Introduction
Bladder urothelial tumors are divided into two main
groups: Non-muscle invasive bladder cancer (NMIBC) and invasive
bladder cancer. NMIBC accounts for 75–80% of primary diagnoses
(1). A transurethral resection of
bladder tumor (TURBT) is the preferred treatment for NMIBC,
however, almost 70% of patients with intermediate- or high-risk
NMIBC relapse within 6–12 months of surgery, and ~25% progress to a
higher stage disease (2,3). Therefore, postoperative adjuvant
intravesical therapy, including intravesical chemotherapy and
immunotherapy, is considered necessary for these patients. The
probabilities of recurrence and progression may reduce to 42–65%
under appropriate intravesical therapy at 5 years (4). Although carcinoma in situ (CIS)
is an NMIBC, it is often poorly differentiated and highly
malignant; therefore, the probability of myometrial invasion is
significantly increased compared with papillary stage Ta and T1
bladder cancer, and Bacillus Calmette-Guérin (BCG) is the
recommended agent for adjuvant intravesical immunotherapy in these
cases.
Programmed death-ligand 1 (PD-L1; also termed B7-H1)
is a member of the B7 family of costimulatory molecules; it is a
cell surface glycoprotein that promotes apoptosis by binding to its
surface receptor, programmed cell death-1 (PD-1), in T cells and B
cells, thereby inhibiting host immune function. PD-L1 has also been
implicated in tumor immune escape (5,6).
OK-432 (also termed Picibanil), which is a
penicillin-killed and lyophilized preparation of a low-virulence
strain of Streptococcus pyogenes (group A), has been
successfully used as an immunotherapeutic agent against numerous
types of malignancies (7–10). It has been reported that OK-432
elicits antitumor effects by stimulating immunocompetent cells,
including macrophages, T cells and natural killer (NK) cells, and
by inducing helper T-cell 1-type cytokines, including interferon
(IFN)-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-6,
IL-8, IL-10, IL-12 and IL-18 (11,12), which
may augment cytotoxic T lymphocytes to antitumor cells.
In the present study, the expression rates of PD-L1
were evaluated in cases of NMIBC patients who had undergone
intravesical immunotherapy, and these expression levels were
compared with patient outcome. The effects of specific cytokines on
PD-L1 expression rates in vitro were also examined, and the
potential mechanism of PD-L1 negativity on the efficacy of
intravesical immunotherapy in patients with NMIBC was
investigated.
Materials and methods
Patient selection and treatment
Tissue samples were collected from 55 patients (43
men and 12 women) with NMIBC who had been treated in our
institution (First Affiliated Hospital of Sun Yat-sen University,
Guangzhou, China) between January 2010 and January 2012. All the
patients had undergone TURBT followed by intravesical immunotherapy
with OK-432. None of the patients had received chemotherapy or
radiotherapy prior to surgery. The mean age of patients was 55
years (range, 28–77 years), and their clinical features are
summarized in Table I. Patients
presenting with muscle-invasive transitional cell carcinoma or
concurrent malignancy of another organ were excluded. Patients with
bladder CIS were also excluded.
 | Table I.Incidence of recurrence and
progression in association with PD-L1. |
Table I.
Incidence of recurrence and
progression in association with PD-L1.
| PD-L1 expression | Patients, n | Recurrences, n
(%) | Progression, n
(%) |
|---|
| Positive | 31 | 15 (48.4) | 5 (16.1) |
| Negative | 24 |
4
(16.7)a | 1
(4.2)a |
| Total | 55 | 19 (34.5) | 6 (10.9) |
The treatment protocol consisted of intravesical
immunotherapy (3 KE Picibanil/30 ml saline) administered 12 times
over 7 months. Instillations were administered every week for 6
weeks and then every 4 weeks for 6 months. Cystoscopy was performed
every 3 months after TURBT, and biopsies were performed if a
suspected lesion was found in order to determine whether tumor
recurrence had occurred. Time-to-first recurrence,
time-to-progression >pT1 stage and toxicity were recorded.
All patients were followed-up for at least 12 months
post-surgery. The present study was approved by the Ethics
Committee of First Affiliated Hospital of Sun Yat-sen University.
Written informed consent was obtained from all patients prior to
enrollment in the present study.
Immunohistochemistry
Tissue samples collected from patients were fixed in
10% dampen formaldehyde solution and embedded in paraffin. In order
to detect expression of PD-L1, 5-µm thick histological sections
were prepared and incubated with normal goat serum (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for 1 h at room
temperature to block endogenous peroxidase activity. Sections were
incubated at 4°C overnight with primary antibodies against PD-L1
(dilution, 1:200; catalog no. ab58810; Abcam, Cambridge, UK).
Immunohistochemical staining was performed using the SABC kit
according to the manufacturer's protocols (Wuhan Boster Biological
Technology, Ltd.). Sections were counterstained with hematoxylin
and then dehydrated, cleared and mounted. To evaluate the
specificity of the reaction, phosphate-buffered saline (PBS) was
used for replacing the primary antibody as the negative control.
