Introduction
Breast cancer is a highly heterogeneous disease,
particularly in its locally advanced form (1). Despite the availability of various
aggressive therapies for breast cancer patients, the mortality
remains high (2). Thus, novel
therapeutics are required for breast cancer patients. Numerous
clinical and experimental studies have established that the
clinical outcome of treatment for breast cancer depends on the
expression of biological predictive markers such as estrogen
receptor (ER) (3). Current effective
prevention strategies for adjuvant therapy in ER-positive breast
cancer include tamoxifen (4).
However, ER-negative breast cancers are more clinically aggressive
compared with ER-positive breast cancers, and their prognosis is
poor due to the lack of hormone receptor (HR)-directed therapies
(1,4).
Since there is no effective cure for ER-negative breast cancer
patients, alternative interventions such as substances derived from
natural herbal sources may be useful to replace the current
regimens (5,6). Therefore, new therapies or strategies
are urgently required for patients with ER-negative and triple
negative breast cancer (TNBC), which does not express any of the
following three receptors: ER, progesterone receptor or human
epidermal growth factor receptor 2 (HER2) (1).
The combination of polyphenols with a
chemotherapeutic drug can result in additive or synergistic effects
in ER-negative breast cancer cells (6). Furthermore, polyphenols induce
restoration of tamoxifen sensitivity in TNBC cells (7).
Green tea is one of the most widely consumed
beverages in the world, and its ingestion in reasonable doses is
considered safe (8). It has been
reported that green tea consumption provides potential protection
against numerous cancers through multiple mechanisms (8). Flavonoids in green tea are dietary
factors that may protect against cancer, and have potent
antioxidant effects (9).
Epidemiological studies suggest that the low incidence of certain
cancers in Asian countries is linked to the regular consumption of
green tea (9,10). There are reports linking green tea
consumption with an improved prognosis in breast cancer (11,12).
Epigallocatechin gallate (EGCG), a major polyphenol
in green tea, has been extensively studied as a bioactive dietary
agent against carcinogenesis, and it appears to act through
multiple signaling pathways, including the mitogen-activated
protein kinase, phosphatidylinositol-3 (PI3) kinase, epidermal
growth factor receptor and nuclear factor-κB signaling pathways
(13–16). EGCG can inhibit breast tumorigenesis
through the ER signaling pathway (17). In addition, EGCG enhances
chemotherapeutic-induced cellular apoptosis in ER-negative
MDA-MB-231 breast cancer cells (18),
suggesting that EGCG exerts its anticancer properties through
acting on ER signal transduction. However, the precise molecular
mechanisms underlying this phenomenon are unclear.
Wnt signals are critical in regulating the normal
development of the mammary gland, and dysregulation of Wnt
signaling causes breast cancer (19).
Wnt signaling cascades can be broadly subdivided into two
categories: The canonical β-catenin signaling pathway and the
non-canonical signaling pathway (19). Wnt/β-catenin signaling is involved in
several stages of growth and differentiation of the mammary gland,
both during embryogenesis and following birth (19). This signaling system is pivotal in
processes involved in the development and pathogenesis of breast
cancer, including angiogenesis and hormonal signaling (20). EGCG induces the disruption of adherent
junction formation and the accumulation of extra-nuclear β-catenin
in MCF-7 cells (19,20). The β-catenin signaling pathway is
involved in EGCG-mediated anticancer protection (21). Therefore, members of the β-catenin
pathway in tissues of breast cancer patients are potential targets
of EGCG.
The present study investigated the anticancer
effects of EGCG, the major active component of green tea (16), on MDA-MB 231 breast cancer cells. In
addition, the expression profiles of the β-catenin signaling
pathway in breast cancer patients were also analyzed. The results
suggest that EGCG inactivates β-catenin signaling in MDA-MB 231
human breast cancer cells. Furthermore, β-catenin was significantly
expressed at higher levels in ER-negative breast cancer patients
compared with patients with ER-positive breast cancer. In summary,
the present findings demonstrate that EGCG could be therapeutically
effective in TNBC patients.
Materials and methods
Study subjects
The present study protocol was approved by the
Institutional Review Board (IRB) of Chonbuk National University
Hospital (Jeonju, Korea; IRB approval no. 2012-07-011). Among
patients who underwent surgery at Chonbuk National University
Hospital for primary breast cancers from June 2008 to July 2009, 74
breast cancer patients were identified and enrolled in the study.
