Introduction
With an increase in life expectancy and changes in
dietary structure, the incidence rate of prostate cancer has shown
an upward trend been in China, at 4.3 per 100,000 according to a
study published in 2008 (1). Although
prostate specific antigen (PSA) tests, digital rectal examination
(DRE), transrectal ultrasound (TRUS) and magnetic resonance imaging
(MRI) have been widely adopted in the diagnosis of numerous
prostate diseases, biopsy is the only current diagnostic technique
for prostate cancer (2). Prostate
biopsy may be performed transrectally, the most common approach
(3), or through the urethra or
perineum. In addition, the level of PSA in blood is often used to
identify prostate cancer, but it is not a definitive indicator as
PSA level can also be affected by other factors such as
inflammation and infection. At present, prostate biopsy is required
for the specific diagnosis of prostate cancer.
However, the number of core samples that should be
taken during prostate biopsy is still debated. It is generally
accepted that increasing the number of samples taken could improve
the detection rate of prostate cancer (4–7), but the
risk of side-effects increase too. In a retrospective study of
3,000 patients, the number of clinical complications increased
significantly with the increase of number of samples taken during
transperineal prostate biopsy (6).
However, if fewer samples are taken this could lead to the
cancerous lesions being missed, under-sampling and an increased
false negative rate (4,5). As a result, some patients would have to
undergo prostate biopsy again.
Previously, imaging techniques-based biopsy has
provided a targeted and advanced modality for prostate cancer
detection (3). Even though an
increasing the number of random samples taken may have only
marginal diagnostic value, increasing the number of targeted core
samples (transperineal template-guided biopsies) taken, which are
only taken from lesions identified and suspected via preoperative
imaging tests to be cancerous, may be a better approach for
transperineal prostate biopsy. The present study aimed to
investigate whether this 12+X modality, as described in the
Methods, for prostate biopsy could be an improvement compared with
the traditional 12-core modality by comparing the detection rate
and number of postoperative complications of the two methods.
Materials and methods
Clinical samples
The data of patients who received TRUS-guided
prostate biopsy for suspected prostate cancer in the General
Hospital of The People's Liberation Army (Beijing, China) between
September 2009 and May 2014 was retrospectively analyzed. The
present stidu was approved by the ethics committee of the General
Hospital of The People's Liberation Army. In total, 1,300 patients
were taken up in the study, with a mean age of 70.5 (34–98) years.
Among these patients, 785 patients were suspected to have prostate
cancer subsequent to the detection of an elevated level of PSA. In
total, 392 patients were suspected to have prostate cancer due to
abnormalities in the prostate detected via ultrasound or MRI; 912
of the cases were accompanied with obstructive symptoms of the
lower urinary tract such as frequent urination, urgent urination,
nocturia and dysuria. PSA levels were monitored twice and a DRE,
transrectal ultrasound and enhanced dynamic MRI were performed on
each patient. The inclusion criteria consisted of patients with:
Total (T)-PSA >10 ng/ml, regardless of free/total (f/t) PSA or
PSA density (PSAD); T-PSA between 4 and 10 ng/ml, with a
concomitant abnormal f/t PSA or PSAD measurement; a palpable and
hard nodule through DRE; and hypoechoic regions of the prostate
identified through TRUS or transabdominal ultrasound (TAUS). The
exclusionary criteria were: A history of prostate cancer;
undergoing anti-androgen therapies; and other variables including
patients with a local skin infection, blood coagulation
dysfunction, diabetes mellitus and/or not well-controlled blood
glucose, or cachexia.
Among the included patients, 1,043 were receiving
their first biopsy. Patients were randomly assigned to the 12-core
group or the 12+X core group (Table
I). For the patients of the 12+X core group, additional core
samples were taken from suspected cancerous lesions identified
through pre-biopsy MRI and ultrasound examinations. The Gleason
scores of the diagnosed patients were tested (8). All patients provided informed consent
prior to the beginning of the study.
 | Table I.Patient information. |
Table I.