Subsequent to being washed twice in PBS, the sections were
incubated with biotinylated secondary antibody (dilution, 1:500;
catalog no. E0466; Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA) for 20 min at room temperature, washed twice in PBS and
then incubated with horseradish-peroxidase (HRP)-labeled
streptavidin (dilution, 1:2,000; catalog no. K5007; Dako; Agilent
Technologies, Inc.) for an additional 20 min. The results were
visualized by fluorescence microscopy (Leica Microsystems GmbH,
Wetzlar, Germany) following chromogenic staining with
diaminobenzidine (Wuhan Boster Biological Technology, Ltd.) and
analyzed using Image-Pro Plus software version 6.0 (Media
Cybernetics, Inc. Rockville, MD, USA). The percentage of
PD-L1-positive tumor cells among the total number of tumor cells
was scored in five randomly selected high-power fields
(magnification, ×400) and percentages >10% were classified as
PD-L1-positive.
Cell culture
Unless otherwise specified, all reagents were
standard laboratory stocks. Human uroepithelial (SV-HUC-1) and
bladder cancer (T24) cell lines were purchased from the Cell Bank
of the Chinese Academy of Sciences (Shanghai, China). The cells
were cultured in Ham's F12 medium and Roswell Park Memorial
Institute-1640 media, respectively, supplemented with 10% fetal
bovine serum and 2% penicillin/streptomycin, and incubated in a
humidified atmosphere of 5% CO2 at 37°C. Cells at
<80% confluence were cultured for 24 h in the presence of IL-2,
IFN-α, IFN-γ (final activity concentration, 1,000 U/ml;
Prospec-Tany TechnoGene, Ltd., East Brunswick, NJ, USA) or PBS as a
control. The cells were harvested for analysis by RT-qPCR and
western blotting.
RT-qPCR
Total RNA (500 ng) was extracted from cultured T24
and SV-HUC-1 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. Reverse transcription was performed using the PrimeScript
RT reagent (Perfect Real Time) kit (Takara Biotechnology Co., Ltd.,
Dalian, China). RT-qPCR was performed using the Roche
capillary-based Light Cycler 2.0 system (Roche Diagnostics,
Indianapolis, IN, USA) and the SYBR Premix Ex Taq™
(Perfect Real Time) kit (Takara Biotechnology Co., Ltd.) under
standard thermocycling conditions (start at 95°C for 15 min and 40
cycles of 95°C for 15 sec, 55°C for 30 sec and 72°C for 30 sec).
PD-L1-specific TaqMan probes and primers were purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). Appropriate dilutions
(1:1,000) of single strand cDNA were prepared for subsequent PCR
using β-actin as the quantitative control. Primers were as follows:
PD-L1 forward, 5′-CACTCATCATTGGCTTTGGTATTTCAG-3′ and reverse,
5′-CGACAGCTCATCTTTGCCTTCTTTG-3′; and β-actin forward,
5′-AGCGGGAAATCGTGCGTGAC-3′ and reverse,
5′-ACTCCTGCTTGCTGATCCACATC-3′. Each experiment was performed in
triplicate and analyzed using the 2−∆∆Cq method of
quantification (13).
Western blot analysis
Cultured SV-HUC-1 and T24 cells were washed three
times in PBS and lysed in 1X SDS buffer. Protein concentrations
were determined using a Bio-Rad DC protein assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's protocol. The proteins were separated by 10%
SDS-PAGE and electrophoretically transferred to Immobilon
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Blocking was performed using 10% non-fat dried milk freshly
prepared in Tween-20 tris-buffered saline [0.1% Tween-20 in 100 mM
tris-CL (pH 7.5) and 0.9% NaCl), which was agitated for 15 min at
room temperature overnight. The membranes were incubated with
primary antibodies against PD-L1 (dilution, 1:200; catalog no.
ab58810; Abcam) and β-actin (dilution, 1:25; catalog no.
SAB5500001; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
overnight at 4°C, followed by incubation with their respective
HRP-conjugated secondary antibodies (1:10,000; catalog no. NA933,
GE Healthcare; NJ, USA) for 2 h at room temperature. Signals were
detected by enhanced chemiluminescence (GE Healthcare Life
Sciences, Chalfont, UK) and developed on X-ray film (Fuji Photo
Film; FUJIFILM Corporation, Tokyo, Japan). Each experiment was
repeated in triplicate.
