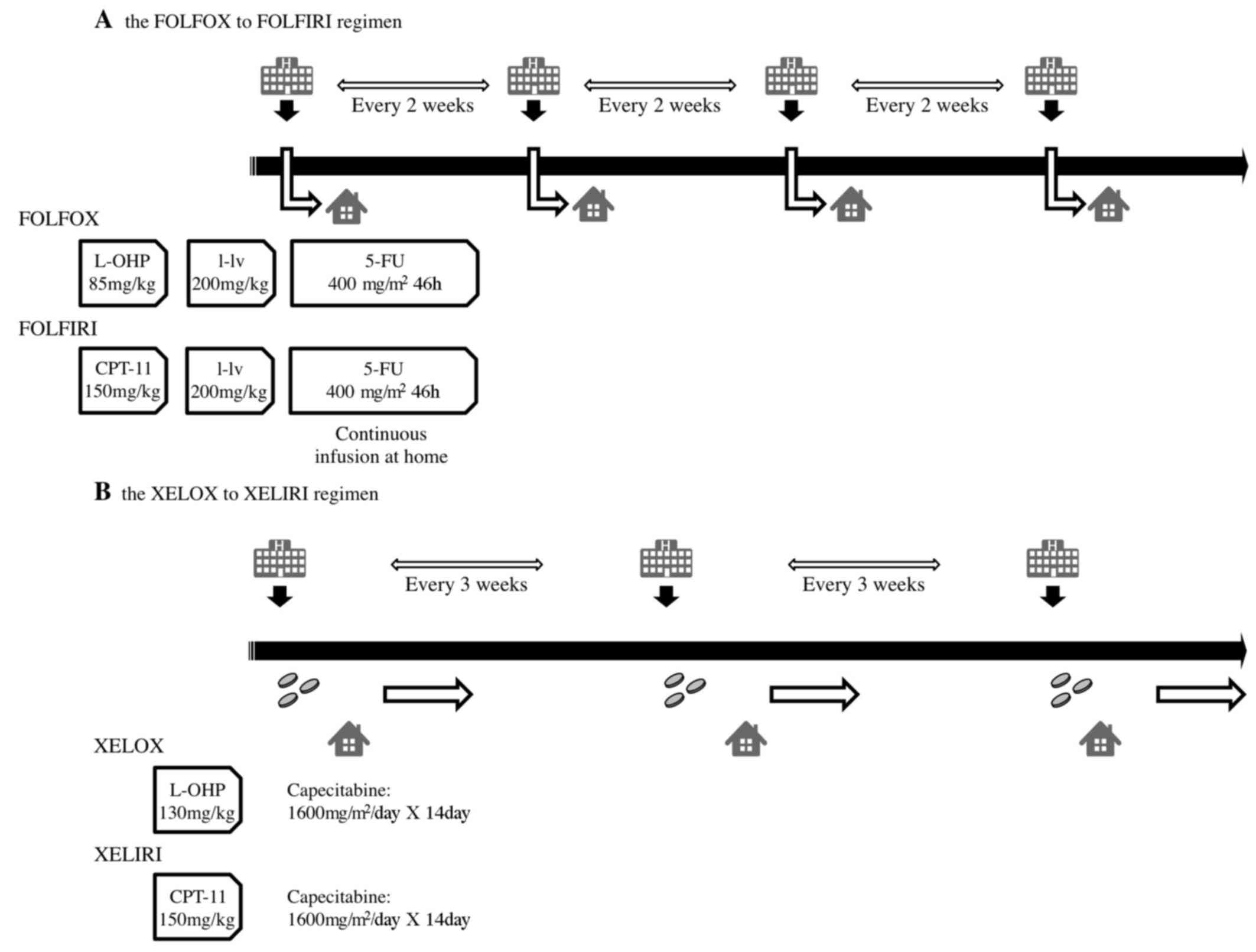
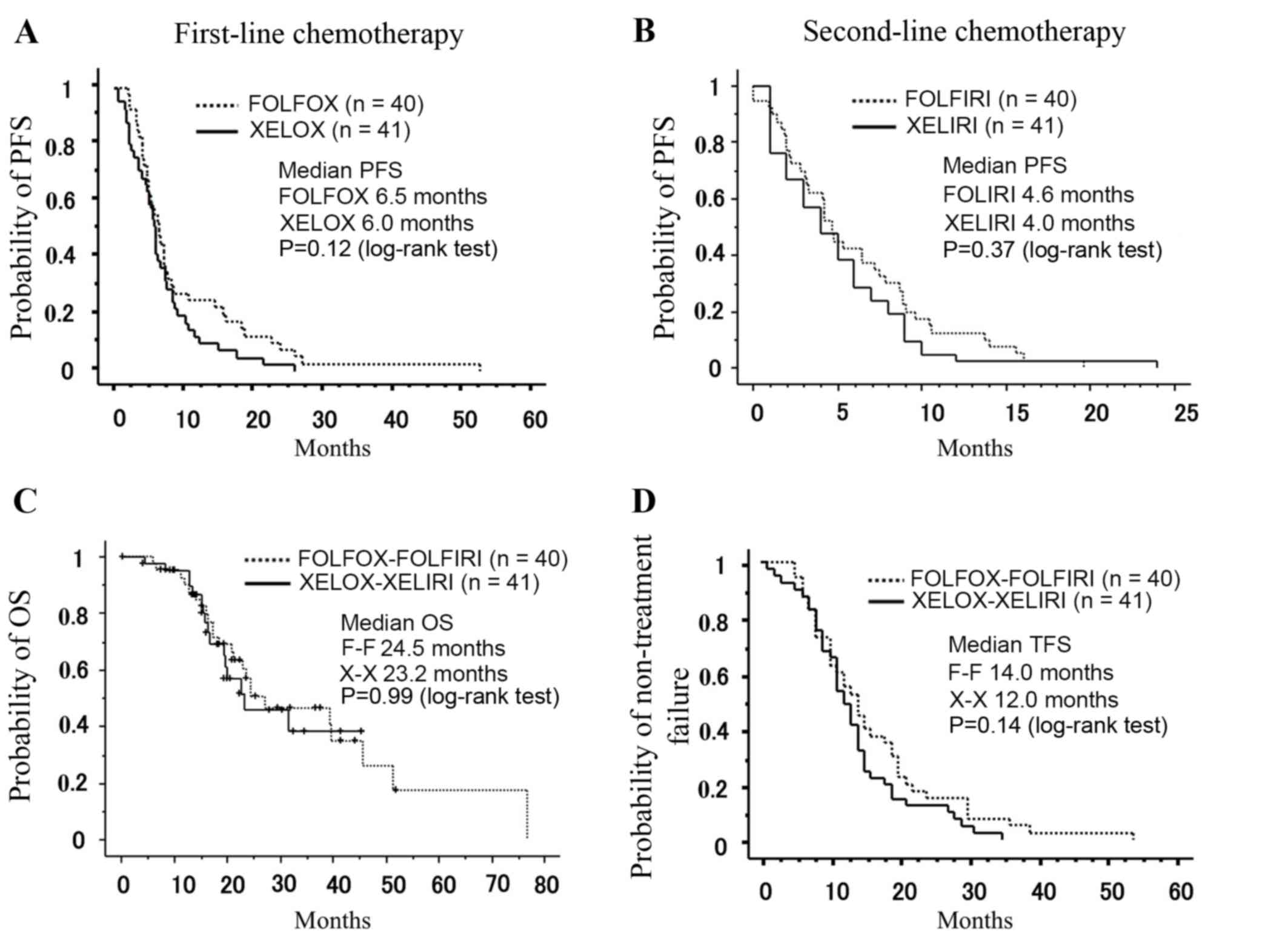
|
1
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arkenau HT, Arnold D, Cassidy J,
Diaz-Rubio E, Douillard JY, Hochster H, Martoni A, Grothey A, Hinke
A, Schmiegel W, et al: Efficacy of oxaliplatin plus capecitabine or
infusional fluorouracil/leucovorin in patients with metastatic
colorectal cancer: A pooled analysis of randomized trials. J Clin
Oncol. 26:5910–5917. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ducreux M, Adenis A, Pignon JP, François
E, Chauffert B, Ichanté JL, Boucher E, Ychou M, Pierga JY,
Montoto-Grillot C and Conroy T: Efficacy and safety of
bevacizumab-based combination regimens in patients with previously
untreated metastatic colorectal cancer: Final results from a
randomised phase II study of bevacizumab plus 5-fluorouracil,
leucovorin plus irinotecan versus bevacizumab plus capecitabine
plus irinotecan (FNCLCC ACCORD 13/0503 study). Eur J Cancer.
