Introduction
Globally, gastric cancer was ranked fifth for cancer
incidence (984,000 cases) and second for cancer-associated
mortality (841,000 mortalities) rates in 2013 (1). Complete surgical removal of the tumor is
the standard treatment for gastric cancer, and it is possible to
cure patients with early-stage disease. However, even following
macroscopic complete removal of the tumor by standard resection
with D2 lymphadenectomy, advanced gastric cancer possesses a poor
clinical outcome. Despite recent advances in systemic chemotherapy,
an optimal global standard has not yet been established for
advanced gastric cancer (2) and there
is no internationally recognized standard or preferred regimen for
the management of the advanced disease stage. In order to improve
outcomes, it is essential to understand the molecular pathogenesis
underlying gastric cancer and to identify robust prognostic or
predictive biomarkers (3).
P21-activated kinases (PAKs) are a family of
serine/threonine kinases that serve as downstream effectors in
several cancer signaling pathways. PAKs are overexpressed,
hyper-activated or amplified in several types of human tumor, which
make them attractive novel therapeutic targets. PAKs are
categorized into group I (PAK1, 2 and 3) and group II (PAK4, 5 and
6), based on their amino acid homologies (4–6). PAK5 is
the latest PAK family member to be identified and is also termed
PAK7 (7–9). PAKs participate in a number of signaling
pathways that are commonly deregulated in human cancer cells. PAK1
is a component of the mitogen-activated protein kinase, JUN
N-terminal kinase, steroid hormone receptor and nuclear factor κB
signaling pathways, which have all been associated with
oncogenesis. Overexpression of PAK1 protein occurs in breast,
colon, ovarian and brain cancer, and PAK4 gene amplification and
protein overexpression was reported in pancreatic cancer (4).
At the Tokyo Medical and Dental University (Tokyo,
Japan), Kobayashi et al (10)
revealed that high PAK4 expression was significantly correlated
with clinicopathological variables associated with tumor
progression. These variables consisted of: Depth of invasion;
metastatic lymph nodes; pathological stage; distant metastasis; or
recurrent disease. High PAK4 expression was significantly
associated with poorer disease-specific survival (DSS) (P<0.001)
and relapse-free survival (RFS) (P<0.001) (10). The role of PAK5 in various types of
cancer has been investigated by several groups. Inhibiting PAK5
expression induced a 7-fold increase in apoptosis in the pancreatic
adenocarcinoma cell line MiaPaCa-2, suggesting that PAK5 is a
kinase that may protect pancreatic cancer cells from apoptosis
(11) PAK5 overexpression assessed
using immunohistochemistry was reported in certain colorectal
cancer types, and PAK5 reduced colorectal carcinoma cell adhesion;
however PAK5 promoted cellular migration on collagen type I,
indicating that PAK5 is involved in colorectal cancer cell
migration and invasion (12).
Immunohistochemical analysis demonstrated that PAK5 expression was
upregulated significantly in different gastric cancer cell lines
and gastric cancer tissue samples, as compared with human embryonic
kidney 293 cells and adjacent normal tissue samples (13). The PAK5 mRNA level was significantly
higher in 25/30 human hepatocellular carcinoma samples compared
with the matched paraneoplastic tissue samples (7). Knockdown of PAK5 inhibited cell
proliferation by inducing cell cycle arrest in
G0/G1 phase in human gastric cancer,
hepatocellular carcinoma and glioma cells (13,14). PAK5
expression in gastric cancer has not yet been comprehensively
investigated in a large sample size.
Using immunohistochemistry, Gu et al
(13) examined 57 specimens of human
gastric cancer, which consisted of 16 cases of T1 and T2 depth of
invasion, and the remaining 41 cases of T3 and T4. It was concluded
that none of the clinicopathological parameters were associated
with PAK5 expression. However, according to this study, the
expression levels of PAK5 were significantly higher in gastric
cancer tissue compared with adjacent normal tissue samples
(P=0.0001). These data suggest that there is a change in PAK5
expression during cancer progression. In the present study, the
association between PAK5 expression and clinicopathological factors
was investigated, and included a relatively high number of patients
with early gastric cancer to investigate whether PAK5 contributes
to gastric cancer progression.