Statistical analysis
All in vitro experiments were performed in
triplicate. Data are presented as the mean ± standard deviation of
three independent repeats. Comparisons between two groups and the
association between PD-L1 mRNA expression level or PD-L1 protein
expression levels and cytokines were analyzed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA), GraphPad Prism 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA) and Microsoft excel
(Microsoft Corporation, Redmond, WA, USA). Significance was
determined using χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Association between PD-L1 and efficacy
of intravesical immunotherapy
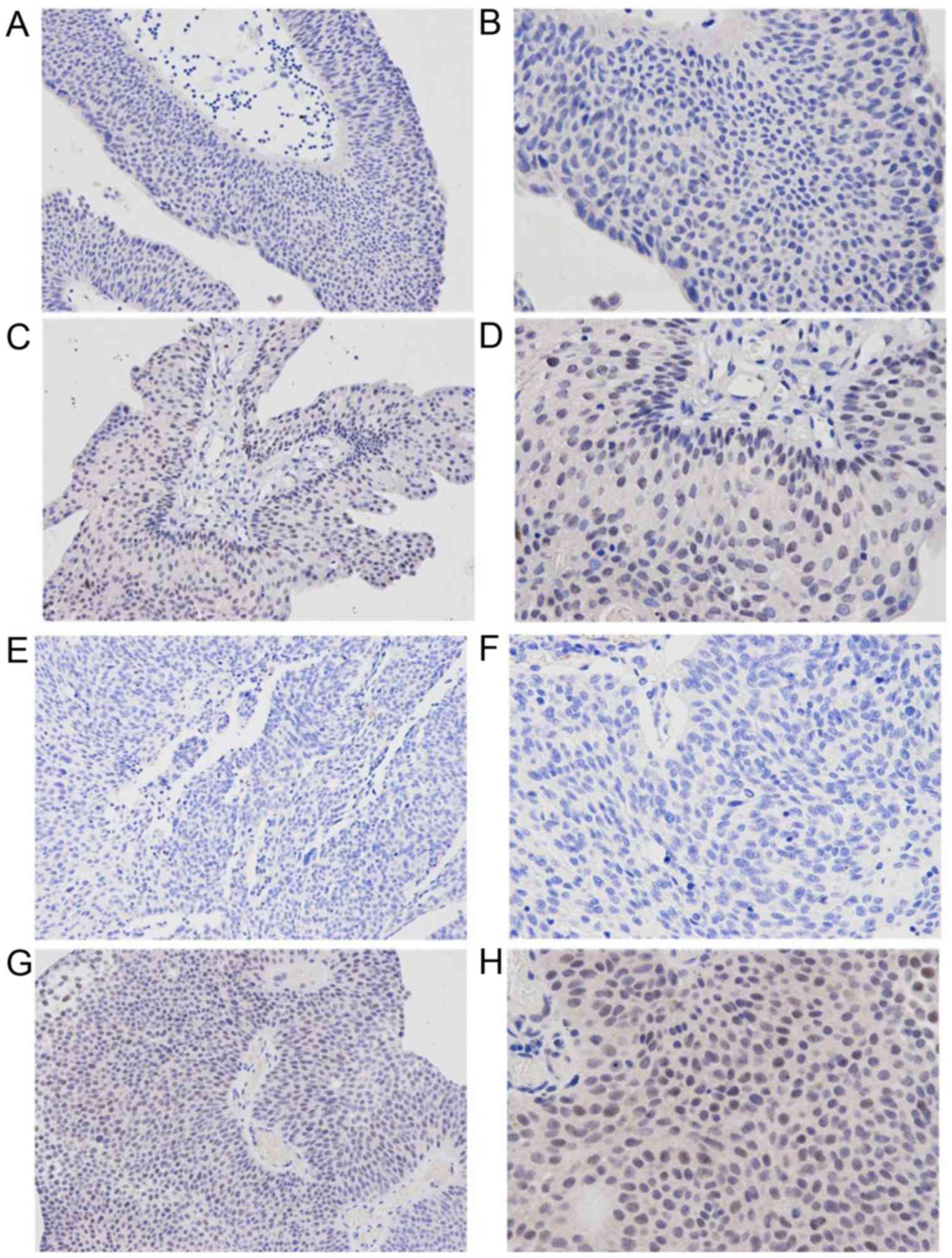
A total of 31/55 (56.4%) NIMIBC specimens stained
positive for PD-L1, with cytoplasmic or membrane staining intensity
ranging from light yellow to brown (Fig.
1). No plasma membrane expression of PD-L1 was observed in the
normal urothelium adjacent to the malignant urothelium. PD-L1
expression was identified to be significantly associated with
pathological grade and histological stage of NMIBC; the percentages
of positive PD-L1 immunostaining were 38.1% (8/21) and 67.6%
(23/34) for pTa and pT1 tumors, respectively (P<0.05).
PD-L1-positive patients who had undergone intravesical
immunotherapy with OK-432 exhibited significantly increased
probabilities of recurrence and progression compared with
PD-L1-negative patients, with recurrence rates of 48.4% (15/31) and
16.7% (4/24), respectively, and progression rates of 16.1% (5/31)
and 4.2% (1/24), respectively (P<0.05; Table I). PD-L1 expression was not associated
with patient sex, and there was no association with patient age or
tumor size (Table II).
 | Table II.Clinical characteristics of patients
in association with PD-L1 expression status. |
Table II.
Clinical characteristics of patients
in association with PD-L1 expression status.
