Introduction
Neuroblastoma (NB) originates from immature
sympathetic neural cells and is one of the most common types of
solid malignant tumors in children. Nearly half of all NB cases
occur in children <2 years old, and the prognosis varies widely,
with outcomes ranging between spontaneous regression and mortality
(1). NB accounts for 7–10% of
childhood malignancies and ~15% of all childhood cancer-associated
mortalities (2,3). Thus, the treatment and management of NB
continues to be a challenge faced by physicians and scientists.
MicroRNAs (miRNAs/miRs) are short non-coding RNAs
that control gene activity by targeting post-transcriptional
expression of specific genes (4).
miRNAs serve an important role in the regulation of fundamental
cellular processes, including proliferation, migration and
differentiation, and are involved in the pathogenesis of NB by
acting as oncogenes or tumor-suppressor genes. For example, miR-23a
promotes NB cell migration and invasion by targeting the cadherin 1
gene (5). miR-338-3p suppresses NB
proliferation, invasion and migration through
phosphatidylinositol-3,4,5-trisphosphate
(PIP3)-dependent rac exchange factor 2 (6). miR-362-5p inhibits the proliferation and
migration of NB cells by targeting phosphatidylinositol-4-phosphate
3-kinase catalytic subunit type 2β (7). miR-145 regulates the gene expression of
hypoxia-inducible factor 2α, thus inhibiting the growth, invasion,
metastasis and angiogenesis of NB cells (8). Although multiple genetic and molecular
lesions have been associated with NB tumorigenesis (6,7), the
molecular mechanisms regulating NB remain unclear.
miR-21 has been suggested to be oncogenic in
multiple types of tumors. Previous studies have demonstrated that
the downregulation of miR-21 suppresses tumor growth and invasion
in breast, glioma, gastric and colon cancer cells by directly
targeting genes for phosphatase tensin homologue (PTEN) and
programmed cell death 4 (PDCD4) (9–12). To the
best of our knowledge, no previous studies have investigated the
role of miR-21 in the NB SK-N-SH cell line, making the present
study the first to examine the association between PTEN and PDCD4
expression, and cell apoptosis in SK-N-SH cells transfected with a
miR-21 inhibitor. Elucidating the molecular mechanisms underlying
NB etiopathogenesis may contribute to the identification of
suitable therapeutic agents for the treatment of patients with
NB.
Materials and methods
Cell culture
SK-N-SH, SH-SY5Y and BE2C cells (American Type
Culture Collection, Manassas, VA, USA) were obtained for use in the
present study. LV3-miR-21 inhibitor-transfected SK-N-SH cells are
denoted as 1381 cells. The cells were cultured through serial
passage in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal
bovine serum (FBS; both from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 IU/ml penicillin and 100 µg/ml streptomycin
(both from Invitrogen; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere with 5% CO2 at 37°C. All
procedures were performed according to internationally accepted
ethical guidelines. The present study was approved by the
Institutional Review Board of the Children's Hospital of Fudan
University (Shanghai, China).
Plasmid construction, lentivirus
packaging and cell infection
A lentiviral vector, pGLV3/H1/green fluorescent
protein (GFP) + Puromycin (pGLV3; Shanghai GenePhama Co., Ltd.,
Shanghai, China), was used to construct the pGLV3-miR-21 inhibitor
plasmid. The miR-21 inhibitor and negative control (NC)
oligonucleotides were synthesized by Shanghai GenePhama Co., Ltd.
(Table I). The miR-21 small hairpin
(sh) DNA double stranded template sequence was synthesized by
Huajin Nano Technology Co., Ltd. (Shanghai, China) using miR-21
inhibitor forward (BamHI) and reverse (EcoRI) primers (Fermentas;
Thermo Fisher Scientific, Inc.). Subsequently, the miR-21 inhibitor
sequence was inserted into the pGLV3 lentivirus plasmid.
pGLV3-shDNA-NC was used as a negative control and was constructed
using forward (BamHI) and reverse (EcoRI) primers.
 | Table I.Sequences of miR-21 inhibitor and
control oligonucleotides, and primers used in their
construction. |
Table I.
Sequences of miR-21 inhibitor and
control oligonucleotides, and primers used in their
construction.
| Oligonucleotide | Sequence |
|---|
| miR-21 inhibitor |
5′-TCAACATCAGTCTGATAAGCTA-3′ |
| Forward,
BamHI |
5′-GATCCTCAACATCAGTCTGATAAGCTACGATTCAACATCAGTCTGATAAGCTAACCGGTTCAACATCAGTCTGATAAGCTATCACTCAACATCAGTCTGATAAGCTATTTTTTGAATT-3′ |
| Reverse,
EcoRI |
5′-ACCGGTTAGCTTATCAGACTGATGTTGAATCGTAGCTTATCAGACTGATGTTGAGAATTCAAAAAATAGCTTATCAGACTGATGTTGAGTGATAGCTTATCAGACTGATGTTGA-3′ |
| miR-NC |
5′-TTCTCCGAACGTGTCACGT-3′ |
| Forward,
BamHI |
5′-GATCCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAACTTTTTTG-3′ |
| Reverse,
EcoRI |
5′-AATTCAAAAAAGTTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAACG-3′ |
The 293T producer cell line (Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences, Shanghai,
China) was cultured in DMEM with 10% FBS, 100 U/ml penicillin and
100 µg/ml streptomycin. Subsequently, 1 day prior to transfection,
the cells (5×105/ml) were seeded into a 15-cm dish.