Materials and methods
Patients
Resected specimens from 279 patients, whose mean age
was 65 years (range, 21–92 years), with a confirmed pathologic
diagnosis of primary gastric cancer, who underwent gastrectomy
between January 2003 and December 2008 at the Tokyo Medical and
Dental University Hospital, were investigated. Surgery included
laparotomy and laparoscopy. There were 213 males and 66 females.
Tumors were histopathologically diagnosed based on the 3rd English
edition of the Japanese Classification of Gastric Carcinoma
(15) guidelines, in which the
description of tumor status as denoted by the tumor node metastasis
(TNM) stage, is identical to that in the 7th edition of the
International Union Against Cancer TNM classification (16).
Papillary and tubular adenocarcinoma were classified
as differentiated, while poorly differentiated adenocarcinoma,
signet-ring cell carcinoma and mucinous adenocarcinoma were
classified as undifferentiated. All patients were followed up every
3–6 months following surgery using serum tumor marker assays and
diagnostic imaging using esophagogastroduodenoscopy, computed
tomography, ultrasonography or magnetic resonance imaging. Patients
with distant metastatic or recurrent disease received chemotherapy
with S-1 (tegafur, gimeracil, oteracil potassium; Taiho
Pharmaceutical Co., Ltd., Tokyo, Japan) 80–120 mg/day, depending on
body surface area, alone or in a combined regimen. The median
follow-up was 61 months (2–111 months). A total of 97 (35%)
patients succumbed, including 83 (30%) who succumbed due to distant
metastases or recurrent disease, and 14 (5%) who succumbed due to
other causes.
Ethical approval
All procedures were in accordance with the ethical
standards of the institutional review board of Tokyo Medical and
Dental University (approval no. 831) and national ethical standards
of the responsible committee on human experimentation and with the
Helsinki Declaration of 1964, and later versions. Written informed
consent was obtained from all patients prior to inclusion in the
present study.
Immunohistochemistry
Deparaffinized sections of formalin-fixed
paraffin-embedded tissue samples were immunohistochemically stained
using the universal Immuno-enzyme Polymer method (Histofine Simple
Stain Max Po multikit; Nichirei Co., Tokyo, Japan). An anti-PAK5
polyclonal rabbit antibody (cat. no. ab110069; dilution, 1:150),
which was applied in a previous study by Fang et al
(7), was purchased from Abcam
(Cambridge, UK) and used as the primary antibody diluted with
Signal Stain® Antibody Diluent (Cell Signaling
Technology, Inc., Danvers, MA, USA). A normal rabbit IgG (cat. no.
sc-2027; dilution, 1:1,000; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) was substituted for the primary antibody for the negative
controls. Strongly and homogeneously stained gastric adenocarcinoma
specimens obtained from the same block were used as positive
controls to reduce any bias from staining conditions.
The 4 µm-thick sections were cut on a microtome,
deparaffinized with xylene and rehydrated in a graded ethanol
series. Antigen retrieval treatment was performed at 98°C in a
microwave oven (MI-77; Azumaya, Tokyo, Japan) for 30 min in pH 6.0,
10 mmol/l citrate buffer (Mitsubishi Gas Chemical Company, Inc.,
Tokyo, Japan). Subsequent to microwaving, the slides were allowed
to cool in the staining jar at room temperature until the buffer
temperature fell below 45°C. The slides were subsequently rinsed
briefly in PBS and endogenous peroxidase was blocked with 15 min
exposure to 3% hydrogen peroxide in methanol at 22°C. Following
washing with PBS at 22°C, the slides were incubated with the
primary antibody at a 1:150 dilution for 25 min under infrared
radiation (MI-77; Azumaya, Tokyo, Japan) at 27°C. They were then
incubated with the second antibody, Histofine Simple Stain Max Po
Multi (Nichirei Co.) for 30 min at 22°C and 3,3′-diaminobenzidine
tetrahydrochloride solution (Histofine Simple Stain DAB Solution;
Nichirei Co.) was applied for color development to visualize the
image. Sections were counterstained with 1% Mayer's hematoxylin
(Wako Pure Chemical Industries, Ltd., Osaka, Japan), dehydrated,
cleared and mounted.