| Age, years (mean ±
SD) | PD-L1-positive
61±10 | PD-L1-negative
64±11 | χ2
analysis n.s. |
|---|
| Sex, n |
|
| n.s. |
|
Male | 24 | 19 |
|
|
Female | 7 | 5 |
|
| Size of tumors,
n |
|
| n.s. |
| <3
cm | 11 | 7 |
|
| ≥3
cm | 20 | 14 |
|
| Histological grade,
n |
|
| P<0.005 |
|
High | 15 | 7 |
|
|
Low | 9 | 24 |
|
| Pathological grade,
n |
|
| P<0.005 |
|
pTa | 8 | 13 |
|
|
pT1 | 23 | 11 |
|
Upregulation of PD-L1 mRNA in T24
bladder cancer cell lines
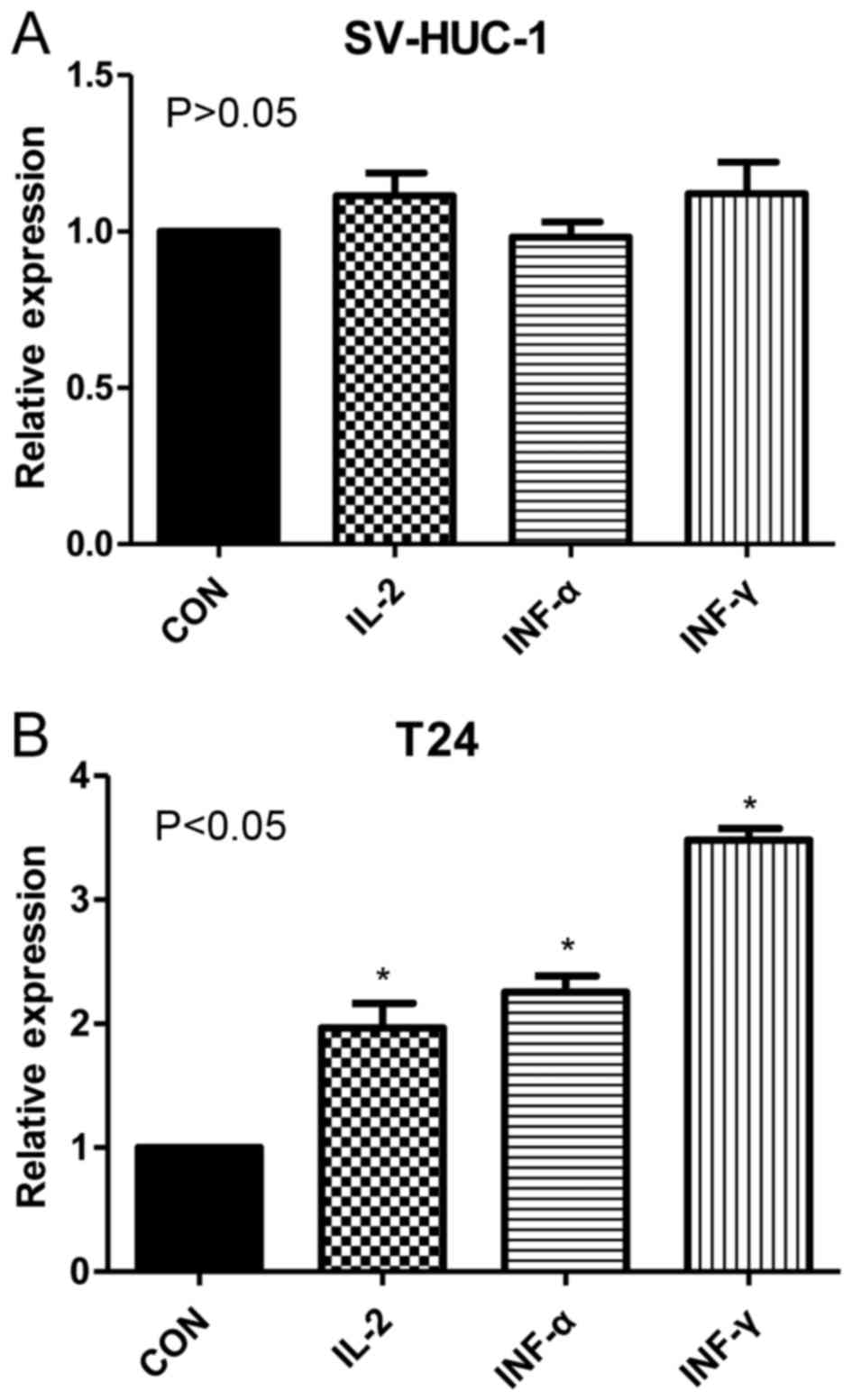
RT-qPCR results demonstrated that relative
expression rates of PD-L1 mRNA were significantly increased in T24
cells treated with IL-2, IFN-α and IFN-γ (1.22±0.16, 1.37±0.21 and
2.45±0.19, respectively) compared with untreated T24 cells
(1.00±0.08; P<0.05). By contrast, no significant differences
were observed between treated and untreated uroepithelial SV-HUC-1
cells (Fig. 2). These results
indicated that the cytokines IL-2, IFN-α and IFN-γ upregulated
PD-L1 mRNA expression in T24 cell lines, but had no significant
effect in SV-HUC-1 cell lines.