Patient information.
| Characteristic | 12-core group | 12+X core group |
|---|
| Number of cases | 363 | 937 |
| Mean age (years) | 71.1 | 70.2 |
| Ethnicity-Han, % | 95.0 | 96.1 |
| Family history of
prostate cancer, % | 3.6 | 3.9 |
| Mean prostate volume,
ml | 39.2 | 37.8 |
| Mean peak value of
TPSA, ng/ml | 15.4 | 16.7 |
| Anomaly in DRE,
% | 66.9 | 65.1 |
| Anomaly in TRUS,
% | 72.1 | 74.2 |
| Anomaly in MRI,
% | 70.5 | 72.4 |
| First biopsy, % | 77.4 | 81.3 |
| Mean surgery time,
min | 17.7 | 21.5 |
| Mean number of core
samples taken | 12 | 15.5 |
Surgery
The coagulation function of patients including
prothrombin time, activated partial thromboplastin time, thrombin
time and fibrinogen was tested by peripheral blood. Routine blood
and urine tests were also performed prior to the biopsy. Cifran
(0.5 g, 4 times a day) and metronidazole (0.5 g, twice a day) were
administered orally 1 day prior to biopsy to prevent infection. On
the day of biopsy, glycerin was administered rectally to facilitate
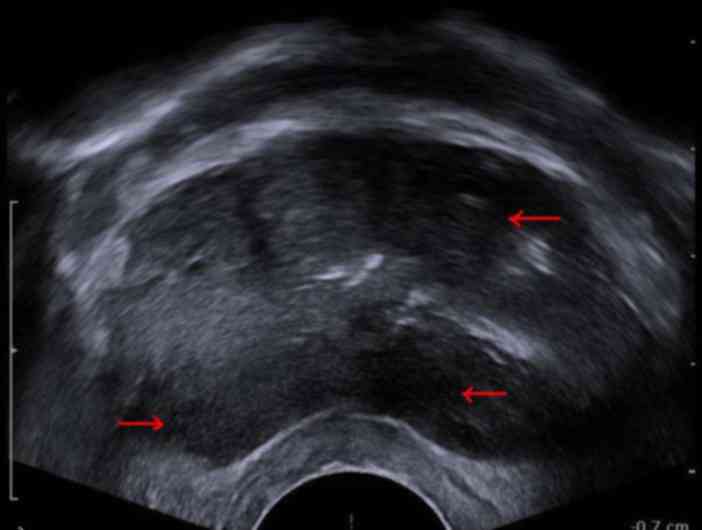
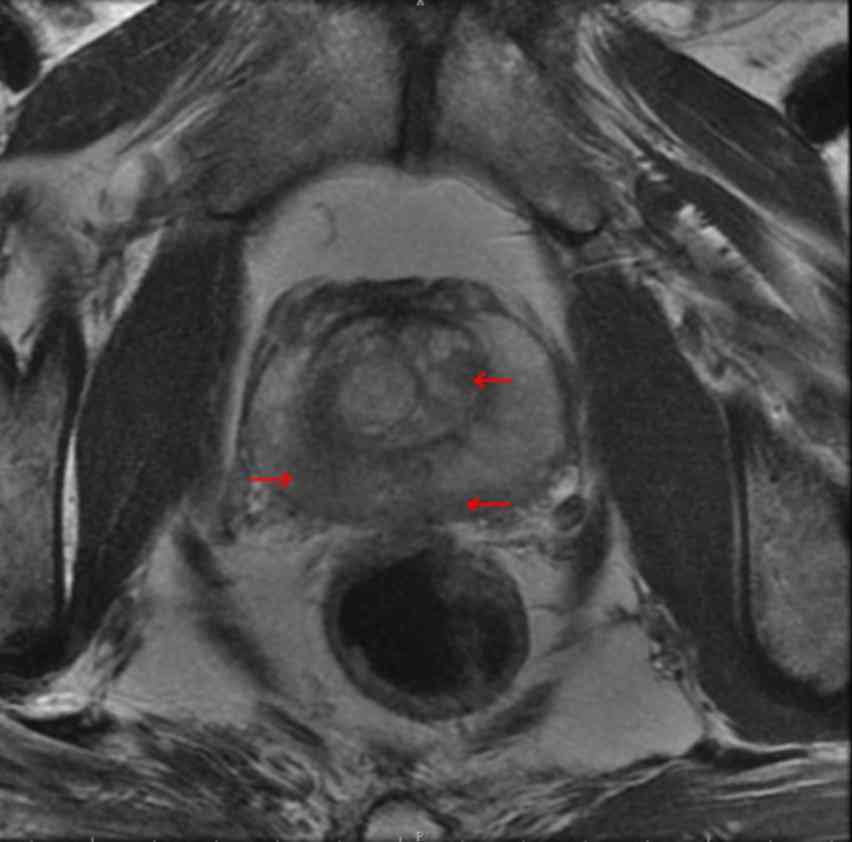
defecation. Signa Excite HD 3.0T MRI machine (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) and a B-K 2102 Ultrasound (BK
Medical ApS, Herlev, Denmark) were used prior to the operation to
obtain images with suspected lesions marked (Figs. 1–2). The
local anesthetic lidocaine (1%) was administered to 973 patients
via injection through the perineal subcutaneous tissue and outside
the prostatic capsule. Caudal block anesthesia was administered to
75 patients via lidocaine (1%) injection. The remaining 252
patients received epidural anesthesia, a mixture of 0.375%
ropivacaine + 1% lidocaine 0.6 ml/kg.