The patients' clinical and pathological characteristics were
analyzed. The tumor-node-metastasis (TNM) stage was determined
according to the 7th edition of the American Joint Committee on
Cancer classification (22). Cancer
and normal tissues (which were located away from the primary tumor
site, histologically confirmed to be free of cancer cells and
obtained from the same patient) were collected from all patients,
who provided informed consent. The fresh tumor and normal
background tissues were snap frozen in liquid nitrogen and stored
at −70°C. Prior to immunoblotting, the tissue specimens were cut
into small pieces and homogenized.
Cells and materials
MDA-MB-231 cells were obtained from the American
Type Culture Collection (Manassas, VA, USA). Cells were cultured in
high glucose-containing Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 10,000 U/ml
penicillin and 10,000 µg/ml streptomycin at 37°C in a 5%
CO2 incubator. EGCG was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). MTT and β-actin antibody (cat.
no. A-5441) were obtained from Sigma-Aldrich (Merck KGaA). The
antibody against β-catenin (cat. no. Sc-59737) and horseradish
peroxidase (HRP)-conjugated immunoglublin (Ig) G were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The
antibodies related to p-AKT (cat. no. 9271) and cyclin D1 (cat. no.
2922) were purchased from Cell Signaling Technology (Beverly, MA,
USA). The PI3 kinase inhibitors LY294002 and wortmannin were
purchased from Merck KGaA. High glucose-containing DMEM, FBS and
PBS were obtained from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Determination of cell viability
Cells were inoculated in a 96-well plate at a
density of 3×104 cells/well, and incubated at 37°C for
24 h to allow attachment. The attached cells were either untreated
or treated with EGCG for 24 h at 37°C. Next, the cells were washed
with PBS prior to the addition of MTT (0.5 mg/ml in PBS), and were
incubated at 37°C for 30 min. The formazan crystals were then
dissolved with dimethyl sulfoxide (100 µl/well), and the absorbance
was detected at 570 nm using a Model 3550 Microplate Reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein extraction
Breast cancer and adjacent normal tissues were
collected immediately following surgery for protein extraction.
These tissue samples were homogenized at 4°C in the presence of
lysis buffer [50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 5 mM EDTA and
1% NP-40]. The homogenates were subsequently centrifuged at 13,000
× g for 30 min at 4°C, and the supernatants were collected. Upon
washing with PBS, MDA-MB-231 cells were treated with EGCG, and then
incubated for 24 h at 37°C. These cells were lysed with ice-cold
M-PER Mammalian Protein Extraction Reagent (Pierce; Thermo Fisher
Scientific, Inc.). The protein concentration was determined using a
DC Protein Assay kit (Bio-Rad Laboratories, Inc.).
Western blot analysis
The cell lysates (10 µg protein) were separated by
10% SDS-PAGE and then transferred to Hybond™ polyvinylidene
fluoride membranes (GE Healthcare Life Sciences, Chalfont, UK).
Each membrane was blocked for 2 h with 5% skim milk and incubated
overnight at 4°C with the β-catenin, β-actin, cyclin D1 or p-AKT
antibody (all diluted at 1:2,500 in 5% skimmed milk/PBS buffer).
HRP-conjugated IgG (1:2,000 dilution) was used as the secondary
antibody for 1 h at 4°C. Protein levels were determined using a
Fujifilm Image Analyzer (Tokyo, Japan). Immunoreactive signal were
visualized with western chemiluminescent HRP substrate (Merck
KGaA). The β-catenin-relative density of the electrophoretic band
was obtained with LAS-1000 Intelligent Dark-Box II (Fujifilm
Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS
version 15.0. (SPSS, Inc., Chicago, IL, USA). Statistical data
analysis was performed using analysis of variance and Duncan's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients' characteristics
The present cohort comprised 74 female patients of
ages ranged from 32 to 78 years (mean, 50.7 years), who were
diagnosed with invasive ductal carcinoma and underwent curative
surgery. In total, 44 (59.5%) patients had T2 tumors on
presentation, 23 (31.1%) patients were T1, 4 (5.4%) patients were
T3 and 3 (4.1%) patients were T4. Lymph node involvement status was
N0 in 33 (44.6%) patients, while 25 (33.8%) patients were N1, 12
(16.2%) were N2 and 4 (5.4%) were N3. In total, 21 (28.4%) patients
were stage I, 34 (45.9%) were stage II and 19 (25.7%) were stage
III. Among all patients, 50 (67.6%) were ER positive and 24 (32.4%)
were ER negative.