Upregulation of PD-L1 protein in T24
cell lines
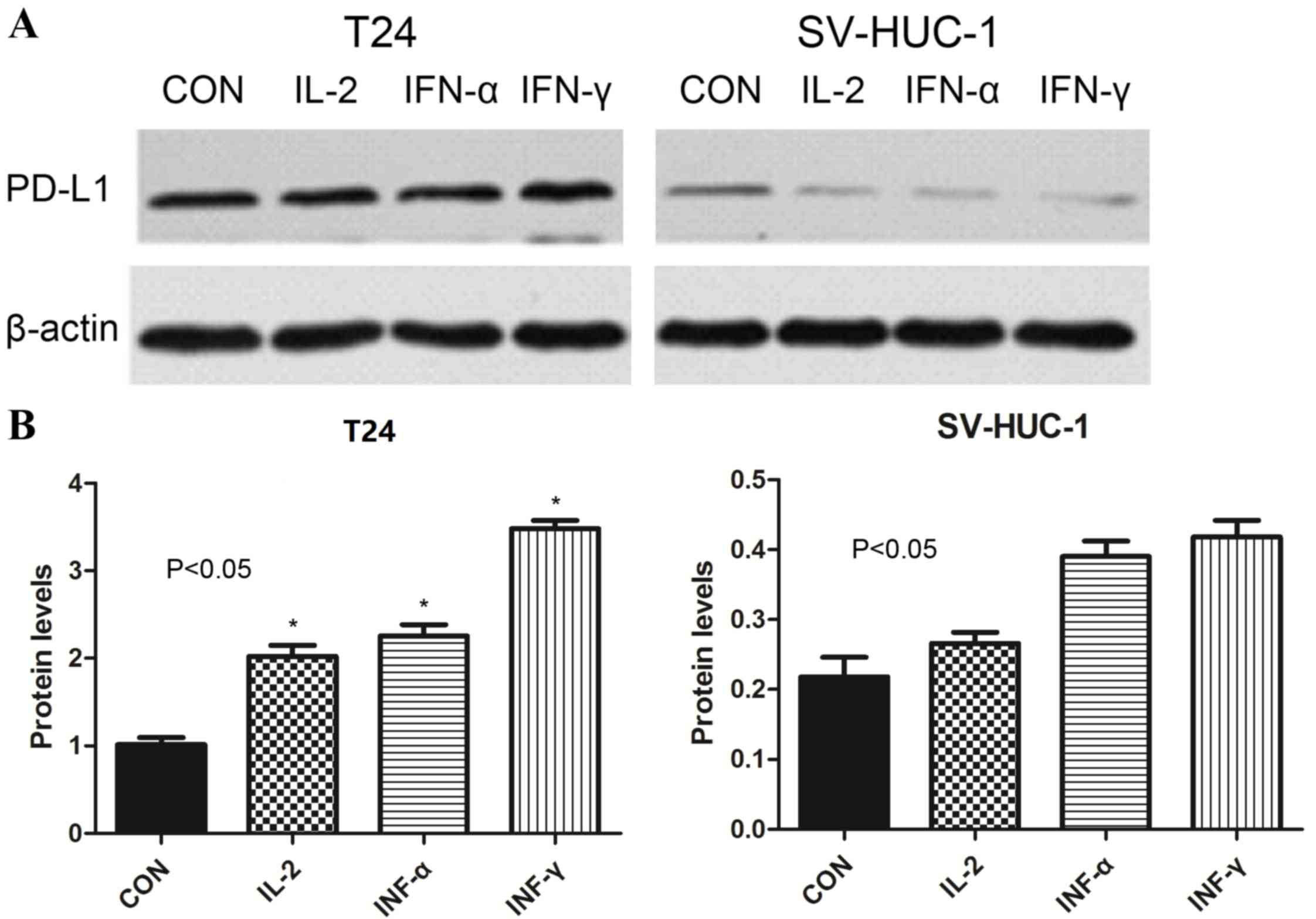
Western blot analysis demonstrated that the levels
of PD-L1 protein were increased in T24 cells treated with IL-2,
IFN-α and IFN-γ (2.1±0.41, 2.77±0.29 and 3.45±0.39, respectively)
compared with untreated cells (1.00±0.08) (P<0.05). By contrast,
the corresponding levels in SV-HUC-1 cells compared with untreated
cells (0.21±0.08) were 0.27±0.05, 0.44±0.11 and 0.37±0.19,
respectively (P>0.05). These findings indicated that the
cytokines IL-2, IFN-α and IFN-γ increased expression of PD-L1
protein in bladder cancer T24 cell lines, but had no significant
effect in non-tumor SV-HUC-1 cell lines (Fig. 3).
Discussion
The majority of patients with NMIBC (>70%)
relapse or progress to a higher level of invasive bladder cancer,
as reflected by increases in the numbers, sizes, stages and grades
of the tumors, as well as the frequency of recurrence and the
presence of CIS (14). Therefore,
postoperative adjuvant intravesical immunotherapy may be
recommended in intermediate- and high-risk patients with NMIBC
(3,14). Tumor immune escape mechanisms may also
be important in recurrence and progression. Immunotherapeutic
agents act by inducing a local immune response to prevent tumor
recurrence (15). BCG has become the
most commonly used intravesical agent and is known to be superior
to other intravesical agents for the prevention of tumor recurrence
(16,17). However, the clinical applications of
BCG have been limited by adverse side effects and difficulties in
its preparation and preservation.
OK-432 (also known as Sapylin) is a biological
reaction modifier derived from the managed and acquired Su-strains
(toxic mutant strain) of human group A hemolytic
Streptococcus. Clinical trials have demonstrated that OK-432
may be effective in the treatment of malignant pleural effusion and
ascites, in solid tumors, including those from lung, stomach, liver
and breast cancer, and in lymphatic cancer, such as metastases
(18). OK-432 has also been shown to
improve and prolong patient survival, alleviate systemic conditions
(particularly those involving the immune function) and reduce the
side effects of chemotherapy and radiotherapy (19–21). In
our center (Department of Urology, First Affiliated Hospital of Sun
Yat-sen University), good results have been achieved in preventing
recurrence and progression of NMIBC by administering OK-432 as an
intravesical immunotherapeutic agent (22). Previous studies have proposed that the
primary antitumor role of OK-432 in bladder carcinoma involves
mobilization of the cellular immune system by activating
macrophages, NK cells, lymphokine-activated killer cells and
various T cells (23,24). Secondary roles include increasing the
binding capacity of these cells to the tumor cells, thereby
enhancing the antitumor effect (25).