The patients were then placed in the lithotomy
position. Following a routine perineal disinfection procedure, a
rectal ultrasound probe (Dual-plane; Computerised Medical System
Company, Inc., St. Louis, MO, USA) was placed in the rectum of the
patients to observe the shape of their prostates. Subsequent to the
fixation of the probe and the perineal template (Centers for
Medicare and Medicaid Services, Baltimore, MD USA), the
ultrasound-guided and perineal template-mapped prostate biopsy was
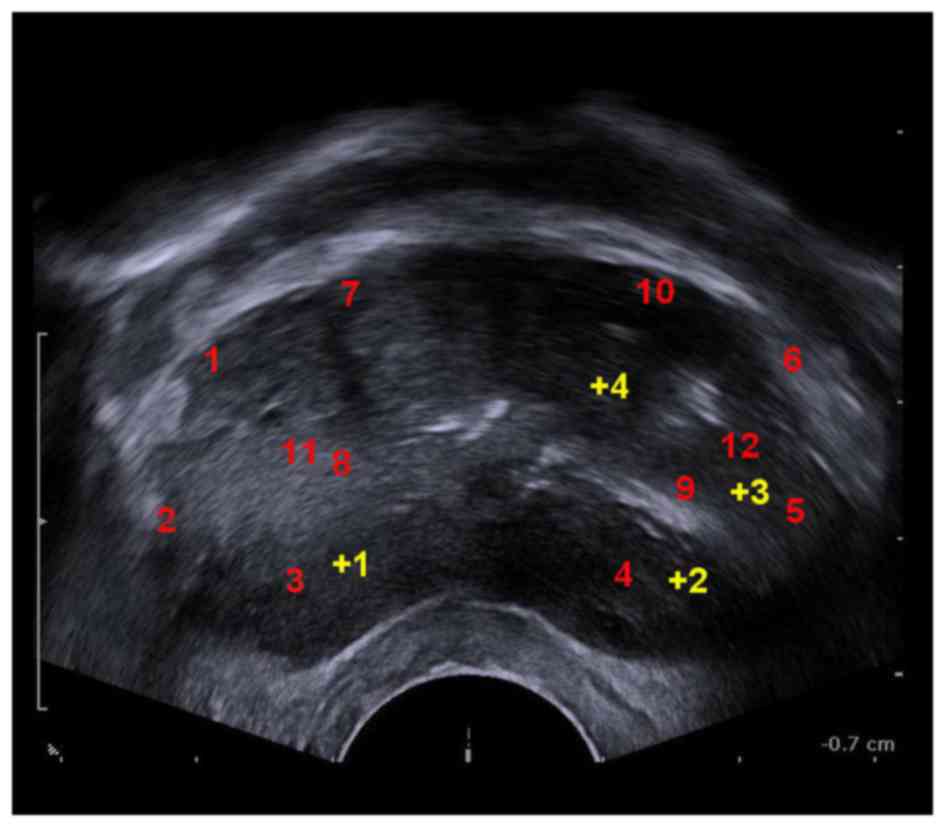
conducted on the patients. For the patients of the 12-core group,
the locations of 12-core samples taken are showed in Fig. 3. For the patients of the 12+X core
group, additional core samples were taken from suspected cancerous
lesions identified through pre-biopsy MRI and ultrasound
examinations (Fig. 3).