pGLV3-miR-21 inhibitor or pGLV3 vectors and packaging plasmids,
including pGag/Pol, pRev and pVSV-G (Shanghai GenePhama Co., Ltd.),
were co-transfected using RNAi-Mate (Shanghai GenePhama Co., Ltd.)
according to the manufacturer's protocol. The supernatant was
collected 72 h post-transfection, centrifuged (2,200 × g at 4°C for
4 min), passed through a 0.45-µm syringe filter and centrifuged
again (50,000 × g at 4°C for 2 h). The viral titer was measured
according to the expression of GFP following the manufacturer's
protocol. The packaged lentiviruses were designated LV3-miR-21
inhibitor and LV3-NC. The sequences of all vectors were verified
through sequence analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA, was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. RT was performed
using gene-specific RT primers from the TaqMan® MicroRNA
Assay kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
the TaqMan MicroRNA Reverse Transcription kit (Takara Bio, Inc.,
Otsu, Japan) according to the manufacturer's protocol. To estimate
the expression of miR-21, the quantification cycle (Cq) values were
normalized using U6 as an internal control. The PCR results were
separated by 2% agarose electrophoresis gel containing ethidium
bromide (0.5 µg/ml) using electrophoresis apparatus (Bulletin
#M1704498; Bio-Rad Laboratories, Inc., Hercules, CA, USA) and a
Visualizer (Bio-Rad GelDoc XR; Bio-Rad Laboratories, Inc.). For the
analysis of PTEN and PDCD4 expression, complementary DNA was
synthesized using PrimeScript™ RT Master Mix (Takara Bio, Inc.)
according to the manufacturer's protocol. RT-qPCR was carried out
using a SYBR Premix Ex Taq™ II (Takara Bio, Inc.). The
housekeeping gene GAPDH was used for normalization. Primers are
illustrated in Table II.
 | Table II.Primer sequences used in quantitative
polymerase chain reactions. |
Table II.
Primer sequences used in quantitative
polymerase chain reactions.
| Primer | Sequence |
|---|
| U6 |
|
|
Forward |
5′-ATTGGAACGATACAGAGAAGATT-3′ |
|
Reverse |
5′-GGAACGCTTCACGAATTTG-3′ |
| miR-21 |
|
|
Forward |
5′-ACGTTGTGTAGCTTATCAGACTG-3′ |
|
Reverse |
5′-AATGGTTGTTCTCCACACTCTC-3′ |
| GAPDH |
|
|
Forward |
5′-GAGTCAACGGATTTGGTCGT-3′ |
|
Reverse |
5′-TTGATTTTGGAGGGATCTCG-3′ |
| PTEN |
|
|
Forward |
5′-GCACTGTTGTTTCACAAGATGATG-3′ |
|
Reverse |
5′-GCAGACCACAAACTGAGGATTG-3′ |
| PDCD4 |
|
|
Forward |
5′-CGACAGTGGGAGTGACGCCCTTA-3′ |
|
Reverse |
5′-CAGACACCTTTGCCTCCTGCACC-3′ |
RT-qPCR was performed using the Rotor-Gene
3000™ system with Rotor Gene Detection software (version
6.1.90; both from Qiagen, Inc., Valencia, CA, USA). The following
thermocycling conditions were performed: 3 min at 95°C; and 40
cycles of 95°C for 15 sec, 58°C for 30 sec and 72°C for 30 sec. All
reactions were performed in triplicate. The 2−∆∆Cq
method (13) was used for the
relative quantification of the gene expression of miR-21, PTEN and
PDCD4.
Western blot (WB) analysis
In order to extract the cellular protein, cells were
lysed on ice for 30 min with radioimmunoprecipitation assay buffer
(50 mmol/l Tris-HCl, pH 7.5; 150 mmol/l NaCl; 0.5% deoxycholate;
and 0.1% SDS). Protein concentration was determined using a Pierce™
BCA Protein Assay kit (Thermo Fisher Scientific Inc.) according to
the manufacturer's protocol. Following denaturation in boiling
water for 5 min, 20-µg samples were separated on 10% SDS-PAGE gels
(Bio-Rad Laboratories, Inc.) and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA).