Interpretation of immunohistochemical
data
The slides were separately evaluated by two
investigators (TA and YT), who were blinded to patient outcome. The
investigators counted whole staining cancer cells of representative
cross-sectional slices. To evaluate discretely distributed cancer
cells, such as in poorly differentiated adenocarcinoma, ≥five
fields were counted/section including the most progressed cell
layers or detached tumor cell groups at the advancing edge of each
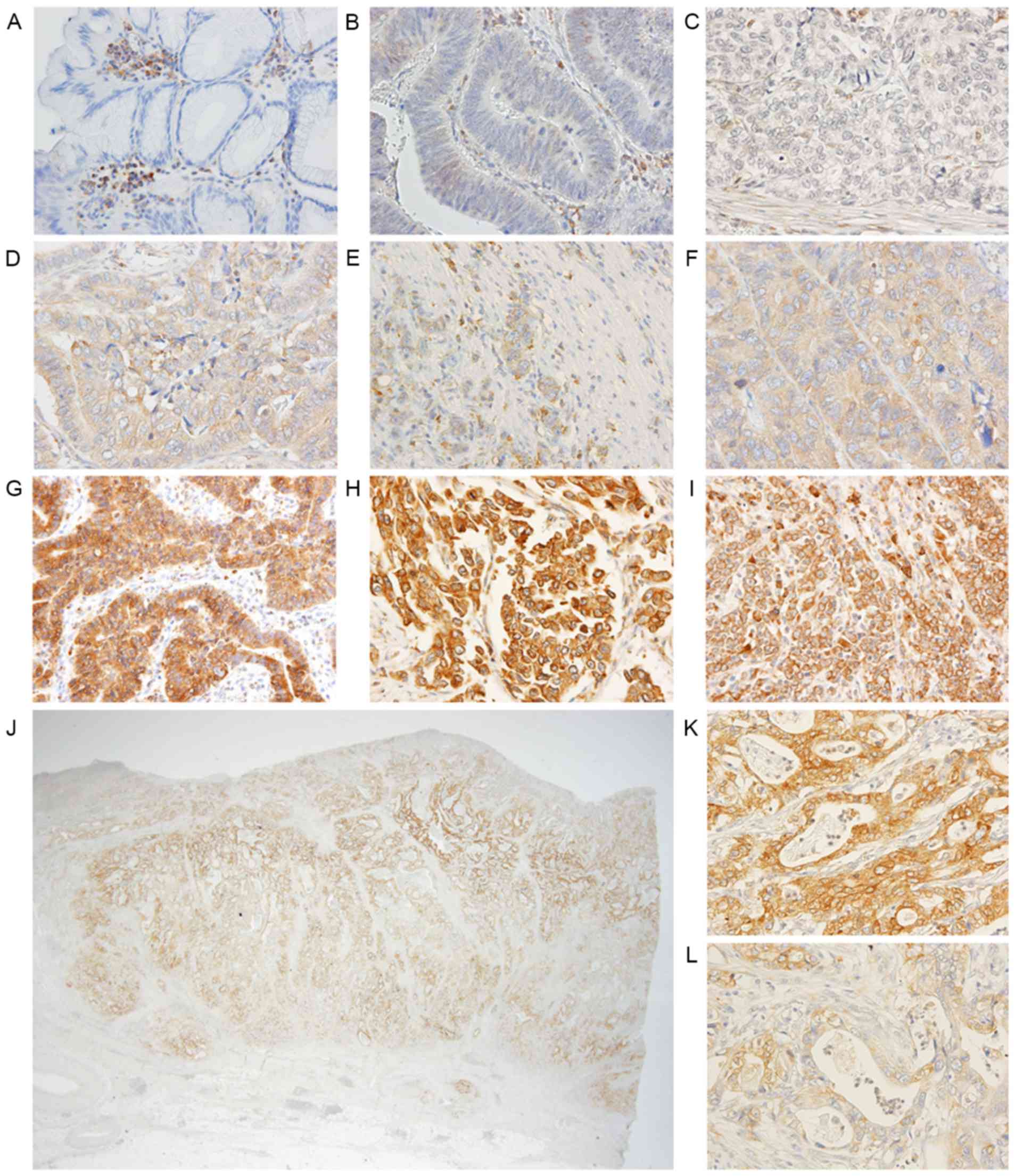
tumor. Staining intensity was scored into 3 grades: 0, none and
very weak (− and ±); 1, weakly positive (+); and 2 strongly
positive (++) (Fig. 1). The
percentage of stained cells (positive frequency) was scored into 4
grades: 1, ≤25%; 2, 26-≤50%; 3, 51-≤75%; and 4, ≥76% cells.