OK-432 not only acts in a similar manner to BCG in stimulating
peripheral blood mononuclear cells to produce cytokines (including
TNF, INF and IL-2, which also perform roles in antitumor
mechanisms), but has been identified to be significantly stronger
than BCG in activating NK cells and inducing the secretion of IL-2,
TNF and INF (26).
In the present study, 55 patients with NMIBC were
divided into 2 groups according to their tumor expression levels of
PD-L1. No significant differences were observed between the 2
groups with respect to patient age, sex, tumor size and grade.
However, OK-432 was revealed to be more effective in preventing
recurrence and progression in patients in the PD-L1-negative group
compared with those in the PD-L1-positive group and historical
controls. Constitutive expression of PD-L1 is usually confined to
macrophage lineage cells (27,28),
however, abnormal expression of PD-L1 has been reported in a
variety of human tumors, including breast, ovarian, lung, colon and
renal cancer, lymphoma and melanoma (29–32). In
the present study, we hypothesized that tumor cells in NMIBC bind
to T-cell surface receptor PD-1, or a putative non-PD-1 receptor,
via PD-L1, to promote antigen-specific T-cell apoptosis or
dysfunction. This may enable the cells to escape immune-mediated
antitumor effects by inhibiting the immune response. Such
interactions between malignant cells and the immune system serve an
important role in tumor formation and development. Furthermore, as
T cells are considered essential for acquired antitumor immunity,
it was concluded that PD-L1 may also serve an important role in the
recurrence and progression of NMIBC. These hypotheses were
supported by observations in the literature. NMIBC is one of the
most responsive human malignancies to immunotherapy, and host
immunity appears to modulate bladder cancer pathogenesis (15). Furthermore, patients with bladder
cancer may manifest acquired immune dysfunction, which appears to
affect lymphocytes and is associated with tumor stage (33–35). It
has become apparent that T-cell-inhibitory PD-L1 is aberrantly
expressed in numerous types of cancer and has been implicated as a
mechanism for tumor cells to evade the host immune system (36,37).
Together, these previous findings indicated that abnormal
expression of PD-L1 in bladder cancer may contribute to the ability
of the tumor to resist host antitumor immune cells, thereby
reducing the benefit of intravesical immunotherapy and promoting
tumor recurrence and progression. As such, PD-L1-positive patients
exhibit poorer responses to treatment with OK-432 compared with
PD-L1-negative patients. Consistent with these findings, the
present immunohistochemistry observations revealed that PD-L1
expression was increased in recurrent cases that had been treated
with OK-432 compared with non-recurrent cases.
The RT-qPCR results indicated that the cytokines
IL-2, IFN-α and IFN-γ were associated with increased expression of
PD-L1 at the mRNA and protein levels in T24 bladder cancer cell
lines in vitro. This effect was not observed in normal
uroepithelial SV-HUC-1 cell lines. IFN-γ may be involved in the
regulation of PD-L1 via certain signaling pathways, including the
Janus kinase (JAK)/signal transducer and activator of transcription
1 (STAT1)/interferon regulatory factor-1 (IRF-1);
phosphoinositide-3 kinase (PI3K)/Akt/mechanistic target of
rapamycin (mTOR)/S6 K and methyl ethyl ketone (MEK)/extracellular
signal-regulated kinase (ERK)/STAT1 pathways (38). Previous studies have confirmed that
the JAK/STATl/IRF-1 and MEK/ERK/STAT1 signaling pathways are
implicated in the association between IFN-γ and PD-L1 (39). In addition, the deletion or mutation
of the phosphatase and tension homolog gene may contribute to an
increase in PD-L1 expression via the PI3K/Akt/mTOR/S6K signaling
pathway (40), and IL-2 is known to
induce the release of IFN-γ (41).
Therefore, we hypothesized that IL-2 may increase the expression of
PD-L1 by mediating the effect of IFN-γ; however, the details and
mechanisms by which IL-2 and IFN-α may increase expression of PD-L1
remain unclear. Based on evidence from previous studies, in
combination with the present RT-qPCR data, it was concluded that
OK-432 stimulated the production of a variety of cytokines,
including IL-2, IFN-α and IFN-γ, which not only perform key roles
in enhancing immunity, but increase the expression of PD-L1,
thereby reducing the antitumor effect.
The antitumor immune response may be reduced by
increased expression of PD-L1 through the inhibition or shielding
of certain antigen-presenting cells (APCs) that are involved in
antigen delivery. Together, these mechanisms may explain why
treatment outcome is poor in PD-L1-positive patients with NMIBC
when OK-432 is administered as a bladder intravesical agent. As
treatment progresses, expression of PD-L1 increases, which directly
inhibits the host antitumor immune system and T cells and may
shield associated APCs, thereby promoting resistance against the
antitumor actions of OK-432 in the treatment of NMIBC.