Oral administration of the aforementioned dose and
concentration of cifran and metronidazole was continued 3 days
post-operatively. A high water intake was recommended for patients
presenting with secondary hematuria. If the symptoms did not
resolve themselves, patients were catheterized until full
remission. Patients that presented with urinary retention were
catheterized for 1 week and orally administered with tamsulosin
hydrochloride in the form of sustained release capsules (0.2 mg, 4
times a day). All patients were followed up 1 month subsequent to
the operation to record their pathological results and any
complications.
Statistical analysis
Statistical analysis was conducted to compare the
differences in PSA, f/t PSA and PSAD between the 2 groups and the
positive rate of different auxiliary examination methods. SPSS 13.0
(SPSS, Inc., Chicago, IL, USA) was used to perform the data
analysis. A χ2 test was conducted to compare the
positive detection rates of 2 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Biopsies were successfully performed on all
patients. The mean number of samples taken from patients in the
12+X core group was 15.5, ranging from 13 to 24 core samples per
patient. The operation time ranged between 15 and 30 min, with a
mean of 20.4 min. Based on the biopsy test results, the presence of
prostate cancer was confirmed for 540 patients (41.5%). Among this
group, 527 patients were found to exhibit adenocarcinoma and 13
patients were affected by other types of cancer. In addition, it
was confirmed that 57 patients displayed prostate intraepithelial
neoplasia (4.4%) and that 703 patients exhibited prostate
hyperplasia and prostatitis (54.1%). The Gleason scores of the
diagnosed patients are shown in Table
II. Compared with the 12-core group, 8.7% patients appeared to
show an increase in Gleason scores in the 12+X core group.
 | Table II.Gleason score of the two groups. |
Table II.
Gleason score of the two groups.
| Gleason Score | 12 core group, % | 12+X core group,
% | P-value |
|---|
| Patients, n | 125 | 415 |
|
| ≤6 | 35.2 | 26.6 | 0.063 |
| 7 | 34.4 | 40.0 | 0.127 |
| 8 | 15.2 | 18.3 | 0.341 |
| 9 | 13.6 | 13.7 | 0.934 |
| 10 |
1.2 |
1.4 | 0.874 |
The positive detection rate of the 12-core group was
34.4% (125/363), while that of the 12+X core group was 44.3%
(415/937). The positive detection rates for the patients are
expressed in Table III. Patients
with abnormalities identified through DRE, TRUS, or MRI were found
to have positive rates of ~24.0, 30.1, and 59.2%, respectively. The
overall incidence rate of complications was 19.0% (247/1,300),
including 6 patients with lidocaine absorption into the blood, 201
patients with post-operative hematuria, 14 patients with perineal
hematoma, 21 patients with acute urinary retention and 5 patients
with urinary tract infections accompanied by fever. The
aforementioned complications were all resolved subsequent to
treatment and did not result in any severe complications. The
incidence rate of post-operative complications for the 12 core and
12+X core groups were 16.8% (61/363) and 19.9% (186/937),
respectively. The difference between the groups was demonstrated to
be insignificant (P=0.175). A comparison of a positive needles
outcome of prostate cancer in the different groups and the
distribution of the lesions are displayed in Table IV.
 | Table III.Positive rates for patients with
different PSA, f/t PSA and PSAD. |
Table III.
Positive rates for patients with
different PSA, f/t PSA and PSAD.
|
| 12 core group | 12+X cores group |
|
|---|
|
|
|
|
|
|---|
| Group | Patients | Positive rate, % | Patients | Positive rate, % | P-value |
|---|
| TPSA, ng/ml |
|
<4 | 22 | 9.1 | 55 | 21.8 |
0.003 |
| 4–10 | 77 | 9.1 | 216 | 21.3 |
0.004 |
|
10–20 | 148 | 16.1 | 373 | 39.7 |
0.015 |
|
>20 | 116 | 61.2 | 293 | 80.5 |
0.041 |
| f/tPSA |
|
≥0.16 | 21 | 9.5 | 56 | 25.0 | <0.001 |
|
<0.16 | 56 | 8.9 | 160 | 20.0 |
0.021 |
| PSAD |
|
≥0.15 | 276 | 15.2 | 734 | 23.4 |
0.037 |
|
<0.15 | 87 | 7.5 | 203 | 10.1 |
0.234 |
| Total | 363 | 34.4 | 937 | 44.3 | 0.039 |
 | Table IV.Comparison of a positive needles
outcome of prostate cancer and the distribution of the lesions. |
Table IV.