Composite scores were derived by addition of the intensity score
and positive frequency score for statistical analysis with respect
to each patient. A composite score of 6 was defined as high
expression, and scores ≤5 were defined as low expression. Any
discrepant evaluations were re-examined simultaneously by the two
investigators and a pathologist at the Tokyo Medical and Dental
University using a double-headed light microscope (BX53; Olympus
Corporation, Tokyo, Japan) and one monitor to achieve
consensus.
Statistical analysis
The χ2 test was used to test possible
associations between PAK5 expression and clinicopathological
factors. Kaplan-Meier curves were plotted to assess the effect of
PAK5 expression on disease-specific survival (DSS) and relapse-free
interval (RFI). Survival curves were compared using the log-rank
test. Multivariable Cox's proportional hazards regression models
were used to assess the prognostic significance of PAK5 expression
and other clinicopathological factors. Statistical analysis was
performed using IBM SPSS software (version 22.0; IBM, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Immunohistochemical analysis of PAK5
expression
PAK5 expression was primarily observed in the
cytoplasm of tumor and non-tumor cells. Although expression was
detected in some nuclei of cancer cells, only cytoplasmic staining
was counted towards a positive frequency score. Cancer cells were
at least weakly stained in 257/279 (92%) tumor samples. PAK5
staining was rarely detected in normal epithelium (Fig. 1). Metaplastic intestinal epithelium
located close to cancer lesions was weakly, but never strongly
stained. Almost all fibroblasts, smooth muscle and muscularis
mucosae were uniformly stained. Of the 279 patients, 44 (15.8%)
exhibited high PAK5 expression. Complete absence of PAK5 staining
in cancer cells was observed in 13 slides of poorly differentiated
adenocarcinoma, 6 slides of signet-ring cell adenocarcinoma and 1
each for mucinous, papillary and tubular adenocarcinoma
samples.
Association between PAK5 expression
and clinicopathological variables
Correlations between the expression of PAK5 and
clinicopathological factors are illustrated in Table I. High expression of PAK5 was
significantly associated with the differentiated pathological type
(differentiated vs. undifferentiated; P<0.001), depth of tumor
invasion (T1 vs. T2-T4; P<0.001), lymph node metastasis (N0 vs.