The membranes were blocked using 5% non-fat milk and
incubated overnight at 4°C with primary rabbit polyclonal
anti-human antibodies directed against PTEN (#9188), PDCD4 (#9535)
or caspase-3 (#9662; all primary antibody dilutions were 1:1,500;
Cell Signaling Technology, Inc., Danvers, MA, USA). Membranes were
incubated for 2 h at room temperature with a goat anti-rabbit
horseradish peroxidase-conjugated immunoglobulin Gc secondary
antibody (#W10809; dilution, 1:3,000; Pierce; Thermo Fisher
Scientific, Inc.). Subsequently, proteins were visualized using an
ECL substrate (Immobilon Western Chemiluminescent HRP substrate;
EMD Millipore) according to the manufacturer's protocol, using a
Bio-Rad Molecular Imager ChemiDoc™ XRS+ with Image Lab™ software
2.0 (Bio-Rad Laboratories, Inc.) and analyzed by ImageJ (version
2.1.4.7; National Institutes of Health, Bethesda, MD, USA).
Membranes were also probed with an anti-human antibody directed
against GAPDH (1:10,000; Shanghai Kangcheng Biological Engineering
Co., Ltd., Shanghai, China) to ensure equal loading of protein. All
experiments were performed in triplicate.
Cell proliferation
Cell proliferation was measured using a cell
counting kit (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Cells were seeded into 96-well plates
(1×103 cells/well) and cultured for 24 h. The viability
of SK-N-SH cells transfected with miR-21 inhibitor or NC was
analyzed every 24 h following transfection. CCK-8 (10 µl) was added
to each well of the 96-well plates and incubated in 37°C for 4 h.
Proliferation rates were determined at 0, 24, 48 and 72 h following
transfection. The optical density was measured at wavelength of 490
nm using a 2550 EIA reader (Bio-Rad Laboratories, Inc.).
Hoechst 33342 staining
The SK-N-SH, SH-SY5Y and BE2C cell lines were
cultured in 6-well tissue culture plates. The culture medium was
removed and the cells were fixed in 4% paraformaldehyde for 10 min
at room temperature. Following washing twice in PBS, the cells were
stained with 10 µM Hoechst 33342 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C for 30 min. The nuclear structure of
the cells was examined using an IX71 fluorescence microscope
(Olympus Corporation, Tokyo, Japan). Quantitative analysis was