49:1236–1245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmiegel W, Reinacher-Schick A, Arnold D,
Kubicka S, Freier W, Dietrich G, Geißler M, Hegewisch-Becker S,
Tannapfel A, Pohl M, et al: Capecitabine/irinotecan or
capecitabine/oxaliplatin in combination with bevacizumab is
effective and safe as first-line therapy for metastatic colorectal
cancer: A randomized phase II study of the AIO colorectal study
group. Ann Oncol. 24:1580–1587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bennouna J, Sastre J, Arnold D, Österlund
P, Greil R, van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C,
et al: Continuation of bevacizumab after first progression in
metastatic colorectal cancer (ML18147): A randomised phase 3 trial.
Lancet Oncol. 14:29–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hamamoto Y, Yamaguchi T, Nishina T,
Yamazaki K, Ura T, Nakajima T, Goto A, Shimada K, Nakayama N,
Sakamoto J, et al: A phase I/II study of XELIRI plus bevacizumab as
second-line chemotherapy for Japanese patients with metastatic
colorectal cancer (BIX Study). Oncologist. 19:1131–1132. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakar B, Gumus M, Basaran M, Argon A,
Ustuner Z, Ustaoglu MA, Saglam S, Guney N, Tenekeci AN and Aykan
NF: XELOX followed by XELIRI or the reverse sequence in advanced
colorectal cancer. Oncology. 73:298–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lassere Y and Hoff P: Management of
hand-foot syndrome in patients treated with capecitabine (Xeloda).
Eur J Oncol Nurs. 8 Suppl 1:S31–S40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Japanese translation of common terminology
criteria for adverse events (CTCAE) and instructions and
guidelines. Int J Clin Oncol. 9(Suppl 3): S1–S82. 2004.(In
Japanese).
|
|
11
|
Balducci L and Beghe C: The application of
the principles of geriatrics to the management of the older person
with cancer. Crit Rev Oncol Hematol. 35:147–154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mabro M, Louvet C, André T, Carola E,
Gilles-Amar V, Artru P, Krulik M and de Gramont A: GERCOR:
Bimonthly leucovorin, infusion 5-fluorouracil, hydroxyurea and
irinotecan (FOLFIRI-2) for pretreated metastatic colorectal cancer.
Am J Clin Oncol. 26:254–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mabro M, Artru P, André T, Flesch M,
Maindrault-Goebel F, Landi B, Lledo G, Plantade A, Louvet C and de
Gramont A: A phase II study of FOLFIRI-3 (double infusion of
irinotecan combined with LV5FU) after FOLFOX in advanced colorectal
cancer patients. Br J Cancer. 94:1287–1292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beretta GD, Petrelli F, Stinco S, Cabiddu
M, Ghilardi M, Squadroni M, Borgonovo K and Barni S: FOLFIRI +
bevacizumab as second-line therapy for metastatic colorectal cancer
pretreated with oxaliplatin: A pooled analysis of published trials.
Med Oncol. 30:4862013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki K, Takaharu K, Muto Y, Ichida K,
Fukui T, Takayama Y, Tsujinaka S, Sasaki J, Horie H, Kawamura YJ,
et al: XELIRI regimen plus continuous treatment with bevacizumab is
well-tolerated and effective in metastatic colorectal cancer
patients in a second-line setting involving the sequential
administration of XELOX and XELIRI. Mol Clin Oncol. 2:827–832.
2014.PubMed/NCBI
|
|
17
|
Chu E, Haller D, Cartwright T, Twelves C,
Cassidy J, Sun W, Saif MW, McKenna E7, Lee S and Schmoll HJ:
Epidemiology and natural history of central venous access device
use and infusion pump function in the NO16966 trial. Br J Cancer.
110:1438–1445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Conroy T, Hebbar M, Bennouna J, Ducreux M,
Ychou M, Llédo G, Adenis A, Faroux R, Rebischung C, Kockler L and
Douillard JY: Quality-of-life findings from a randomised phase-III
study of XELOX vs FOLFOX-6 in metastatic colorectal cancer. Br J
Cancer. 102:59–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perrocheau G, Bennouna J, Ducreux M,
Hebbar M, Ychou M, Lledo G, Conroy T, Dominguez S, Faroux R,
Florentin V and Douillard JY: Cost-minimisation analysis in
first-line treatment of metastatic colorectal cancer in France:
XELOX versus FOLFOX-6. Oncology. 79:174–180. 2010. View Article : Google Scholar : PubMed/NCBI
|