Expression of β-catenin in breast
cancer patients
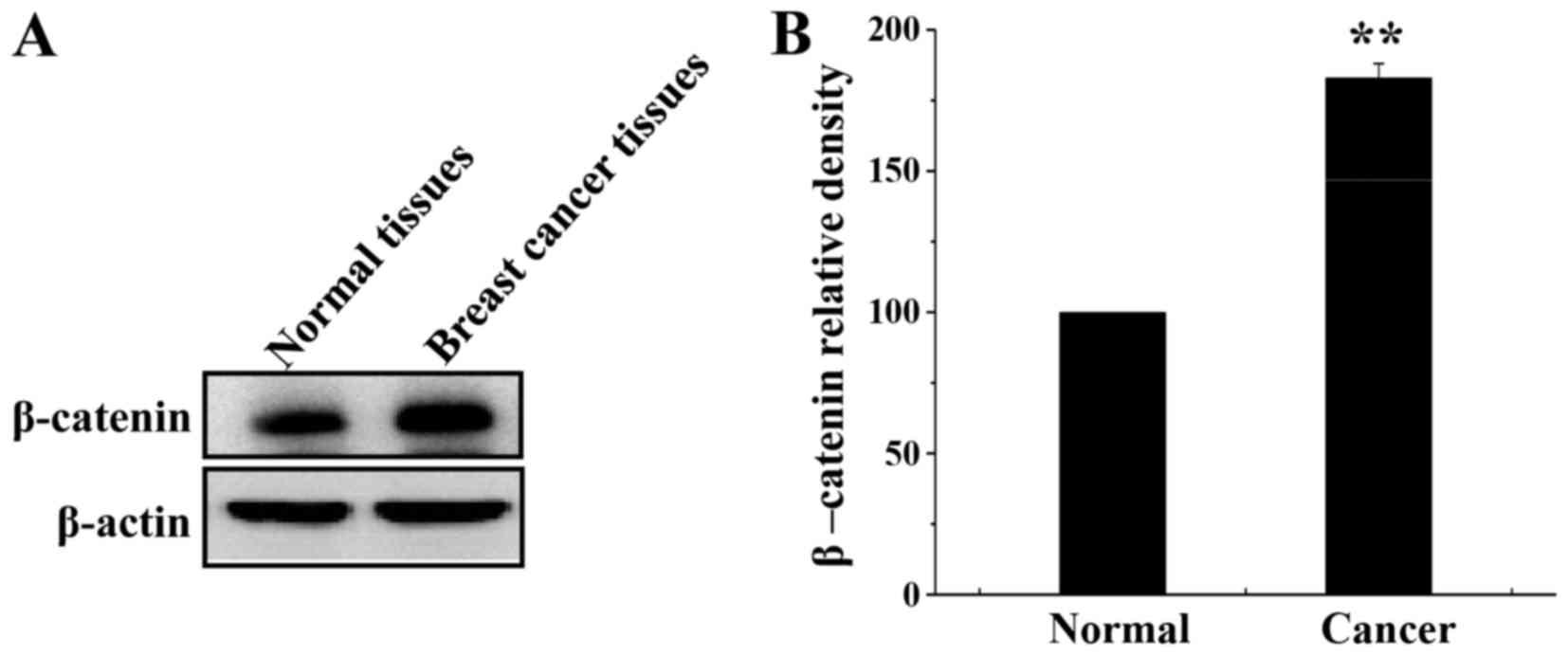
β-catenin expression was analyzed in breast cancer
and normal tissues by western blotting. β-catenin was overexpressed
in breast cancer tissue compared with its expression in normal
tissue (Fig. 1A and B).
Association between β-catenin
expression and patients' clinicopathological features
β-catenin expression in tumor cells was scored as 3+
(an intensity of >2/3 of the adjacent normal epithelium), 2+
(1/3-2/3 of the adjacent normal epithelium) and 1+ (<1/3 of the
adjacent normal epithelium). In total, 14 (18.9%) patients had 1+
β-catenin expression, 28 (37.8%) had 2+ expression and 32 (43.2%)
had 3+ expression. β-catenin expression was associated with lymph
node metastasis (P=0.04), TNM stage (P=0.03) and ER status
(P<0.01) (Table I). Other factors
were not associated with β-catenin expression.
 | Table I.Expression of β-catenin according to
the clinicopathological factors of breast cancer. |
Table I.
