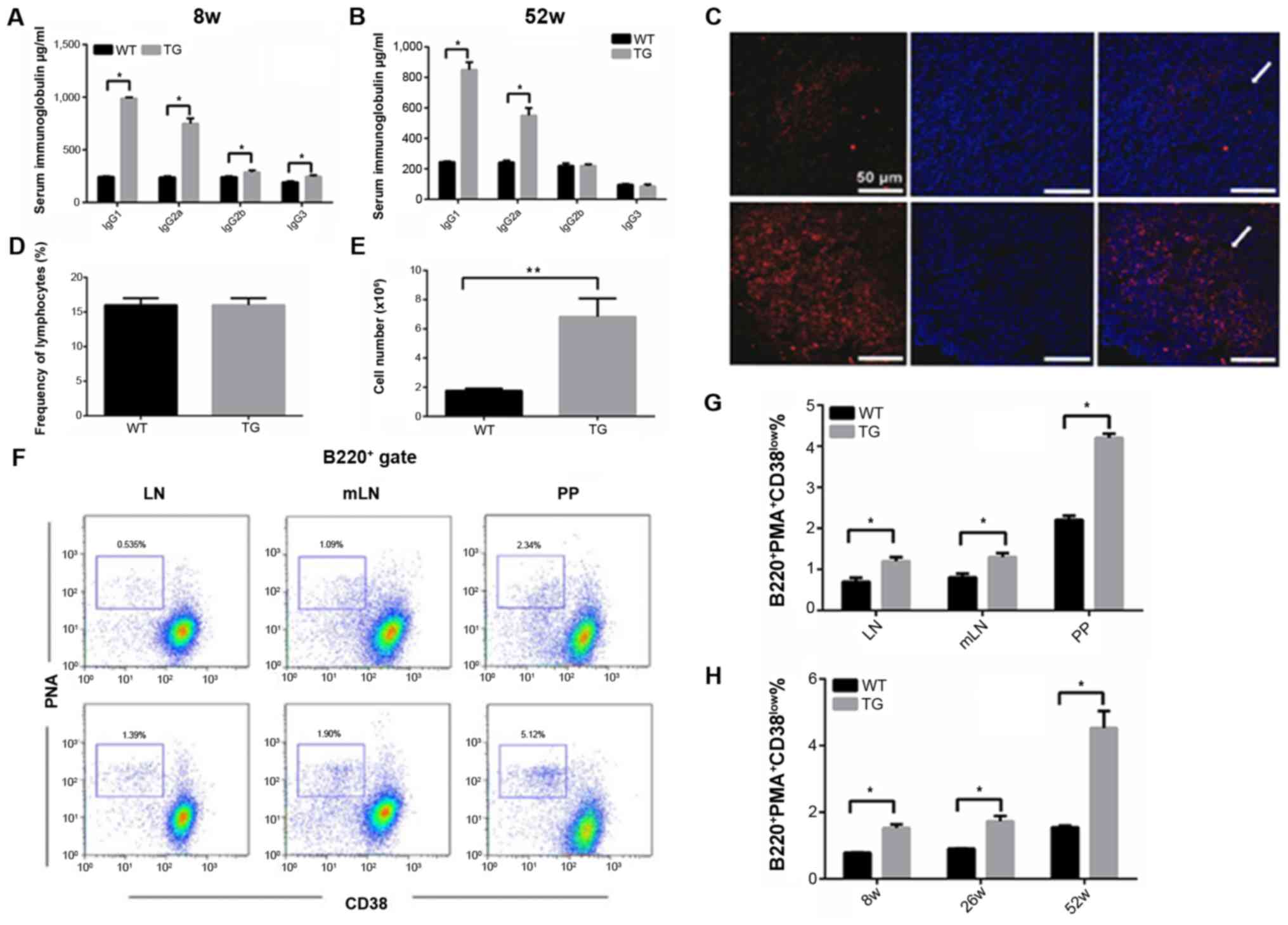
The levels of IgGl, IgG2b, IgG2a, and IgG3 in the
serum of 8-week old transgenic mice were 3–6 times higher than
those in the wild-type mice. IgGl and IgG2a serum levels in 52-week
old transgenic mice were still higher compared with the wild-type
mice, however IgG2b and IgG3 levels were normal in 52-week old
transgenic mice (Fig. 3A and B).