performed by counting green fluorescent (apoptosis-positive) cells
under ×400 magnification in three independent fields. The values
are expressed as the percentage of apoptotic cells relative to the
total number of cells per field.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed using SPSS
statistical software (version 17.0; SPSS, Inc., Chicago, IL, USA).
The significance of differences between groups was analyzed using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
miR-21 inhibitor downregulates miR-21
in SK-N-SH cells
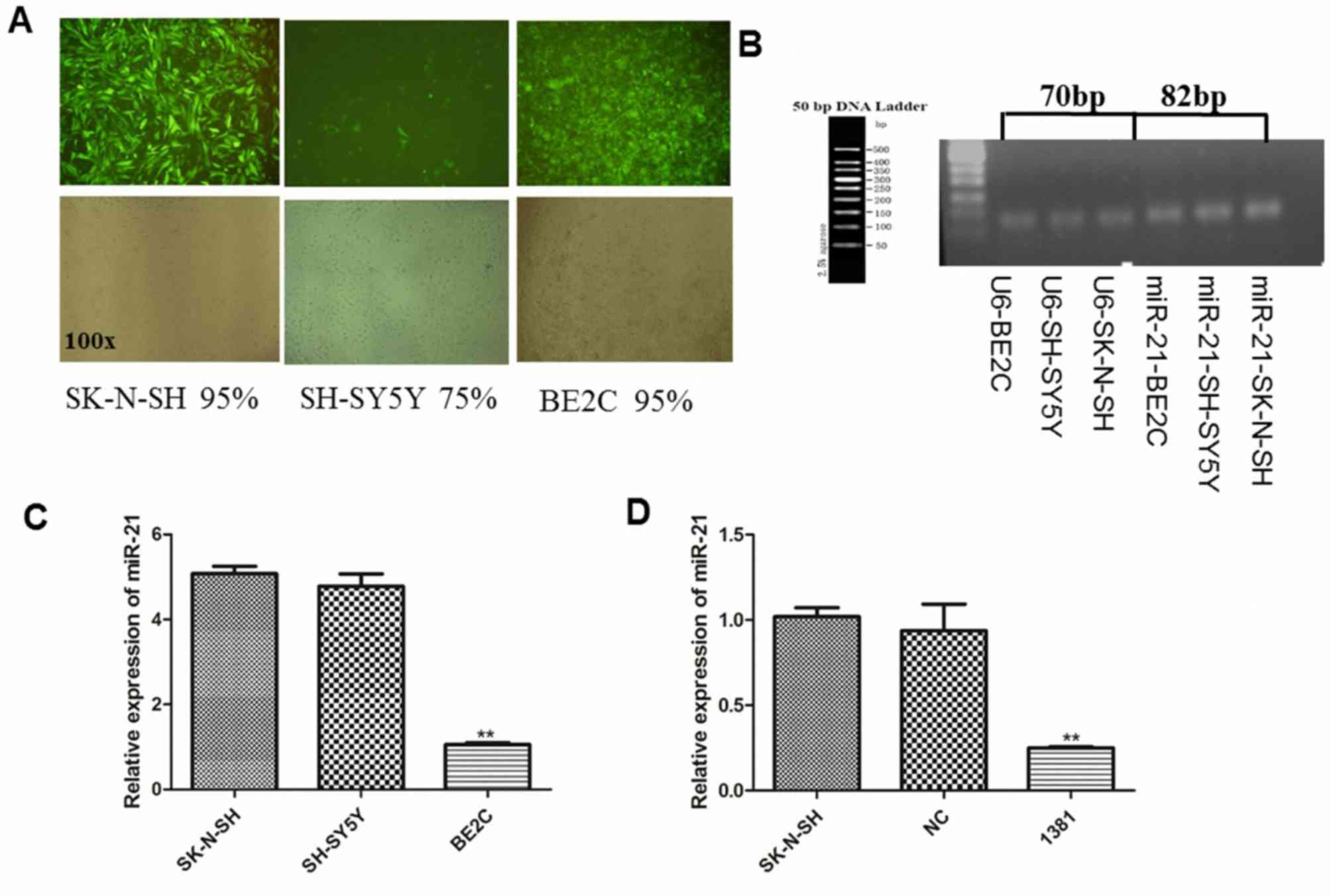
The infection efficiency of lentivirus in SK-N-SH,
SH-SY5Y and BE2C cells was estimated at 95, 75 and 95%,
respectively through fluorescence microscopy (Fig. 1A). The expression of miR-21 was
detected in all three cell lines. The results of 2% agarose gel
electrophoresis demonstrated that the size of the PCR products was
correct (U6, 70 bp; miR-21, 82 bp), without any non-specific bands,
and that only specifically amplified products were present
(Fig. 1B).
Statistical analysis demonstrated no significant
difference between miR-21 expression in SK-N-SH and SH-SY5Y cells
(Fig. 1C). However, miR-21 expression
in BE2C cells was significantly lower compared with that in SK-N-SH
and SH-SY5Y cell lines (both P<0.01; Fig. 1C). Based on its miR-21 expression and
infection efficiency, the SK-N-SH cell line was selected for
further experiments. Following transfection, the expression of
miR-21 was determined by RT-qPCR. The expression of miR-21 was
significantly diminished in LV3-miR-21 inhibitor-transfected cells
(1381) compared with that in untransfected SK-N-SH and
LV3-NC-transfected cells (both P<0.01; Fig. 1D).
miR-21 inhibitor upregulates messenger
RNA (mRNA) and protein expression of PTEN and PDCD4 in 1381
cells
Following miR-21 inhibitor transfection, the
expression of PTEN and PDCD4 protein in 1381 cells was increased
compared with that in the control SK-N-SH and NC groups, in which
PTEN and PDCD4 expression was similar (Fig. 2A). Quantification of triplicate WB
analysis demonstrated a significant increase in the expression of
PTEN (Fig. 2B) and PDCD4 (Fig. 2C) protein compared with that in the NC