N1-N3; P<0.001), presence of distant metastasis or recurrence
(present vs. absent; P=0.038), and advanced tumor stage (I vs.
II–IV; P=0.001). None of the 30 signet-ring cell adenocarcinoma
slides exhibited high PAK5 expression.
 | Table I.Correlations between PAK5 expression
and clinicopathological factors of patients with gastric
cancer. |
Table I.
Correlations between PAK5 expression
and clinicopathological factors of patients with gastric
cancer.
|
|
| PAK5 expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | Patients, n | Low (n=235) | High (n=44) | P-value |
|---|
| Age |
|
|
|
|
| ≥70
years | 110 | 92 | 18 | 0.826 |
| <70
years | 169 | 143 | 26 |
|
| Sex |
|
|
|
|
| Male | 213 | 174 | 39 | 0.037 |
|
Female | 66 | 61 |
5 |
|
| Location |
|
|
|
|
| L, M | 224 | 192 | 32 | 0.170 |
| U | 55 | 43 | 12 |
|
| Pathological
type |
|
|
|
|
|
Differentiated | 129 | 97 | 32 | <0.001 |
|
Undifferentiated | 150 | 138 | 12 |
|
| Depth of
invasion |
|
|
|
|
| T1 | 116 | 110 |
6 | <0.001 |
|
T2/T3/T4 | 163 | 125 | 38 |
|
| Lymph node
metastasis |
|
|
|
|
| N0 | 144 | 132 | 12 | <0.001 |
|
N1/N2/N3 | 135 | 103 | 32 |
|
| Distant metastasis or
recurrence |
|
|
|
|
|
Negative | 195 | 170 | 25 | 0.038 |
|
Positive | 84 | 65 | 19 |
|
| Stage |
|
|
|
|
| I | 133 | 122 | 11 | 0.001 |
|
II/III/IV | 146 | 113 | 33 |
|
Association between DSS and RFI
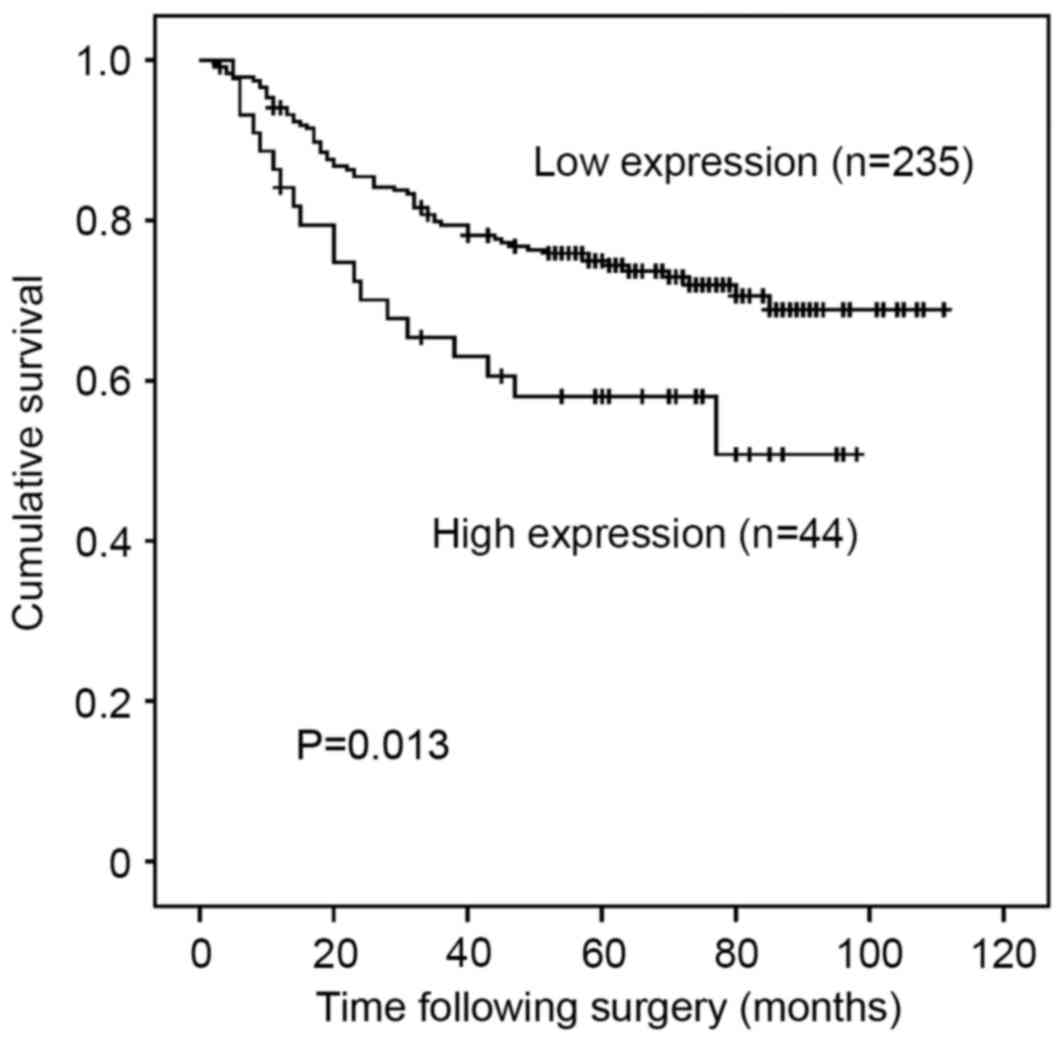
High expression of PAK5 was significantly associated
with poorer DSS (P=0.013; Fig. 2).
The 5-year DSS rate was 58.0% in patients with high expression of
PAK5 and 74.8% in those with low expression. Upper stomach lesion,
undifferentiated type of cancer, depth of invasion of tumor,
positive lymph node metastases, positive distant metastases,
advanced pathological stages and high expression of PAK5 were
significantly associated with poorer DSS following univariate
analysis (Table II). However, high
expression of PAK5 was not an independent prognostic factor [hazard
ratio (HR)=1.14; 95% confidence interval (CI), 0.65–2.00; P=0.659]
following multivariate Cox's proportional hazards regression
analysis adjusted for the following clinical prognostic factors:
Localization of tumor; histopathological type; depth of invasion;
lymph node metastases; and distant metastases (Table II).
 | Table II.Prognostic factors in univariate and
multivariate Cox's proportional hazard regression models for
DSS. |
Table II.