Comparison of a positive needles
outcome of prostate cancer and the distribution of the lesions.
| Distribution | 12 core group,
% | 12+X core group,
% | P-value |
|---|
| Positive needle,
n |
4.3 |
5.1 | 0.254 |
|
Fronta | 84.1 | 85.3 | 0.763 |
|
Backa | 80.4 | 84.2 | 0.127 |
Discussion
With the wide application of TRUS in clinical
practice, TRUS-guided prostate biopsy has become the gold standard
for diagnosis of prostate cancer (9).
Hodge introduced the TRUS-guided 6 core biopsy in 1989 (7). This technique was later criticized for
its high false negative rate, which was as high as 20–30% (10). Therefore, 8-, 10-, 12- and 13-core and
saturated puncture techniques were proposed to improve the
detection rate for prostate cancer. Numerous studies have confirmed
the diagnostic value of an increase in the number of samples taken
for the detection of prostate cancer (6,7,11,12).
However, alternative studies found that a slight increase in the
number of cores would not significantly increase the detection rate
of prostate cancer (13,14). A possible explanation for such a
dispute might be associated with the fact that initial biopsies
mostly took samples randomly. The present study found that taking
additional samples from prostate sites suspected to be cancerous,
identified using pre-operative imaging tests, provided an improved
biopsy modality. The 12+X core biopsy modality, which combined
random puncture with targeted puncture, enhanced detection rate by
28.8% with an average of only 3.5 additional samples taken.
Chen et al (13) found that ~80% patients with prostate
cancer exhibited multifocal distribution, mostly located in the
peripheral zone of the prostate. Small-sized cancerous lesions were
mainly located in the apex zone. For prostate cancer patients with
normal or slightly elevated PSA, most lesions were located in the
apex zone (14). Vis et al
(15) conducted an in vitro
simulation experiment on the specimens taken from 40 patients that
underwent radical prostatectomy and found that the positive rate
was higher in transperineal prostate biopsies compared with
transrectal prostate biopsies. The comparatively lower false
negative rate of perineal prostate biopsy may be explained by the
fact that during a perineal prostate biopsy the needle will
penetrate the apex region of the prostate. More tissue can be
obtained from the peripheral zone of the prostate, which is also
the predilection site of prostate cancer (16). However, with respect to transrectal
prostate biopsies, more tissue will be obtained from the
transitional zone of the prostate, and the peripheral zone of the
prostate is more likely to be missed. However, there are also
studies suggesting that there is no significant difference between
these two approaches in terms of the false negative rate (17–19). As a
result, a widely accepted perspective is that the number of
puncture cores is more important than the biopsy approach taken
(20).
Perineal prostate biopsy was chosen in the present
study because this approach allows the use of perineal templates.
The use of perineal templates in perineal prostate biopsies can
overcome the deviation away from the site of interest caused by
using a long needle, and the needle parallel to the probe can avoid
injuring the rectum. Additionally, an even distribution of needle
gage between 0.5 and 1 cm may effectively reduce false negative
rate, theoretically (21). The
present study suggests other benefits of transperineal prostate
biopsy. Firstly, disinfecting perineal skin is much easier than
disinfecting that of the rectum. As a result, the rate of
biopsy-associated infection can be greatly reduced, and the risk of
post-operative severe infection can be effectively controlled via
the administration of prophylactic antibiotics taken one day prior
to the operation (22). Secondly, due
to the reduced rate of complications associated with transperineal
prostate biopsy, increasing the number of samples taken would be
more advisable compared with alternative prostate biopsies. In the
present study, more samples were taken in 12+X core group compared
with the 12-core group, but the incidence rate of complications did
not increase significantly between groups.