and SK-N-SH groups (both P<0.01). In addition, RT-qPCR analysis
revealed significantly increased expression of PTEN (Fig. 2D) and PDCD4 (Fig. 2E) mRNA in 1381 cells compared with
that in the control cell group (both P<0.01).
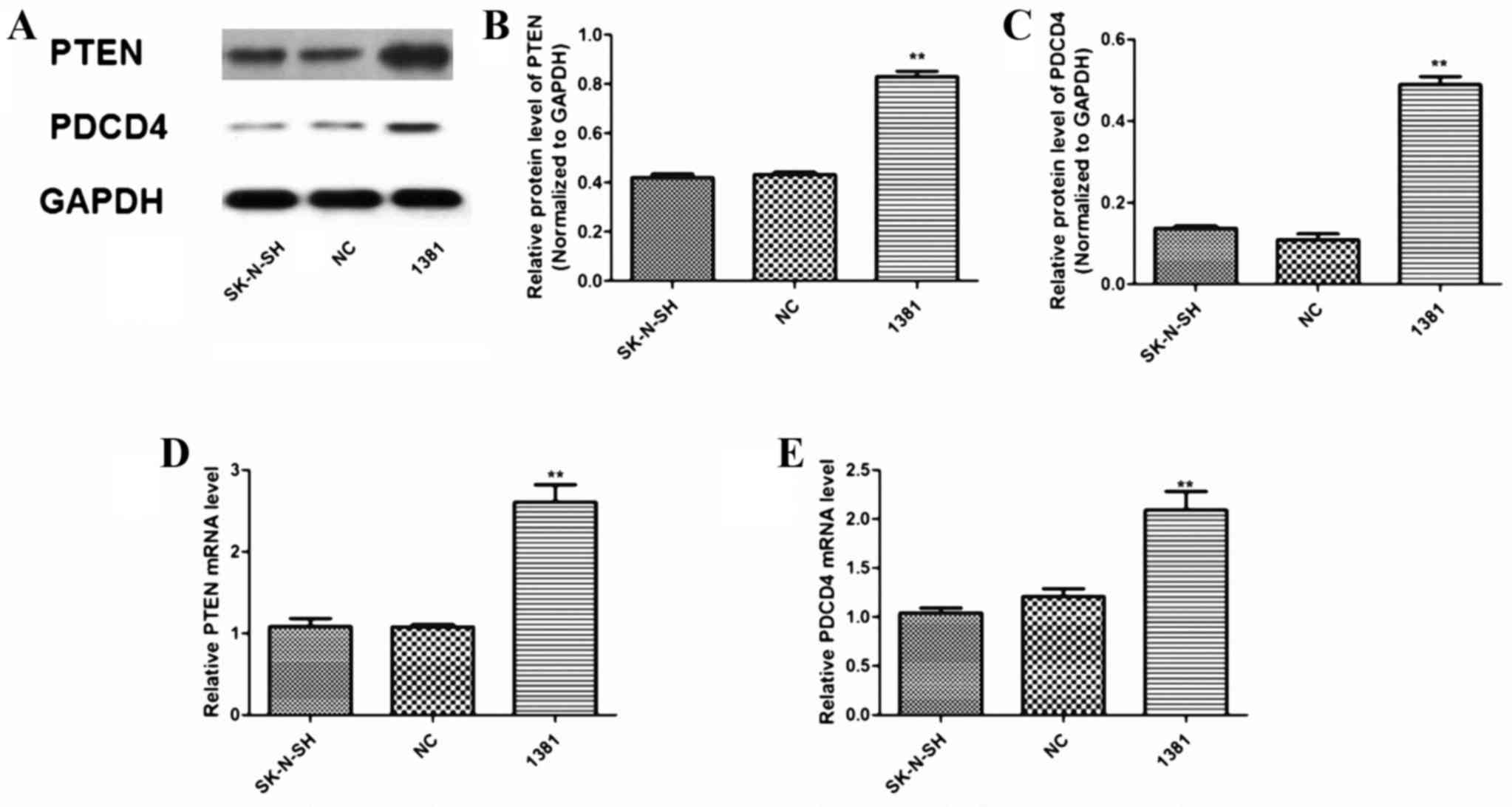 | Figure 2.Expression of PTEN and PDCD4 protein
and mRNA in 1381 cells transfected with a miR-21 inhibitory
oligonucleotide. (A) Representative WB analysis of protein
expression in untransfected SK-N-SH, NC-transfected and 1381 cells
(left to right lanes, respectively). Quantitation of triplicate WB
analysis demonstrated that expression of (B) PTEN and (C) PDCD4 was
significantly increased when miR-21 was inhibited in 1381 cells.
RT-qPCR analysis of (D) PTEN and (E) PDCD4 mRNA expression relative
to U6 demonstrated a significant increase in 1381 cells compared
with that in untransfected SK-N-SH cells and cells transfected with
NC. **P<0.01 vs. NC and SK-N-SH. NC, negative control; miR,
microRNA; 1381, SK-N-SH cells transfected with miR-21 inhibitor;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; WB, western blot; PDCD4, programmed cell death 4; PTEN,
phosphatase and tensin homologue; mRNA, messenger RNA. |
miR-21 inhibitor reduces proliferation
and induces apoptosis in 1381 cells
Hoechst 33342 nucleic acid staining was performed on
untransfected cells and cells transfected with LV3-NC and
LV3-miR-21 inhibitor. Fig. 3A
illustrates the characteristic appearance, including nuclear
chromatin condensation and nuclear fragmentation, in apoptotic 1381
cells. Transfection with the miR-21 inhibitor resulted in a
significant increase in the number of apoptotic 1381 cells
(12.45±1.99%) compared with the basal apoptosis level observed in
the NC (3.04±0.68%) and untransfected SK-N-SH (3.65±0.46%) groups
(both P<0.01; Fig. 3B). The CCK-8
assay revealed that transfection with the miR-21 inhibitor
significantly inhibited the proliferation of 1381 cells compared
with the cell viability noticed in the untransfected SK-N-SH and NC
groups (P<0.05; Fig. 3C).
Furthermore, WB analysis (Fig. 3D)
demonstrated a significant increase in the expression of activated
caspase-3 protein in 1381 cells compared with that in NC and
SK-N-SH cells (P<0.01; Fig.
3E).