Prognostic factors in univariate and
multivariate Cox's proportional hazard regression models for
DSS.
|
| Univariate
(Log-rank) | Multivariate |
|---|
|
|
|
|
|---|
| Clinicopathological
factors | 5-year DSS (%) | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
| ≥70
years | 67 | 0.100 |
|
|
|
| <70
years | 75.5 |
|
|
|
|
| Sex |
|
|
|
|
|
|
Male | 75.6 | 0.878 |
|
|
|
|
Female | 67.1 |
|
|
|
|
| Location |
|
|
|
|
|
| L,
M | 76.5 | 0.020 |
1 |
| 0.029 |
| U | 58.1 |
| 1.74 | 1.06–2.85 |
|
| Pathological
type |
|
|
|
|
|
|
Differentiated | 81.6 | 0.003 |
1 |
|
0.447 |
|
Undifferentiated | 65 |
| 1.21 | 0.74–2.00 |
|
| Depth of
invasion |
|
|
|
|
|
| T1 | 97.3 | <0.001 |
1 |
| <0.001 |
|
T2/T3/T4 | 64.7 |
| 6.60 | 2.29–18.99 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
Negative (N0) | 94.2 | <0.001 |
1 |
| <0.001 |
|
Positive (N1/2/3) | 57.5 |
| 5.26 | 2.51–11.04 |
|
| Distant
metastasis |
|
|
|
|
|
|
Negative | 79.4 | <0.001 |
1 |
| <0.001 |
|
Positive |
0.0 |
| 5.79 | 3.42–9.81 |
|
| PAK5 |
|
|
|
|
|
|
Low | 74.8 | 0.014 |
1 |
|
0.659 |
|
High |
58 |
| 1.14 | 0.65–2.00 |
|
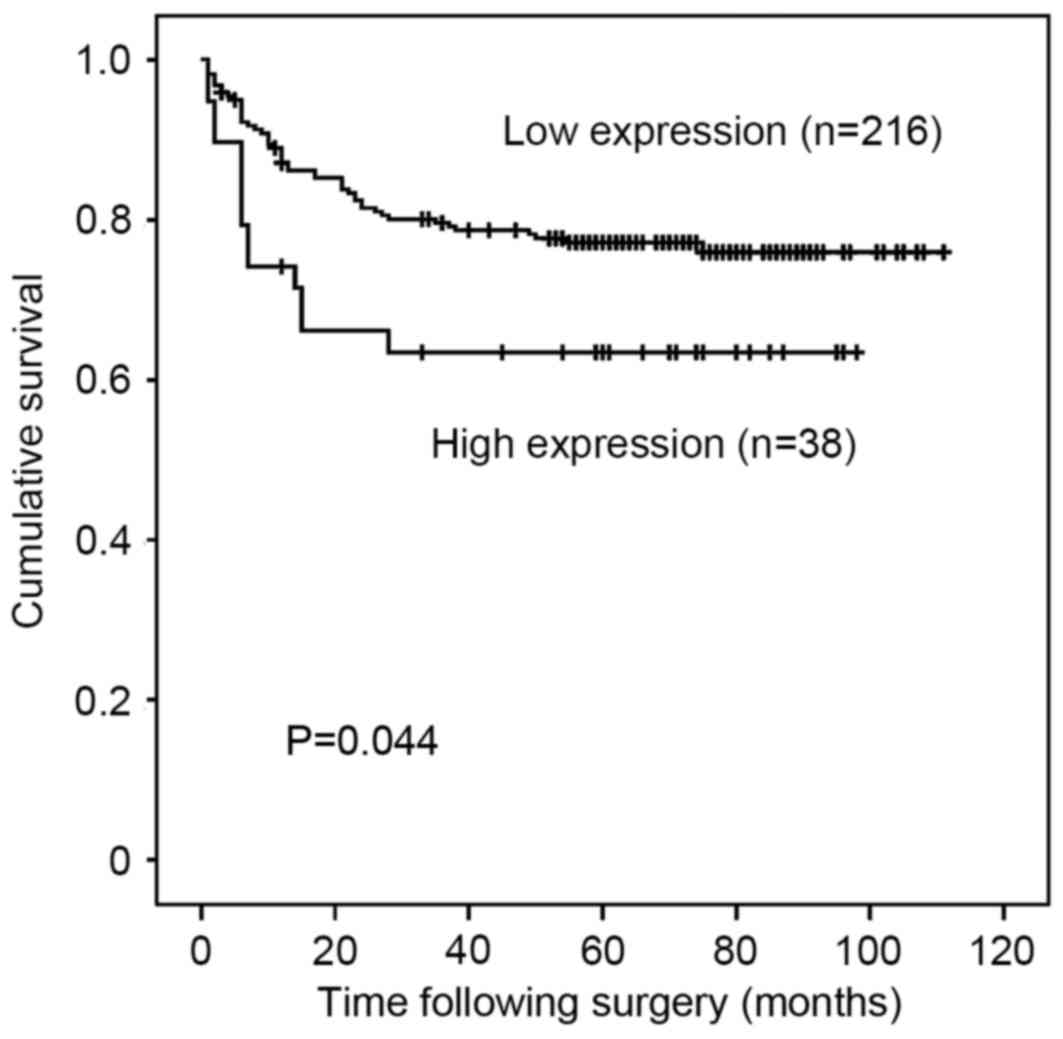
For patients with stage I to III disease (n=254),
high PAK5 expression was significantly associated with poorer RFI
on univariate analysis (P=0.044; Fig.
3). However, following multivariate analysis adjusted for
location of the tumor, histopathology, depth of invasion and lymph
node metastases, PAK5 expression was not an independent prognostic
factor for RFI (HR=0.96; 95% CI, 0.48–1.91; P=0.909) (Table III).
 | Table III.Prognostic factors in univariate and
multivariate Cox's proportional-hazards regression models for RFI
in stage I to III. |
Table III.
Prognostic factors in univariate and
multivariate Cox's proportional-hazards regression models for RFI
in stage I to III.
|
| Univariate
(Log-rank) | Multivariate |
|---|
|
|
|
|
|---|
| Clinicopathological
factor | 5-year RFI (%) | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
| ≥70
years | 76 | 0.833 |