The interaction between bladder cancer cells and the
immune system is a complex and dynamic process, therefore
additional in vitro and in vivo mechanistic studies
are required to fully elucidate the role of PD-L1 in bladder cancer
and its effect on immunotherapy. However, the present study
demonstrated that abnormal expression of PD-L1 is associated with
recurrence and progression in bladder cancer and that it may
compromise the antitumor effect of intravesical immunotherapy.
The expression of the negative costimulatory
molecule PD-L1 is negatively-associated with intravesical
immunotherapeutic outcome in patients with NMIBC. It was
hypothesized that the mechanism involves upregulation of PD-L1
mediated by specific cytokines, thereby compromising the immune
response, ultimately leading to tumor recurrence and progression.
The present findings may contribute to future improvements in the
efficacy of adjuvant intravesical therapy in patients with NMIBC
according to the expression of PD-L1.
References
|
1
|
Irani J, Bernardini S, Bonnal JL, Chauvet
B, Colombel M, Davin JL, Laurent G, Lebret T, Maidenberg M,
Mazerolles C, et al: Urothelial tumors. Prog Urol. 17:1065–1098.
2007.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Milojevic B, Dzamic Z, Kajmakovic B,
Petronic D Milenkovic and Grujicic S Sipetic: Urothelial carcinoma:
Recurrence and risk factors. J BUON. 20:391–398. 2015.PubMed/NCBI
|
|
3
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eifler JB, Scarpato KR and Clark PE:
Management of noninvasive bladder cancers. Curr Opin Oncol.
27:185–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seliger B and Quandt D: The expression,
function, and clinical relevance of B7 family members in cancer.
Cancer Immunol Immunother. 61:1327–1341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi L, Chen SH, Yang LJ and Li Y: The role
of PD-1 and PD-L1 in T-cell immune suppression in patients with
hematological malignancies. J Hematol Oncol. 6:742013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wada H, Isobe M, Kakimi K, Mizote Y,
Eikawa S, Sato E, Takigawa N, Kiura K, Tsuji K, Iwatsuki K, et al:
Vaccination with NY-ESO-1 overlapping peptides mixed with Picibanil
OK-432 and montanide ISA-51 in patients with cancers expressing the
NY-ESO-1 antigen. J Immunother. 37:84–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tano T, Okamoto M, Kan S, Bando T, Goda H,
Nakashiro K, Shimodaira S, Koido S, Homma S, Fujita T, et al:
Immunochemoradiotherapy for patients with oral squamous cell
carcinoma: Augmentation of OK-432-induced helper T cell 1 response
by 5-FU and X-ray irradiation. Neoplasia. 15:805–814. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamoto Y, Mizukoshi E, Kitahara M,
Arihara F, Sakai Y, Kakinoki K, Fujita Y, Marukawa Y, Arai K,
Yamashita T, et al: Prolonged recurrence-free survival following
OK432-stimulated dendritic cell transfer into hepatocellular
carcinoma during transarterial embolization. Clin Exp Immunol.
163:165–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoo C, Do HA, Jeong IG, Park H, Hwang JJ,
Hong JH, Cho JS, Choo MS, Ahn H and Kim CS: Efficacy of dendritic
cells matured early with OK-432 (Picibanil), prostaglandin E2, and
interferon-alpha as a vaccine for a hormone refractory prostate
cancer cell line. J Korean Med Sci. 25:1284–1290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujimoto T, Duda RB, Szilvasi A, Chen X,
Mai M and O'Donnell MA: Streptococcal preparation OK-432 is a
potent inducer of IL-12 and a T helper cell 1 dominant state. J
Immunol. 158:5619–5626. 1997.PubMed/NCBI
|
|
12
|
Saito M, Ebina T, Koi M, Yamaguchi T,
Kawade Y and Ishida N: Induction of interferon-gamma in mouse
spleen cells by OK-432, a preparation of Streptococcus pyogenes.
Cell Immunol. 68:187–192. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sylvester RJ, van der Meijden APM,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: A
combined analysis of 2596 patients from seven EORTC trials. Eur
Urol. 49:466–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alexandroff AB, Nicholson S, Patel PM and
Jackson AM: Recent advances in bacillus Calmette-Guerin
immunotherapy in bladder cancer. Immunotherapy. 2:551–560. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gandhi NM, Morales A and Lamm DL: Bacillus
Calmette-Guérin immunotherapy for genitourinary cancer. BJU Int.
112:288–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A and Palou-Redorta J; European Association of
Urology (EAU), : EAU guidelines on non-muscle-invasive urothelial
carcinoma of the bladder. Eur Urol. 54:303–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian K, Han B, Shen Y, Li C and Xuan Y:
Investigation on immune function and chest drainage in patients
with thoracic malignancies using the streptococcal agent Sapylin. J
Cancer Res Ther. 10:1030–1032. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Gao E, Liu X, Ye Z, Chen Y, Li Q,
Qu J, Dai X, Wang O, Pan Y and Zhang X: Effectiveness of OK-432
(Sapylin) to reduce seroma formation after axillary lymphadenectomy
for breast cancer. Ann Sur Oncol. 20:1500–1504. 2013. View Article : Google Scholar
|
|
20
|
Matsubara N, Itoh K, Mukai H and Nagai S:
Long-term outcome of pleurodesis with OK-432 in metastatic breast
cancer: A new risk model for success from an analysis of 75 cases.