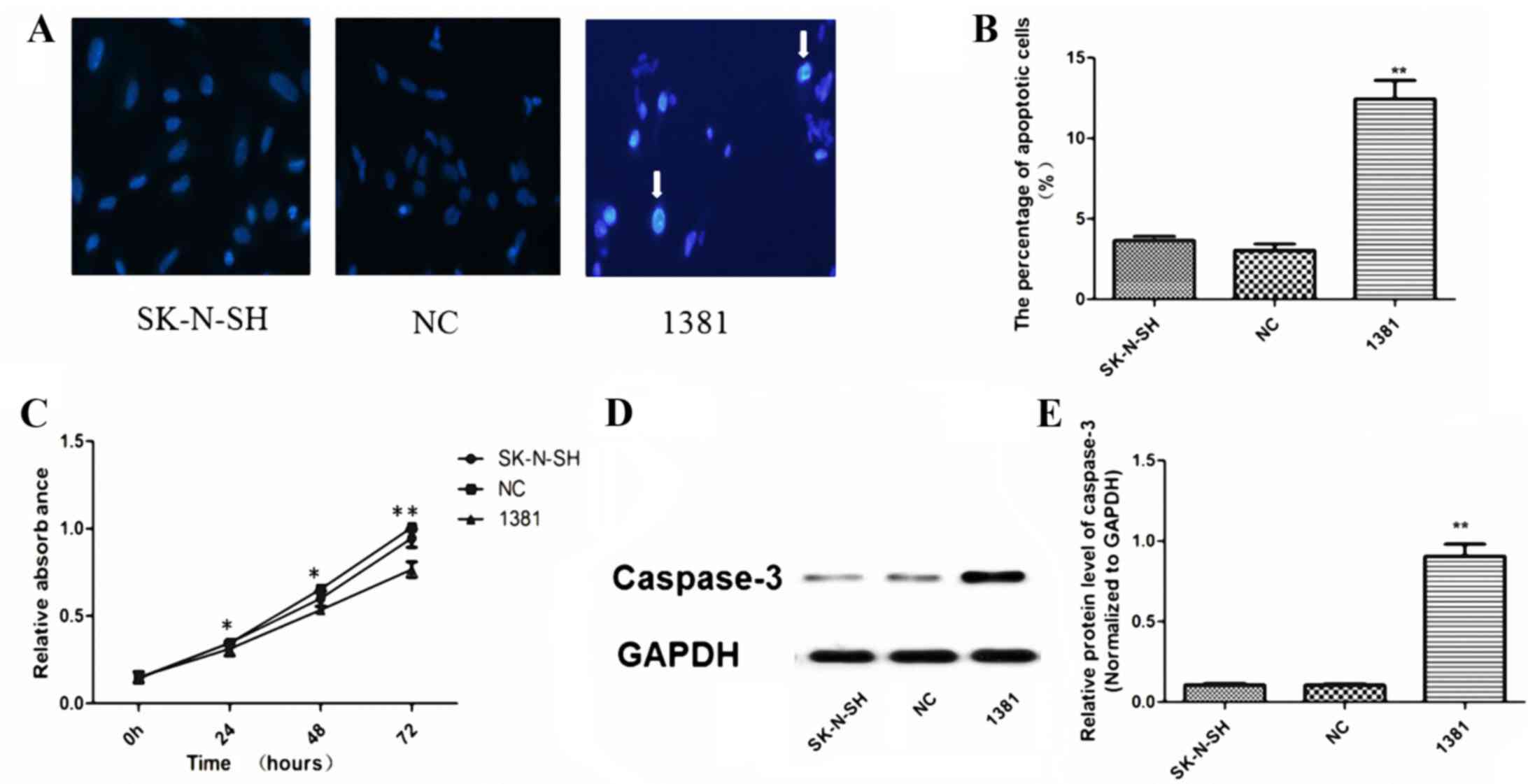 | Figure 3.Apoptosis, proliferation and caspase-3
activation in 1381 cells. (A) Hoechst 33342 nucleic acid staining
in untransfected SK-N-SH, NC-transfected and 1381 cells. Arrows
indicate the characteristic appearance of nuclear chromatin
condensation and nuclear fragmentation in apoptotic 1381 cells.
Magnification, ×400. (B) The percentage of apoptotic cells in the
1381 group was significantly increased compared with that in NC and
untransfected SK-N-SH cells (**P<0.01). (C) CCK-8 assay
demonstrated that the miR-21-inhibitor significantly reduced the
proliferation of 1381 cells compared with the viability of SK-N-SH
and NC cells at 24, 48 and 72 h. WB analysis illustrating a (D)
representative image from triplicate experiments and (E)
quantitative results, indicating a significantly increased level of
activated caspase-3 protein when miR-21 was inhibited in 1381
cells, compared with that in NC and SK-N-SH cells. *P<0.05,
**P<0.01 vs. NC and SK-N-SH. NC, negative control; miR,
microRNA; 1381, SK-N-SH cells transfected with miR-21 inhibitor;
WB, western blot; CCK, cell counting kit. |
Discussion
miRNAs are endogenous, 19–22-nucleotide long,
non-coding RNAs that can negatively regulate protein expression by
inducing the degradation of target mRNAs, inhibiting their
translation or both, by specifically binding to the 3′ untranslated
regions of target mRNAs (4). Although
increasing evidence suggests that a novel class of miRNAs can
regulate various target genes, including oncogenes and tumor
suppressors, the role of miRNAs in NB remains unclear (14).
Previous evidence indicates that miR-21 participates
in the development and progression of various types of human
tumors, including glioblastoma, hepatocellular, lung, colon, and
prostate cancer (15,16). miR-21 is encoded by chromosome
17q23.2, which is frequently involved in unbalanced translocations
in NB cell lines (15). In addition,
miR-21 is among the most frequently detected miRNAs in primary NB
tumors and NB cell lines (17,18).