|
|
|
| <70
years | 77.2 |
|
|
|
|
| Sex |
|
|
|
|
|
|
Male | 75 | 0.725 |
|
|
|
|
Female | 76.5 |
|
|
|
|
| Location |
|
|
|
|
|
| L,
M | 81.1 | 0.002 |
1 |
| 0.014 |
| U | 59.3 |
| 2.00 | 1.15–3.46 |
|
| Pathological
type |
|
|
|
|
|
|
Differentiated | 84.8 | 0.003 |
1 |
| 0.310 |
|
Undifferentiated | 69.4 |
| 1.38 | 0.74–2.55 |
|
| Depth of
invasion |
|
|
|
|
|
| T1 | 96.5 | <0.001 |
1 |
| 0.001 |
|
T2/T3/T4 | 61.7 |
| 6.30 | 2.20–18.06 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
Negative (N0) | 95.0 | <0.001 |
1 |
| <0.001 |
|
Positive (N1/2/3) | 54.6 |
| 6.92 | 3.02–15.84 |
|
| PAK5 |
|
|
|
|
|
|
Low | 79.3 | 0.044 |
1 |
| 0.909 |
|
High | 65.4 |
| 0.96 | 0.48–1.91 |
|
Discussion
The present study demonstrated that high expression
of PAK5 was significantly associated with depth of tumor invasion,
lymph node metastasis, distant metastasis and recurrence in
patients with gastric cancer. Furthermore, high expression of PAK5
was revealed to be significantly associated with poorer DSS and
RFI. However, high expression of PAK5 was not identified as an
independent prognostic factor following multivariate analysis. A
previous study that investigated the potential associations between
PAK5 and gastric cancer reported that none of the
clinicopathological parameters were associated to PAK5 expression
(13). This discrepancy may be
associated with the different scoring systems and methodologies
used to measure PAK5 status. In the current study, no PAK5 staining
was detected in normal epithelium, and metaplastic intestinal
epithelium located close to cancer lesions was weakly stained in
some cases, but never strongly stained. In this respect, the data
of the present study are similar to the findings of Gu et al
(13), who reported that the
immunohistochemical expression level of PAK5 was significantly
higher in cancer tissue compared with adjacent normal tissue
samples. Overall, these findings remain consistent with an
important role for PAK5 in gastric cancer progression.