In miR-21 transgenic mice, spontaneous germinal
center reaction was detected in peripheral lymphoid organs
(Fig. 3C), and the proportion of B
cells in the germinal center in the miR-146 transgenic mice was
significantly higher than that in the wild-type mice (Fig. 3D and E). Similar results were observed
in the mesenteric lymph nodes, PP globules, and spleen (Fig. 3F and G).
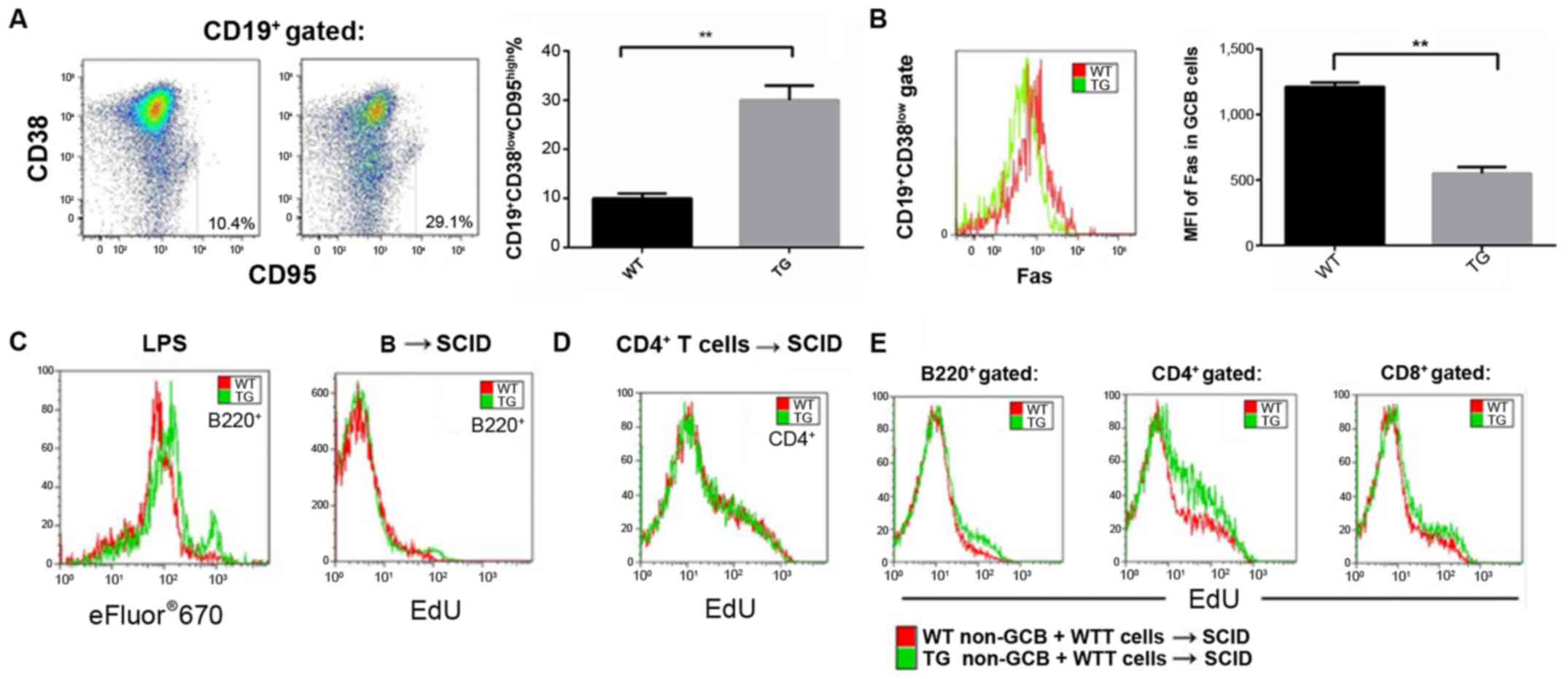
miR-21 downregulates the Fas protein
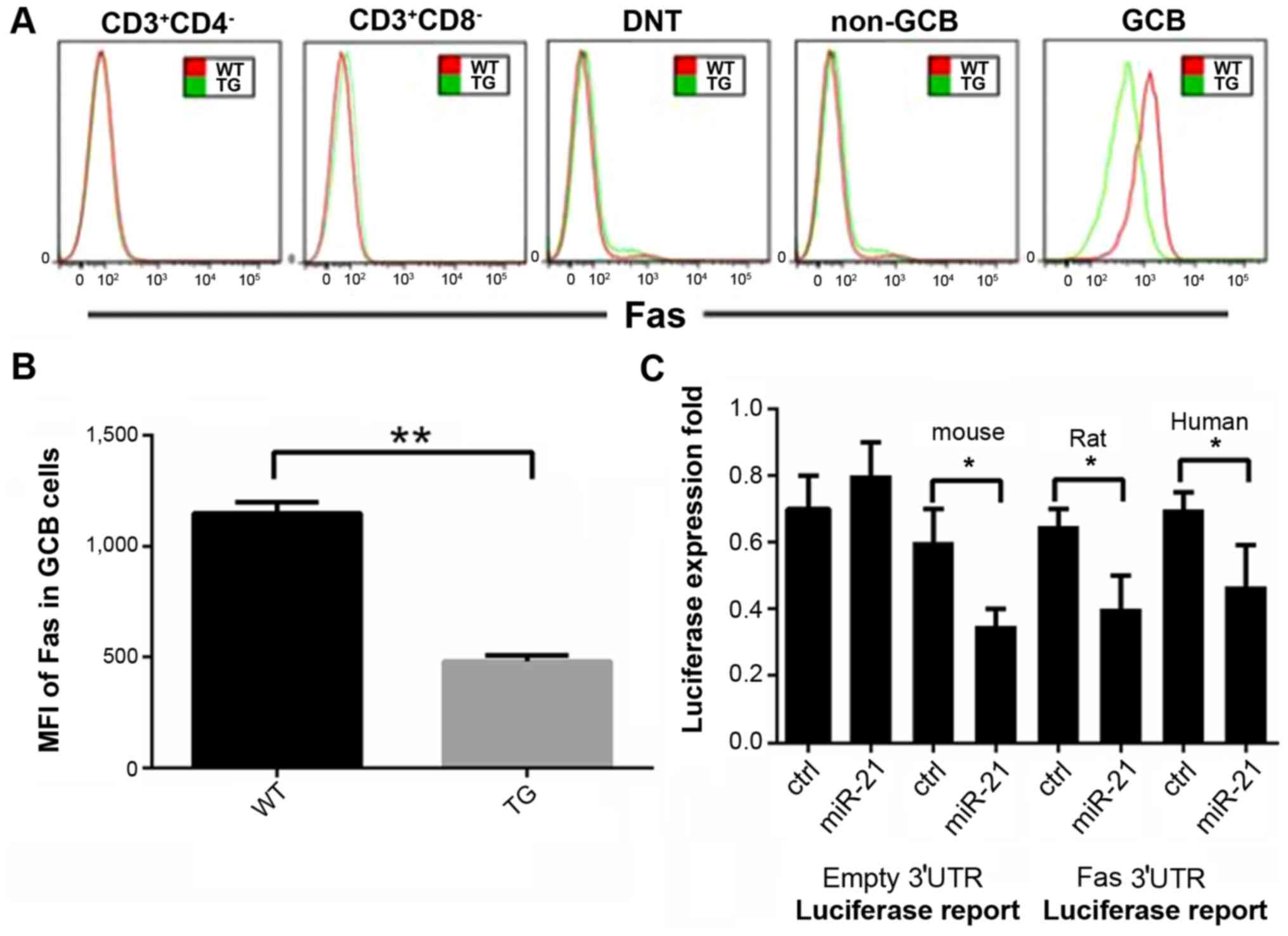
expression in GCB cells
Fas protein expression in various subsets of the
lymphocytes including CD3+CD4+ T cells,
CD3+CD8+ T cells,
CD3+CD4+CD8 cells, TCRaIB+DNT
cells, B220+PNA+CD38low non-GCB
cells, B220+PNA and CD38high GCB cells were
evaluated in the transgenic mice, using flow cytometry. High Fas
protein expression was only detected in the GCB cell subsets in
wild-type mice (Fig. 4A). miR-21
overexpression effectively reduced Fas protein expression in GCB
cells. Compared to wild-type mice, the Fas average fluorescence
intensity in the GCB cells in the transgenic mice decreased by more
than 50% (Fig. 4B). Our results
demonstrated that Fas was the direct target of miR-21 (Fig. 4C).