Expression of β-catenin according to
the clinicopathological factors of breast cancer.
|
| β-catenin expression,
n |
|
|---|
|
|---|
| Characteristics | 1+(n=14) | 2+(n=28) | 3+(n=32) | P-value |
|---|
| Tumor size |
|
|
| 0.21 |
| T1 | 4 | 11 | 8 |
|
| T2 | 8 | 15 | 21 |
|
| T3 | 2 | 1 | 1 |
|
| T4 | 0 | 1 | 2 |
|
| Lymph node |
|
|
| 0.04 |
| N0 | 9 | 11 | 13 |
|
| N1 | 3 | 11 | 11 |
|
| N2 | 1 | 4 | 7 |
|
| N3 | 1 | 2 | 1 |
|
| TNM stage |
|
|
| 0.03 |
| I | 5 | 9 | 7 |
|
| II | 4 | 13 | 17 |
|
|
III | 5 | 6 | 8 |
|
| ER status |
|
|
| 0.01 |
|
Negative | 0 | 13 | 11 |
|
|
Positive | 14 | 15 | 21 |
|
Effect of EGCG on MDA-MB-231 cell
viability
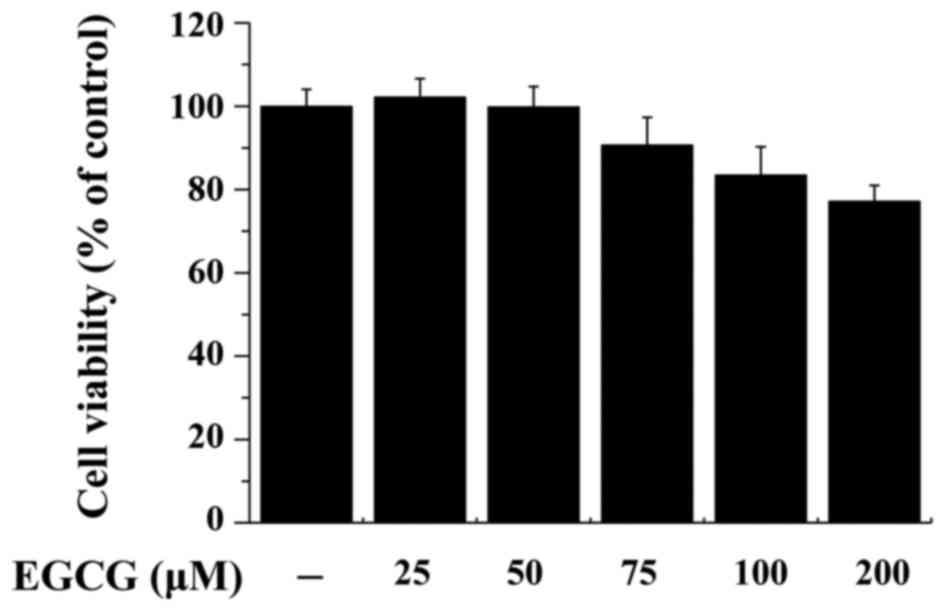
MDA-MB-231 cells were treated with EGCG (0–200 µM)
for 24 h, and toxicity was analyzed using an MTT assay. EGCG
decreased MDA-MB-231 cell viability in a dose-dependent manner
(Fig. 2).