Previous studies highlight the clinical significance
of DRE, TRUS, MRI and PSA as indicators in the diagnosis of
prostate cancer (23,24). The present study suggests that
high-field MRI could greatly enhance the detection rate of prostate
cancer. The close analysis of images obtained from an MRI of the
prostate helped identify suspected cancerous lesions, and
TRUS-guided prostate biopsies targeting these lesions significantly
enhanced the detection rate of prostate cancer. PSA is still a
clinically important index for diagnosing prostate cancer. The
positive detection rate is significantly higher for patients with
T-PSA ≥10 ng/ml, T-PSA between 4 and 10 ng/ml, F/T PSA <0.16 and
PSAD ≥0.15. Therefore, the aforementioned indicators should be
fully analyzed prior to the selection and implementation of biopsy,
especially for patients with hyperplasia of the prostate gland and
PSA values in the gray area. In previous years, with the rapid
development of auxiliary imaging techniques, new high-accuracy
biopsy techniques have emerged, including MRI-guided prostate
biopsy, transrectal TRUS and MRI image fusion-guided prostate
biopsy, real-time ultrasound elastography-guided prostate biopsy
and ultrasound histoscanning, which can significantly enhance the
detection rate of prostate cancer (25–27).
The present study demonstrates that images obtained
from enhanced MRI facilitate the analysis and identification of
cancer lesions. The TRUS-guided transperineal prostate 12+X core
biopsy with template can significantly enhance the detection rate
of prostate cancer, due to the combination of random puncture with
targeted puncture. In addition, this biopsy modality exhibits good
accuracy and safety. Therefore, the technique presented in the
present study is advisable for clinical practice.
Acknowledgements
The present study was supported by the Clinical
research support fund of the General Hospital of The People's
Liberation Army (grant no. 2015FC-TSYS-2008). The authors thank
Professor Zhang for technical support and assistance during the
surgical procedure.
References
|
1
|
Peng P, Gong YM, Bao PP, Ke JZ, Xiang YM,
Zhang ML and Zheng Y: Estimates and prediction of prostate cancer
incidence, mortality and prevalence in China, 2008. Zhonghua Liu
Xing Bing Xue Za Zhi. 33:1056–1059. 2012.(In Chinese). PubMed/NCBI
|
|
2
|
Carter HB: American Urological Association
(AUA) guideline on prostate cancer detection: Process and
rationale. BJU Int. 112:543–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simmons LA, Ahmed HU, Moore CM, Punwani S,
Freeman A, Hu Y, Barratt D, Charman SC, Van der Meulen J and
Emberton M: The PICTURE study-prostate imaging (multi-parametric
MRI and Prostate HistoScanning™) compared to transperineal
ultrasound guided biopsy for significant prostate cancer risk
evaluation. Contemp Clin Trials. 37:69–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abd TT, Goodman M, Hall J, Ritenour CW,
Petros JA, Marshall FF and Issa MM: Comparison of 12-core versus
8-core prostate biopsy: Multivariate analysis of large series of US
veterans. Urology. 77:541–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoon BI, Shin TS, Cho HJ, Hong SH, Lee JY,
Hwang TK and Kim SW: Is it effective to perform two more prostate
biopsies according to prostate-specific antigen level and prostate
volume in detecting prostate cancer? Prospective study of 10-core
and 12-core prostate biopsy. Urol J. 9:491–497. 2012.PubMed/NCBI
|
|
6
|
Pepe P and Aragona F: Morbidity after
transperineal prostate biopsy in 3000 patients undergoing 12 vs 18
vs more than 24 needle cores. Urology. 81:1142–1146. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hodge KK, McNeal JE, Terris MK and Stamey
TA: Random systematic versus directed ultrasound guided transrectal
core biopsies of the prostate. J Urol. 142:71–75. 1989.PubMed/NCBI
|
|
8
|
Zakian KL, Sircar K, Hricak H, Chen HN,
Shukla-Dave A, Eberhardt S, Muruganandham M, Ebora L, Kattan MW,
Reuter VE, et al: Correlation of proton MR spectroscopic imaging
with gleason score based on step-section pathologic analysis after
radical prostatectomy. Radiology. 234:804–814. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eskicorapci SY, Baydar DE, Akbal C,
Sofikerim M, Günay M, Ekici S and Ozen H: An extended 10-core
transrectal ultrasonography guided prostate biopsy protocol
improves the detection of prostate cancer. Eur Urol. 45:444–449.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Sutter Ph, Coibion M, Vosse M, Hertens
D, Huet F, Wesling F, Wayembergh M, Bourdon C and Autier Ph: A
multicentre study comparing cervicography and cytology in the
detection of cervical intraepithelial neoplasia. Br J Obstet
Gynaecol. 105:613–620. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bjurlin MA and Taneja SS: Standards for
prostate biopsy. Curr Opin Urol. 24:155–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rochester MA, Griffin S, Chappell B and
McLoughlin J: A prospective randomised trial of extended core
prostate biopsy protocols utilizing 12 versus 15 cores. Urol Int.