miR-21 strengthens homeostatic
proliferation of lymphocytes
Natural B cells or CD4+ T cells from
transgenic and wild-type mice were transferred into the SCID
recipient mice with immunodeficiency. Homeostatic proliferation of
lymphocytes was studied (EdU incorporation assay). Seven days after
adoptive transfer, the proliferation level of the natural B cells
originating from the transgenic mice was significantly higher than
those B cells originating from the wild-type mice. However, no
significant difference was observed in the case of CD4+
T cells (Fig. 5).
Discussion
It has been shown that miR-21, miR-132, and miR-155
levels are upregulated when the THP cells are stimulated by LPS.
Other reports demonstrated that miR-21 was a NF-κB-dependent
natural immune negative feedback regulatory factor (20), and the major target genes were shown
to be TRAF6 and IRAK-1/2 (21). It
has been reported that, miR-21 gene knockout mice usually suffer
from spontaneous autoimmune disorders and die before reaching
maturity. Following characteristics were observed in these mice: i)
Spleen and lymph node enlargement, ii) inflammatory infiltration in
multiple organs, and iii) imbalance in peripheral immune tolerance
of T cells. Moreover, myeloid cell proliferation and peripheral
lymphoid organs tumors might occur in these mice.
T cell survival, activation and apoptosis rate in
transgenic mice with overexpressed miR-21 were not different
compared with wild-type mice. It seems that miR-21 regulates the T
cell mediated immune response via a negative feedback mechanism.
TCR transfers the antigen-activated signals to T cells and the
three signal pathways implicated in this induction are: NFAT, AP-1
and NF-κB (22). Imbalance of NF-κB
in T cells may lead to immuno-inflammatory responses mediated by T
cells and malignant tumor genesis (23). We detected a large number of DNT cells
in the spleen. This observation along with other observations such
as enlargement of lymph nodes and spleens and presence of
inflammatory cell infiltration in liver and lung were in line with
the characteristics of human autoimmune lymphoid hyperplasia
syndrome (ALPS).
Although spontaneous ALPS occurred in miR-21
transgenic mice, the majority of ALPS patients may be affected by
non-malignant enlargement of lymph nodes and spleen at a tender
age. In these cases, the number of DNT cells increased
significantly and IgG level in the serum was considerably elevated
(24).
Spleen and lymph gland enlargement is often observed
in 3-week old miR-21 transgenic mice and a peak is usually reached
at 4 weeks after birth. In all young transgenic mice, we observed
an increase in the number of DNT cells in the spleen, while IgGl,
IgG2b, IgG2a, and IgG3 levels in 8-week old transgenic mice were
higher than those in wild-type mice. These conditions were relieved
at 52 weeks. miR-21 transgenic mice had an extremely high morbidity
of pulmonary and hepatic inflammatory cell infiltration.
Clinically, 4–5% of the ALPS patients have infiltrative pulmonary
lesions and liver dysfunctions.
Genetic defects in typical ALPS patients mainly
occur in the apoptosis pathway. These defects directly break the
lymphocytes homeostasis and immune tolerance (25). Prior reports have shown that
Fas gene mutation and Fas protein downregulation are the
major causes for ALPS onset (26).
Here, we demonstrated that miR-21 overexpression can downregulate
the Fas expression in the GCB cells and finally lead to ALPS in
transgenic mice. However, in transgenic mice, Fas dysfunction
occurs in B cells with highly-expressed miR-21.