Effect of EGCG on β-catenin,
phosphorylated (p)-Akt and cyclin D1 expression in MDA-MB-231
cells
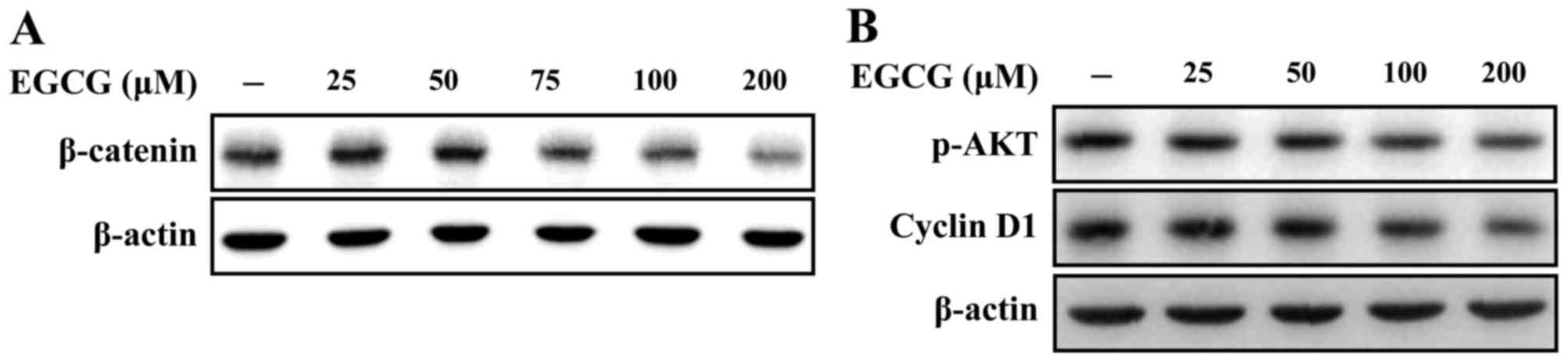
Western blot analysis indicated that treatment of
MDA-MB-231 cells with EGCG for 24 h suppressed β-catenin expression
in a dose-dependent manner (Fig. 3A).
To investigate the effect of EGCG on the phosphorylation of Akt and
cyclin D1 activity, western blotting was performed. Treatment of
MDA-MB-231 cells with EGCG for 24 h decreased p-Akt and cyclin D1
expression (Fig. 3B).
Effect of EGCG treatment with PI3
kinase inhibitors on β-catenin expression in MDA-MB-231 cells
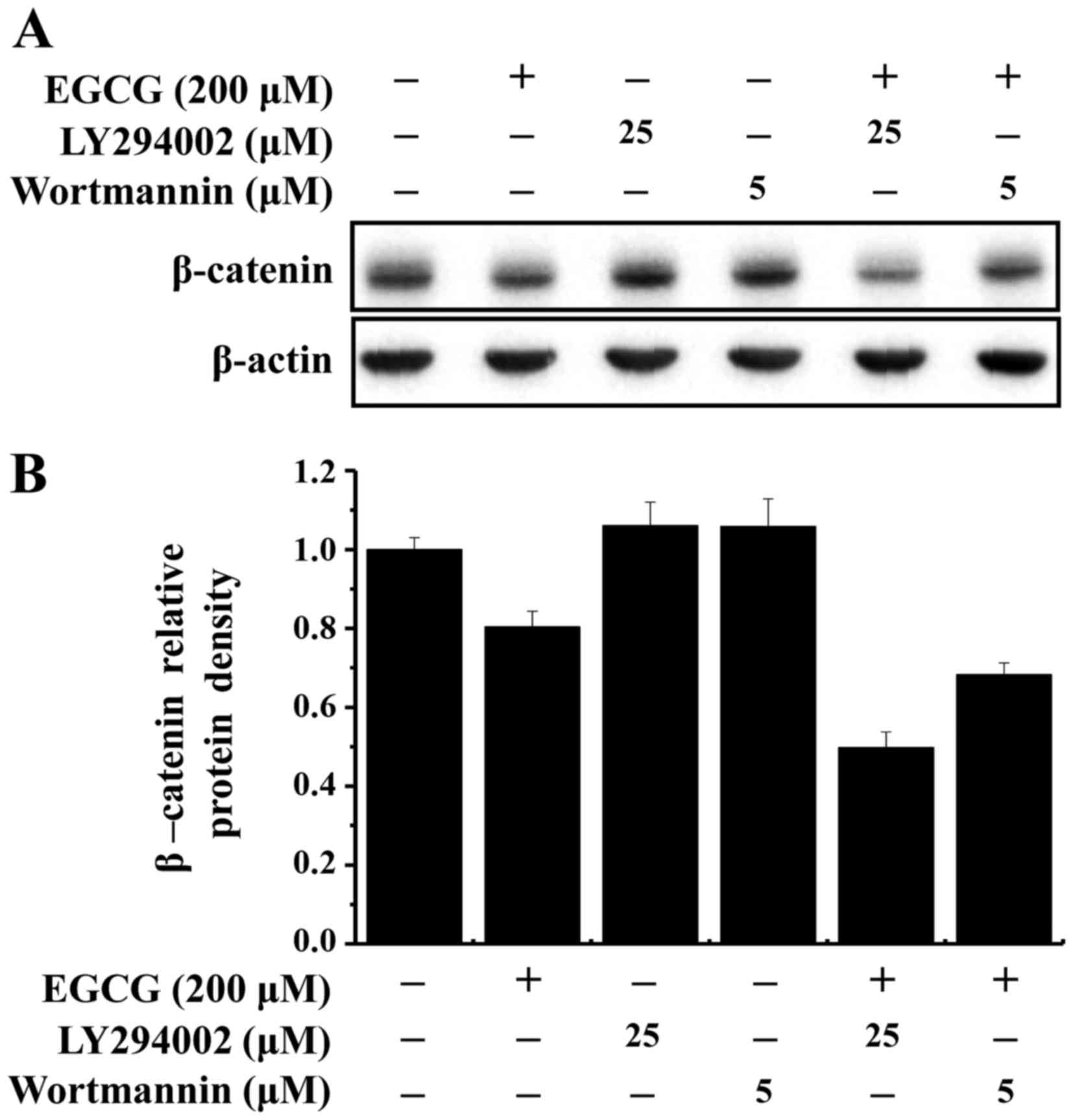
PI3 kinase-dependent signaling pathway may be
involved in the regulation of β-catenin expression, in combination
with other signal transduction pathways. MDA-MB-231 cells were
pretreated with PI3 kinase inhibitors for 1 h (25 µM LY294002 or 5
µM wortmannin), and EGCG was added for 24 h. EGCG with LY294002 or
wortmannin exhibited an additive effect on β-catenin suppression in
MDA-MB-231 cells (Fig. 4A and B).
These results suggest that EGCG inhibited the growth of MDA-MB-231
cells through inactivation of the β-catenin signaling pathway.
Discussion
Breast cancer is the most frequently diagnosed
cancer in women worldwide and the second most common cause of
cancer mortality (23). Advances in
cancer genomics have clarified the intrinsic subtypes of breast
cancer, resulting in targeted treatment, including endocrine
therapy and anti-HER2 therapy for patients with breast cancer who
have HR- or HER2-positive tumors (1,4). The
success of target treatment has been integral to the survival
improvement in patients with breast cancer (24). However, TNBC patients, who account for
15–20% of all breast cancer patients, have no target treatment and
suffer shorter survival than patients with other subtypes of breast
cancer (25). Additionally, TNBC is
remarkably heterogeneous at the transcriptional level, and this is
the main barrier for improving survival and developing target
treatment for patients with TNBC (25). Thus, there are numerous studies on new
drugs or combination treatments with conventional drugs for TNBC,
including research into alternative treatments with natural herbal
regimens such as polyphenols (5,6).
Furthermore, polyphenols induce restoration of tamoxifen
sensitivity in MDA-MB-231 cells (7).