83:155–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen ME, Johnston DA, Tang K, Babaian RJ
and Troncoso P: Detailed mapping of prostate carcinoma foci: Biopsy
strategy implications. Cancer. 89:1800–1809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan W, Li H, Zhou Y, Huang Z, Rong S, Xia
M, Ji Z, Chen J and Jiang Y: Prostate carcinoma spatial
distribution patterns in Chinese men investigated with systematic
transperineal ultrasound guided 11-region biopsy. Urol Oncol.
27:520–524. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vis AN, Boerma MO, Ciatto S, Hoedemaeker
RF, Schröder FH and van der Kwast TH: Detection of prostate cancer:
A comparative study of the diagnostic efficacy of sextant
transrectal versus sextant transperineal biopsy. Urology.
56:617–621. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Engelbrecht MR, Huisman HJ, Laheij RJ,
Jager GJ, van Leenders GJ, Hulsbergen-Van De Kaa CA, de la Rosette
JJ, Blickman JG and Barentsz JO: Discrimination of prostate cancer
from normal peripheral zone and central gland tissue by using
dynamic contrast-enhanced MR imaging. Radiology. 229:248–254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Udeh EI Amu, OC Nnabugwu II and Ozoemena
O: Transperineal versus transrectal prostate biopsy: Our findings
in a tertiary health institution. Niger J Clin Pract. 18:110–114.
2015.PubMed/NCBI
|
|
18
|
Chang DT, Challacombe B and Lawrentschuk
N: Transperineal biopsy of the prostate-is this the future? Nat Rev
Urol. 10:690–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bittner N, Merrick GS, Butler WM, Bennett
A and Galbreath RW: Incidence and pathological features of prostate
cancer detected on transperineal template guided mapping biopsy
after negative transrectal ultrasound guided biopsy. J Urol.
190:509–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wagenlehner FM, Pilatz A, Waliszewski P,
Weidner W and Johansen TE: Reducing infection rates after prostate
biopsy. Nat Rev Urol. 11:80–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moore CM, Robertson NL, Arsanious N,
Middleton T, Villers A, Klotz L, Taneja SS and Emberton M:
Image-guided prostate biopsy using magnetic resonance
imaging-derived targets: A systematic review. Eur Urol. 63:125–140.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fiard G, Hohn N, Descotes JL, Rambeaud JJ,
Troccaz J and Long JA: Targeted MRI-guided prostate biopsies for
the detection of prostate cancer: Initial clinical experience with
real-time 3-dimensional transrectal ultrasound guidance and
magnetic resonance/transrectal ultrasound image fusion. Urology.
81:1372–1378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Villers A: Words of wisdom. Re: Improving
detection of clinically significant prostate cancer: MRI/TRUS
fusion-guided prostate biopsy. Eur Urol. 65:1218–1219. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Panebianco V, Sciarra A, Marcantonio A,
Forte V, Biondi T, Laghi A and Catalano C: Conventional imaging and
multiparametric magnetic resonance (MRI, MRS, DWI, MRP) in the
diagnosis of prostate cancer. Q J Nucl Med Mol Imaging. 56:331–342.
2012.PubMed/NCBI
|
|
25
|
Correas JM, Tissier AM, Khairoune A,
Khoury G, Eiss D and Hélénon O: Ultrasound elastography of the
prostate: State of the art. Diagn Interv Imaging. 94:551–560. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schiffmann J, Fischer J, Tennstedt P,
Beyer B, Böhm K, Michl U, Graefen M and Salomon G: Comparison of
prostate cancer volume measured by HistoScanningTm and final
histopathological results. World J Urol. 32:939–944. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clyne M: Prostate cancer: Visual
estimation versus software fusion for MRI-targeted biopsy. Nat Rev
Urol. 11:52014. View Article : Google Scholar : PubMed/NCBI
|