In our transgenic mice, a decrease in Fas expression
on the surface of the natural B cells allowed an easy
transformation into GCB cells. It seems that Fas downregulation in
GCB cells may disturb lymphocyte homeostasis and excessive lymphoid
hyperplasia. Furthermore, Fas downregulation in GCB cells can
promote the survival of mature T cells. Usually, activated T cells
lose their CD4 or CD8 co-receptors, therefore an accumulation in
DNT cells may occur. These observations supported the idea that
miR-21 is involved in ALPS pathogenesis and may provide a new
explanation of ALPS pathogenesis, particularly in cases with viral
infection and chronic inflammation. We believe that the miR-21
deficient mice experienced an autoimmune disease similar to
ALPS.
References
|
1
|
Halász T, Horváth G, Pár G, Werling K,
Kiss A, Schaff Z and Lendvai G: miR-122 negatively correlates with
liver fibrosis as detected by histology and FibroScan. World J
Gastroenterol. 21:7814–7823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Croci S, Zerbini A, Boiardi L, Muratore F,
Bisagni A, Nicoli D, Farnetti E, Pazzola G, Cimino L, Moramarco A,
et al: MicroRNA markers of inflammation and remodelling in temporal
arteries from patients with giant cell arteritis. Ann Rheum Dis.
20:78462015.
|
|
3
|
Punga AR, Andersson M, Alimohammadi M and
Punga T: Disease specific signature of circulating miR-150-5p and
miR-21-5p in myasthenia gravis patients. J Neurol Sci. 356:90–96.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu XN, Ye YX, Niu JW, Li Y, Li X, You X,
Chen H, Zhao LD, Zeng XF, Zhang FC, et al: Defective PTEN
regulation contributes to B cell hyperresponsiveness in systemic
lupus erythematosus. Sci Transl Med. 6:246ra99. 2014. View Article : Google Scholar
|
|
5
|
Quinn SR and O'Neill LA: The role of
microRNAs in the control and mechanism of action of IL-10. Curr Top
Microbiol Immunol. 380:145–155. 2014.PubMed/NCBI
|
|
6
|
Ma X, Zhou J, Zhong Y, Jiang L, Mu P, Li
Y, Singh N, Nagarkatti M and Nagarkatti P: Expression, regulation
and function of microRNAs in multiple sclerosis. Int J Med Sci.
11:810–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang ZM, Fang M, Wang JP, Cai PC, Wang P
and Hu LH: Clinical relevance of plasma miR-21 in new-onset
systemic lupus erythematosus patients. J Clin Lab Anal. 28:446–451.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haider BA, Baras AS, McCall MN, Hertel JA,
Cornish TC and Halushka MK: A critical evaluation of microRNA
biomarkers in non-neoplastic disease. PLoS One. 9:e895652014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smigielska-Czepiel K, van den Berg A,
Jellema P, van der Lei RJ, Bijzet J, Kluiver J, Boots AM, Brouwer E
and Kroesen BJ: Comprehensive analysis of miRNA expression in
T-cell subsets of rheumatoid arthritis patients reveals defined
signatures of naive and memory Tregs. Genes Immun. 15:115–125.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chafin CB, Regna NL, Hammond SE and Reilly
CM: Cellular and urinary microRNA alterations in NZB/W mice with
hydroxychloroquine or prednisone treatment. Int Immunopharmacol.
17:894–906. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bao H, Hu S, Zhang C, Shi S, Qin W, Zeng
C, Zen K and Liu Z: Inhibition of miRNA-21 prevents fibrogenic
activation in podocytes and tubular cells in IgA nephropathy.