The Wnt signaling pathway is an important
developmental pathway that is frequently dysregulated in human
cancer (19). Wnt ligands primarily
signal via membrane-bound Frizzled receptors through a number of
different but interconnected signaling pathways, including the
β-catenin, Wnt/Ca2+ and planar-cell polarity signaling
pathways (19). β-catenin is a
transcriptional activator that activates target genes in the
nucleus (25). Free cytoplasmic
β-catenin may translocate to the nucleus and activate the Wnt
signaling pathway, which contributes to tumorigenesis and
progression in numerous organs, including the breast (25). Aberrant expression of β-catenin is
associated with increased invasion, metastasis and poor prognosis
in patients with breast cancer (26).
Consistent with this, the present study revealed that β-catenin is
overexpressed in breast cancer tissue compared with its expression
in normal tissue, and that β-catenin expression is associated with
lymph node metastasis, high TNM stage and ER-negative status.
Flavonoids are low-molecular weight, plant-derived
compounds present in fruits, vegetables, herbs, tea and wine
(20). They are divided into
different classes, including polyphenols, which are particularly
concentrated in green tea (Camellia sinensis), accounting
for 30–40% of its dry weight, while other flavonoids are present
only in small quantities in green tea (15). EGCG, the most abundant polyphenol in
green tea, is linked to the majority of health benefits associated
with green tea consumption (8,9). Green tea
and its major constituent, EGCG, have been extensively studied as
potential treatments for a variety of diseases, including cancer
(14,15). EGCG exerts its anticancer effects
through multiple mechanisms, including anti-oxidation, induction of
apoptosis, inhibition of angiogenesis and metastasis (10). Epidemiological data suggest that EGCG
protects against hormone-associated cancers, including breast
cancer (15). EGCG can prevent and
inhibit breast tumorigenesis independently of the ER status
(16), and is cytotoxic toward breast
cancer cells regardless of their ER status. Following treatment
with EGCG, cell numbers were significantly lower in ER-positive and
ER-negative cell lines compared with those in the control (7,18). In the
present study, EGCG decreased MDA-MB-231 cell viability in a
dose-dependent manner.