Furthermore, NB cell lines that have been established from human NB
cells exhibit similar cellular heterogeneity. Based on the
morphological appearance, biochemical properties and growth
patterns, three major cell types have been identified in NB cell
lines. These have been designated as neuroblastic,
substrate-adherent and non-neuronal, and intermediate-type NB cells
(19). SH-SY5Y, SK-N-SH, and BE2C
cell lines represent the above three types, respectively (19). The SK-N-SH cell line was selected
following consideration of its miR-21 expression and the infection
efficiency of the lentivirus into the target cells.
PTEN and PDCD4 are target genes of miR-21 (20), which were validated by the three
following target prediction programs: PicTar (http://pictar.mdc-berlin.de/), TargetScan (http://www.targetscan.org) and miRanda (http://www.microrna.org). PDCD4 suppresses several
proteins that regulate translation and cell proliferation, and has
been implicated in tissue invasion and proliferation (20,21). PTEN
is a phosphatase that maintains low levels of PIP3 by
conversion to phosphatidylinositol 4,5-bisphosphate. When PTEN
fails to maintain this homeostasis, PIP3 levels increase
and activate the AKT serine/threonine kinase (Akt) signaling
pathway. Activation of the Akt signaling pathway leads to several
effects, including promotion of cell growth and proliferation, and
inhibition of apoptosis (20,21). For example, miR-21 promotes cell
growth and migration by targeting PDCD4 gene expression in Kazakh
esophageal squamous cell carcinoma (22). Similarly, overexpression of miR-21 in
cervical cancer promotes the proliferation and migration of cells
via inhibition of PTEN (23). The
results of the present study have demonstrated that the
downregulation of miR-21 following transfection with a
miR-21-inhibitor results in a significant increase in PTEN and
PDCD4 mRNA and protein expression compared with that observed in
untransfected and NC-transfected cells. Furthermore, miR-21
inhibitor transfection led to a significant reduction in tumor cell
growth and induction of apoptosis compared with the effects
observed in untransfected and NC-transfected cells. Thus, this
result suggests that the reduction of miR-21 activates the
caspase-3 signaling pathway, possibly mediated by PTEN/PDCD4
induction, to subsequently inhibit cell proliferation and induce
apoptosis.
Chan et al (24) demonstrated that miR-21 is commonly and
markedly upregulated in human glioblastoma, and that inhibiting
miR-21 expression leads to caspase-3/caspase-7 activation and
associated apoptotic cell death in multiple glioblastoma cell
lines. Zhou et al (25)
reported that the reduction of miR-21 by antisense oligonucleotides
activates the caspase-9 and caspase-3 signaling pathways, possibly
mediated by multiple potential target genes, and subsequently
induce glioma cell apoptosis. Recently, White et al
(26) demonstrated that endothelial
apoptosis in pulmonary hypertension is controlled by the
miR-21/PDCD4/caspase-3 axis. Li et al (27) reported that, in ovarian cancer A2780
cells, icariin substantially decreased miR-21 expression, increased
the expression levels of target proteins PTEN and
reversion-inducing-cysteine-rich protein with kazal motifs,
suppressed cell proliferation, accelerated apoptosis and increased
caspase-3 activity, compared with the effects observed in the
untreated control group. The results of the current study indicate
that miR-21 regulates the potential targets PTEN/PDCD4 to activate
the caspase-3 signaling pathway. However, the mechanism underlying
miR-21-mediated regulation of the caspase-3 signaling pathway
remains unclear and warrants further investigation.
In conclusion, the present study has demonstrated
that miR-21 expression is downregulated in NB cells, and has
revealed that the inhibition of miR-21 can promote cell apoptosis
and inhibit proliferation by upregulating tumor-suppressive
PTEN/PDCD4 expression via caspase-3 activation. To the best of our
knowledge, the present study is the first to confirm that miR-21
regulates PTEN/PDCD4 in NB. These results suggest that miR-21 is an
effective therapeutic target in the treatment of patients with
NB.
Acknowledgements
The present study was supported by the Shanghai
Committee of Science and Technology (grant nos. 15411961900 and
12431900205, awarded to Professor Kai Li and Dr Xiaolong Zhao,
Department of Endocrinology, Huashan Hospital of Fudan University,
Shanghai, China, respectively).
Glossary
Abbreviations
Abbreviations:
|
PDCD4
|
programmed cell death 4
|
|
PTEN
|
phosphatase and tensin homologue
|
|
NB
|
neuroblastoma
|
|
miRs
|
microRNAs
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
sh
|
small hairpin
|
|
WB
|
western blot
|
|
GFP
|
green fluorescent protein
|
|
PIP3
|
phosphatidylinositol
3,4,5-triphosphate
|
|
Akt
|
AKT serine/threonine kinase
|
References
|
1
|
Sharp SE, Gelfand MJ and Shulkin BL:
Pediatrics: Diagnosis of neuroblastoma. Semin Nucl Med. 41:345–353.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu H, Zheng J, Xiao X, Zheng S, Dong K,
Liu J and Wang Y: Environmental endocrine disruptors promote
invasion and metastasis of SK-N-SH human neuroblastoma cells. Oncol
Rep. 23:129–139. 2010.PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng L, Yang T, Kuang Y, Kong B, Yu S,
Shu H, Zhou H and Gu J: MicroRNA-23a promotes neuroblastoma cell
metastasis by targeting CDH1. Oncol Lett. 7:839–845.