Int J Clin Oncol. 17:470–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J, Liu X, Cui F, Chen G, Guan Y and He
J: The efficacy of the inhalation of an aerosolized Group A
streptococcal preparation in the treatment of lung cancer. Chin J
Cancer Res. 24:346–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun XZ and QIU SP: Intravesical
instillation of picibanil in prophylaxis of local recurrence after
resecion of bladder cancer and its mechanism. China Journal of
Modern Medicine. 49–51. 54:2004.
|
|
23
|
Tian YF, Tang K, Guan W, Yang T, Xu H,
Zhuang QY and Ye ZQ: OK-432 suppresses proliferation and metastasis
by tumor associated macrophages in bladder cancer. Asian Pac J
Cancer Prev. 16:4537–4542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sudo T, Aruga A, Shimizu K, Matsushita N
and Takasaki K: OK432-activated natural killer cells enhanced
trastuzumab (Herceptin (R)-mediated antibody-dependent cellular
cytotoxicity in patients with advanced cancer. Anticancer Res.
26:4327–4333. 2006.PubMed/NCBI
|
|
25
|
Okamoto M, Ohe G, Furuichi S, Nishikawa H,
Oshikawa T, Tano T, Ahmed SU, Yoshida H, Moriya Y, Matsubara S, et
al: Enhancement of anti-tumor immunity by lipoteichoic acid-related
molecule isolated from OK-432, a streptococcal agent, in athymic
nude mice bearing human salivary adenocarcinoma: Role of natural
killer cells. Anticancer Res. 22:3229–3239. 2002.PubMed/NCBI
|
|
26
|
Nakayama F, Iwagaki H, Gouchi A, Hizuta A,
Isozaki H, Takakura N and Tanaka N: Effect of streptococcal lyzate
OK-432 on peripheral blood mononuclear cells in gastric cancer
patients. J Med. 29:199–215. 1998.PubMed/NCBI
|
|
27
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen C, Qu QX, Huang JA, Zhu YB, Ge Y,
Wang Q and Zhang XG: Expression of programmed-death receptor
ligands 1 and 2 may contribute to the poor stimulatory potential of
murine immature dendritic cells. Immunobiology. 212:159–165. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang Y, Zhang SD, McCrudden C, Chan KW,
Lin Y and Kwok HF: The prognostic significance of PD-L1 in bladder
cancer. Oncol Rep. 33:3075–3084. 2015.PubMed/NCBI
|
|
30
|
Huang Y, Zhang SD, McCrudden C, Chan KW,
Lin Y and Kwok HF: The presence of programmed death 1
(PD-1)-positive tumor-infiltrating lymphocytes is associated with
poor prognosis in human breast cancer. Breast Cancer Res Treat.
139:667–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maine CJ, Aziz NH, Chatterjee J, Hayford
C, Brewig N, Whilding L, George AJ and Ghaem-Maghami S: Programmed
death ligand-1 over-expression correlates with malignancy and
contributes to immune regulation in ovarian cancer. Cancer Immunol
Immunother. 63:215–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Afreen S and Dermime S: The
immunoinhibitory B7-H1 molecule as a potential target in cancer:
Killing many birds with one stone. Hematol Oncol Stem Cell Ther.
7:1–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Inman BA, Sebo TJ, Frigola X, Dong H,
Bergstralh EJ, Frank I, Fradet Y, Lacombe L and Kwon ED: PD-L1
(B7-H1) expression by urothelial carcinoma of bladder and
BCG-induced granulomata: Associations with localized stage
progression. Cancer. 109:1499–1505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boorjian SA, Sheinin Y, Crispen PL, Farmer
SA, Lohse CM, Kuntz SM, Leibovich BC, Kwon ED and Frank I: T-cell
coregulatory molecule expression in urothelial cell carcinoma:
Clinicopathologic correlations and association with survival. Clin
Cancer Res. 14:4800–4808. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xing L, Ping L, Bo Z, Keqin Z, Fengshuo J
and Yanfeng L: Phenotype and metergasis of dendritic cells from
peripheral blood of bladder carcinoma patients. J Third Mil Med
Univ. 901–904. 2013.
|
|
36
|
Dong HD, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dunn GP, Koebel CM and Schreiber RD:
Interferons, immunity and cancer immunoediting. Nat Rev Immunol.
6:836–848. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu JZ, Hamrouni A, Wolowiec D, Coiteux V,
Kuliczkowski K, Hetuin D, Saudemont A and Quesnel B: Plasma cells
from multiple myeloma patients express B7-H1 (PD-L1) and increase
expression after stimulation with IFN-{gamma} and TLR ligands via a
MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 110:296–304.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parsa AT, Waldron JS, Panner A, Crane CA,
Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et
al: Loss of tumor suppressor PTEN function increases B7-H1
expression and immunoresistance in glioma. Nat Med. 13:84–88. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Camisaschi C, Filipazzi P, Tazzari M,
Casati C, Beretta V, Pilla L, Patuzzo R, Maurichi A, Cova A, Maio
M, et al: Effects of cyclophosphamide and IL-2 on regulatory CD4+ T
cell frequency and function in melanoma patients vaccinated with
HLA-class I peptides: Impact on the antigen-specific T cell
response. Cancer Immunol Immunother. 62:897–908. 2013. View Article : Google Scholar : PubMed/NCBI
|