β-catenin is an important protein in the progression
of multiple epithelial malignancies (20). A previous study treating MCF-7 cells
with EGCG observed a reduction in β-catenin protein content and
messenger RNA expression (13). In
the present study, western blot analysis indicated that the protein
levels of β-catenin in whole cell lysates of MDA-MB-231 cells were
significantly reduced following incubation with EGCG for 24 h in a
dose-dependent manner.
PI3 kinases serve key roles in cell proliferation,
migration, apoptosis, gene expression and differentiation (27). Numerous proteins have been identified
as direct or indirect downstream targets of PI3 kinase, and the
most explored effector of the PI3 kinase signaling pathway is Akt
(28). Akt, also known as protein
kinase B, is a serine/threonine kinase that is pivotal in cellular
metabolism, growth and survival (28). The Akt signaling pathway is also a
target for flavonoids such as EGCG (14). A previous report indicated that EGCG
inhibits the PI3 kinase/Akt/mammalian target of rapamycin signaling
pathway (29). The current study
noticed that EGCG effectively inhibited p-Akt. Furthermore, the
reduction in p-Akt by EGCG pretreatment was dose dependent. Cyclin
D1 is a member of the cyclin protein family, and is involved in
regulating cell cycle progression (14). The synthesis of cyclin D is initiated
during G1, and drives the G1/S phase transition (14). Khan et al (14) reported that EGCG decreased the
expression of cyclin D1. The present study also demonstrated that
incubation with EGCG for 24 h reduced cyclin D1 expression in
MDA-MB-231 cells.
Soluble β-catenin has the ability to bind PI3
kinase, which may mediate β-catenin stabilization (27). Thus, the inhibition of PI3 kinase may
be responsible for the reduced expression of p-Akt and β-catenin
observed in the present study. A previous study indicated that the
PI3 kinase-dependent signaling pathway may be involved in the
regulation of β-catenin expression, together with other signal
transduction pathways (29).
Therefore, the present study investigated the effect of PI3 kinase
and EGCG on β-catenin expression, and the results revealed that
inhibition of PI3 kinase with LY294002 and wortmannin displayed an
additive effect with EGCG by considerably reducing the expression
of β-catenin. These data may account for the anticancer effect of
EGCG via β-catenin inhibition in MDA-MB-231 cells.
In conclusion, the present study provided
associations between β-catenin expression and poor prognostic
factors of breast cancer, and suggested that EGCG inactivates the
β-catenin signaling pathway in MDA-MB 231 breast cancer cells. To
the best our knowledge the current study is the first to
demonstrate that EGCG suppresses cell proliferation and disrupts
adherence junction formation via inhibition of the β-catenin
signaling pathway in the MDA-MB 231 cell line. In summary, the
present findings suggest that EGCG could be considered a potential
treatment drug for TNBC patients.
Acknowledgements
The present study was supported by grants from the
Biomedical Research Institute, Chonbuk National University Hospital
in 2011 and a National Research Foundation (NRF) of Korea grant
provided by the Korean government (grant no.
NRF-2013R1A1A2011718).
References
|
1
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roodi N, Bailey LR, Kao WY, Verrier CS,
Yee CJ, Dupont WD and Parl FF: Estrogen receptor gene analysis in
estrogen receptor-positive and receptor-negative primary breast
cancer. J Natl Cancer Inst. 87:446–451. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gadducci A, Biglia N, Sismondi P and
Genazzani AR: Breast cancer and sex steroids: Critical review of
epidemiological, experimental and clinical investigations on
etiopathogenesis, chemoprevention and endocrine treatment of breast
cancer. Gynecol Endocrinol. 20:343–360. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sartippour MR, Pietras R, Marquez-Garban
DC, Chen HW, Heber D, Henning SM, Sartippour G, Zhang L, Lu M,
Weinberg O, et al: The combination of green tea and tamoxifen is
effective against breast cancer. Carcinogenesis. 27:2424–2433.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sak K: Chemotherapy and dietary
phytochemical agents. Chemother Res Pract.