2014.PubMed/NCBI
|
|
6
|
Chen X, Pan M, Han L, Lu H, Hao X and Dong
Q: miR-338-3p suppresses neuroblastoma proliferation, invasion and
migration through targeting PREX2a. FEBS Lett. 587:3729–3737. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu K, Yang L, Chen J, Zhao H, Wang J, Xu S
and Huang Z: miR-362-5p inhibits proliferation and migration of
neuroblastoma cells by targeting phosphatidylinositol 3-kinase-C2β.
FEBS Lett. 589:1911–1919. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Pu J, Qi T, Qi M, Yang C, Li S,
Huang K, Zheng L and Tong Q: MicroRNA-145 inhibits the growth,
invasion, metastasis and angiogenesis of neuroblastoma cells
through targeting hypoxia-inducible factor 2 alpha. Oncogene.
33:387–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang G, Wang JJ, Tang HM and To ST:
Targeting strategies on miRNA-21 and PDCD4 for glioblastoma. Arch
Biochem Biophys. 580:64–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qi L, Bart J, Tan LP, Platteel I, Sluis
Tv, Huitema S, Harms G, Fu L, Hollema H and Berg Av: Expression of
miR-21 and its targets (PTEN, PDCD4, TM1) in flat epithelial atypia
of the breast in relation to ductal carcinoma in situ and invasive
carcinoma. BMC Cancer. 9:1632009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026.
2012.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gentilin E, Uberti E Degli and Zatelli MC:
Strategies to use microRNAs as therapeutic targets. Best Pract Res
Clin Endocrinol Metab. 30:629–639. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krichevsky AM and Gabriely G: miR-21: A
small multi-faceted RNA. J Cell Mol Med. 13:39–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfeffer SR, Yang CH and Pfeffer LM: The
role of miR-21 in cancer. Drug Dev Res. 76:270–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Afanasyeva EA, Hotz-Wagenblatt A, Glatting
KH and Westermann F: New miRNAs cloned from neuroblastoma. BMC
Genomics. 9:522008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buechner J, Henriksen JR, Haug BH, Tomte
E, Flaegstad T and Einvik C: Inhibition of mir-21, which is
up-regulated during MYCN knockdown-mediated differentiation, does
not prevent differentiation of neuroblastoma cells.
Differentiation. 81:25–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang S, Zheng J, Xiao X, Xu T, Tang W, Zhu
H, Yang L, Zheng S, Dong K, Zhou G and Wang Y: SOX2 promotes
tumorigenicity and inhibits the differentiation of I-type
neuroblastoma cells. Int J Oncol. 46:317–323. 2015.PubMed/NCBI
|
|
20
|
Buscaglia LE and Li Y: Apoptosis and the
target genes of microRNA-21. Chin J Cancer. 30:371–380. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Huang K and Yu J: Inhibition of
microRNA-21 upregulates the expression of programmed cell death 4
and phosphatase tensin homologue in the A431 squamous cell
carcinoma cell line. Oncol Lett. 8:203–207. 2014.PubMed/NCBI
|
|
22
|
Liu T, Liu Q, Zheng S, Gao X, Lu M, Yang
C, Dai F, Sheyhidin I and Lu X: MicroRNA-21 promotes cell growth
and migration by targeting programmed cell death 4 gene in Kazakh's
esophageal squamous cell carcinoma. Dis Markers. 2014:2328372014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Zhang W, Lv Q and Zhu D:
Overexpression of miR-21 promotes the proliferation and migration
of cervical cancer cells via the inhibition of PTEN. Oncol Rep.
33:3108–3116. 2015.PubMed/NCBI
|
|
24
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou X, Zhang J, Jia Q, Ren Y, Wang Y, Shi
L, Liu N, Wang G, Pu P, You Y and Kang C: Reduction of miR-21
induces glioma cell apoptosis via activating caspase 9 and 3. Oncol
Rep. 24:195–201. 2010.PubMed/NCBI
|
|
26
|
White K, Dempsie Y, Caruso P, Wallace E,
McDonald RA, Stevens H, Hatley ME, van Rooij E, Morrell NW, MacLean
MR and Baker AH: Endothelial apoptosis in pulmonary hypertension is
controlled by a microRNA/programmed cell death 4/caspase-3 axis.
Hypertension. 64:185–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Jiang K and Zhao F: Icariin
regulates the proliferation and apoptosis of human ovarian cancer
cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol
Rep. 33:2829–2836. 2015.PubMed/NCBI
|