Biochem Biophys Res Commun. 444:455–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Peng W, Ouyang X, Li W and Dai Y:
Circulating microRNAs as candidate biomarkers in patients with
systemic lupus erythematosus. Transl Res. 160:198–206. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mycko MP, Cichalewska M, Machlanska A,
Cwiklinska H, Mariasiewicz M and Selmaj KW: MicroRNA-301a
regulation of a T-helper 17 immune response controls autoimmune
demyelination. Proc Natl Acad Sci USA. 109:pp. E1248–E1257. 2012;
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stagakis E, Bertsias G, Verginis P, Nakou
M, Hatziapostolou M, Kritikos H, Iliopoulos D and Boumpas DT:
Identification of novel microRNA signatures linked to human lupus
disease activity and pathogenesis: miR-21 regulates aberrant T cell
responses through regulation of PDCD4 expression. Ann Rheum Dis.
70:1496–1506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo
X, Li J, Zhou H, Tang Y and Shen N: MicroRNA-21 and microRNA-148a
contribute to DNA hypomethylation in lupus CD4+ T cells
by directly and indirectly targeting DNA methyltransferase 1. J
Immunol. 184:6773–6781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruan Q, Wang T, Kameswaran V, Wei Q,
Johnson DS, Matschinsky F, Shi W and Chen YH: The microRNA-21-PDCD4
axis prevents type 1 diabetes by blocking pancreatic beta cell
death. Proc Natl Acad Sci USA. 108:pp. 12030–12035. 2011;
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bostjancic E and Glavac D: Importance of
microRNAs in skin morphogenesis and diseases. Acta Dermatovenerol
Alp Pannonica Adriat. 17:95–102. 2008.PubMed/NCBI
|
|
18
|
Alisi A, Da Sacco L, Bruscalupi G,
Piemonte F, Panera N, de Vito R, Leoni S, Bottazzo GF, Masotti A
and Nobili V: Mirnome analysis reveals novel molecular determinants
in the pathogenesis of diet-induced nonalcoholic fatty liver
disease. Lab Invest. 91:283–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dey N, Das F, Mariappan MM, Mandal CC,
Ghosh-Choudhury N, Kasinath BS and Choudhury GG: MicroRNA-21
orchestrates high glucose-induced signals to TOR complex 1,
resulting in renal cell pathology in diabetes. J Biol Chem.
286:25586–25603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bergman P, James T, Kular L, Ruhrmann S,
Kramarova T, Kvist A, Supic G, Gillett A, Pivarcsi A and Jagodic M:
Next-generation sequencing identifies microRNAs that associate with
pathogenic autoimmune neuroinflammation in rats. J Immunol.
190:4066–4075. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fognani E, Giannini C, Piluso A, Gragnani
L, Monti M, Caini P, Ranieri J, Urraro T, Triboli E, Laffi G, et
al: Role of microRNA profile modifications in hepatitis C
virus-related mixed cryoglobulinemia. PLoS One. 8:e629652013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu WD, Pan HF, Li JH and Ye DQ:
MicroRNA-21 with therapeutic potential in autoimmune diseases.
Expert Opin Ther Targets. 17:659–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koelsch KA, Webb R, Jeffries M, Dozmorov
MG, Frank MB, Guthridge JM, James JA, Wren JD and Sawalha AH:
Functional characterization of the MECP2/IRAK1 lupus risk haplotype
in human T cells and a human MECP2 transgenic mouse. J Autoimmun.
41:168–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimura J, Ichii O, Miyazono K, Nakamura T,
Horino T, Otsuka-Kanazawa S and Kon Y: Overexpression of Toll-like
receptor 8 correlates with the progression of podocyte injury in
murine autoimmune glomerulonephritis. Sci Rep. 4:72902014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong L, Wang X, Tan J, Li H, Qian W, Chen
J, Chen Q, Wang J, Xu W, Tao C, et al: Decreased expression of
microRNA-21 correlates with the imbalance of Th17 and Treg cells in
patients with rheumatoid arthritis. J Cell Mol Med. 18:2213–2224.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van der Geest KS, Smigielska-Czepiel K,
Park JA, Abdulahad WH, Kim HW, Kroesen BJ, van den Berg A, Boots
AM, Lee EB and Brouwer E: SF Treg cells transcribing high levels of
Bcl-2 and microRNA-21 demonstrate limited apoptosis in RA.
Rheumatology (Oxford). 54:950–958. 2015. View Article : Google Scholar : PubMed/NCBI
|