2012:2825702012.PubMed/NCBI
|
|
7
|
Chisholm K, Bray BJ and Rosengren RJ:
Tamoxifen and epigallocatechin gallate are synergistically
cytotoxic to MDA-MB-231 human breast cancer cells. Anticancer
Drugs. 15:889–897. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Graham HN: Green tea composition,
consumption, and polyphenol chemistry. Prev Med. 21:334–350. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakachi K, Matsuyama S, Miyake S, Suganuma
M and Imai K: Preventive effects of drinking green tea on cancer
and cardiovascular disease: Epidemiological evidence for multiple
targeting prevention. Biofactors. 13:49–54. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurahashi N, Sasazuki S, Iwasaki M, Inoue
M and Tsugane S; JPHC Study Group, : Green tea consumption and
prostate cancer risk in Japanese men: A prospective study. Am J
Epidemiol. 167:71–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang CS, Landau JM, Huang MT and Newmark
HL: Inhibition of carcinogenesis by dietary polyphenolic compounds.
Annu Rev Nutr. 21:381–406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang M, Holman CD, Huang JP and Xie X:
Green tea and the prevention of breast cancer: A case-control study
in Southeast China. Carcinogenesis. 28:1074–1078. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu YC and Liou YM: The anticancer effects
of (−)-Epigalocathine-3-gallate on the signaling pathways
associated with membrane receptors in MCF-7 cells. J Cell Physiol.
226:2721–2730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khan N, Afaq F, Saleem M, Ahmad N and
Mukhtar H: Targeting multiple signaling pathways by green tea
polyphenol (−)-epigallocatechin-3-gallate. Cancer Res.
66:2500–2505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stuart EC, Scandlyn MJ and Rosengren RJ:
Role of epigallocatechin gallate (EGCG) in the treatment of breast
and prostate cancer. Life Sci. 79:2329–2336. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thangapazham RL, Singh AK, Sharma A,
Warren J, Gaddipati JP and Maheshwari RK: Green tea polyphenols and
its constituent epigallocatechin gallate inhibits proliferation of
human breast cancer cells in vitro and in vivo. Cancer Lett.
245:232–241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goodin MG, Fertuck KC, Zacharewski TR and
Rosengren RJ: Estrogen receptor-mediated actions of polyphenolic
catechins in vivo and in vitro. Toxicol Sci. 69:354–361. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roy AM, Baliga MS and Katiyar SK:
Epigallocatechin-3-gallate induces apoptosis in estrogen
receptor-negative human breast carcinoma cells via modulation in
protein expression of p53 and Bax and caspase-3 activation. Mol
Cancer Ther. 4:81–90. 2005.PubMed/NCBI
|
|
19
|
Boras-Granic K and Wysolmerski JJ: Wnt
signaling in breast organogenesis. Organogenesis. 4:116–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amado NG, Fonseca BF, Cerqueira DM, Neto
VM and Abreu JG: Flavonoids: Potential Wnt/beta-catenin signaling
modulators in cancer. Life Sci. 89:545–554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loh YN, Hedditch EL, Baker LA, Jary E,
Ward RL and Ford CE: The Wnt signaling pathway is upregulated in an
in vitro model of acquired tamoxifen resistant breast cancer. BMC
Cancer. 13:1742013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FI and Trotti A III: Part VII breastAJCC Cancer Staging
Manual. 7th. Springer; New York, NY: pp. 347–376. 2010
|
|
23
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piccart-Gebhart MJ, Procter M,
Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga
J, Bell R, Jackisch C, et al: Trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer. N Engl J Med.
353:1659–1672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
López-Knowles E, Zardawi SJ, McNeil CM,
Millar EK, Crea P, Musgrove EA, Sutherland RL and O'Toole SA:
Cytoplasmic localization of beta-catenin is a marker of poor
outcome in breast cancer patients. Cancer Epidemiol Biomarkers
Prev. 19:301–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nelson CM and Chen CS: Cell-cell signaling
by direct contact increases cell proliferation via a PI3K-dependent
signal. FEBS Lett. 514:238–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toker A and Newton AC: Cellular signaling:
Pivoting around PDK-1. Cell. 103:185–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peairs A, Dai R, Gan L, Shimp S, Rylander
MN, Li L and Reilly CM: Epigallocatechin-3-gallate (EGCG)
attenuates inflammation in MRL/lpr mouse mesangial cells. Cell Mol
Immunol. 7:123–132. 2010.PubMed/NCBI
|