PAK5 has been reported to be expressed predominantly
in the brain, and to promote neurite outgrowth by interacting with
GTPases, cell division control protein 42 homolog (Cdc42) and Rac,
in a signaling pathway that is antagonistic to Rho (9,17). In the
human brain, PAK5 contributes to microtubule stability by
inactivating serine/threonine-protein kinase MARK (MARK) through
binding to the catalytic domain, consequently preventing the
MARK-induced phosphorylation of Tau (6,18). The
sub-cellular localization of PAK5 appears to be associated with
some of its functions. The presence of the Cdc42/Rac interactive
binding domain within PAK5 prevents its accumulation in the
nucleus, and PAK5 binds to the Rho family small G proteins, RhoD
and RhoH, in addition to Cdc42 (19).
The interactions of PAK5 with Cdc42 and RhoD target it to different
compartments within the cell (19).
Endogenous PAK5 shuttles between mitochondria and the nucleus, and
induces resistance to apoptosis by phosphorylating Bcl2-associated
agonist of cell death in the mitochondria (20,21). The
role of PAK5 in the resistance to apoptosis may underlie our
results hypothesizing that PAK5 may be involved in cancer
metastasis.
As is the case with numerous cancers, heterogeneity
is prevalent in gastric cancer tissue. Despite this, certain
proteins that are heterogeneously expressed in gastric cancer
cells, including erb-b2 receptor tyrosine kinase 2 (HER2), are
targets of clinically effective anticancer drugs (22). Only 10–20% of all patients with
gastric cancer overexpress HER2 (23,24), and
HER2 expression differs significantly by histological subtype with
a high correlation between HER2 expression and intestinal
histologic type (23). In the present
study, similar to HER2, PAK5 expression differed significantly by
histological type, although some tumors contained differentiated
and undifferentiated cancer cells, consistent with a complex
underlying molecular pathogenesis that is at present poorly
understood. Although several effective and specific small molecule
pan-PAK inhibitors, or inhibitors of group I or II PAKs,
particularly PAK1 and PAK4, are in advanced stages of preclinical
testing (25), the development and
efficacy of PAK5-specific inhibitors has not yet been reported.
In conclusion, high expression of PAK5 was
significantly associated with differentiated pathological type,
depth of tumor invasion, lymph node metastasis, presence of distant
metastasis or recurrence, and therefore with advanced tumor stage,
and poorer DSS and RFI rates. Overall, the data of the current
study suggest that PAK5 is a potential drug target in gastric
cancer.
Acknowledgements
The authors would like to thank Dr Takumi Akashi,
pathologist at the Tokyo Medical and Dental University, for his
advice and great help in the interpretation of the
immunohistochemical data.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lordick F, Lorenzen S, Yamada Y and Ilson
D: Optimal chemotherapy for advanced gastric cancer: Is there a
global consensus? Gastric Cancer. 17:213–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim C, Mulder K and Spratlin J: How
prognostic and predictive biomarkers are transforming our
understanding and management of advanced gastric cancer.
Oncologist. 19:1046–1055. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar R, Gururaj AE and Barnes CJ:
p21-activated kinases in cancer. Nat Rev Cancer. 6:459–471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ye DZ and Field J: PAK signaling in
cancer. Cell Logist. 2:105–116. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wells CM and Jones GE: The emerging
importance of group II PAKs. Biochem J. 425:465–473. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang ZP, Jiang BG, Gu XF, Zhao B, Ge RL
and Zhang FB: P21-activated kinase 5 plays essential roles in the
proliferation and tumorigenicity of human hepatocellular carcinoma.
Acta Pharmacol Sin. 35:82–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao ZS and Manser E: PAK and other
Rho-associated kinases-effectors with surprisingly diverse
mechanisms of regulation. Biochem J. 386:201–214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pandey A, Dan I, Kristiansen TZ, Watanabe
NM, Voldby J, Kajikawa E, Khosravi-Far R, Blagoev B and Mann M:
Cloning and characterization of PAK5, a novel member of mammalian
p21-activated kinase-II subfamily that is predominantly expressed
in brain. Oncogene. 21:3939–3948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi K, Inokuchi M, Takagi Y, Otsuki
S, Fujimori Y, Sato Y, Yanaka Y, Higuchi K, Aburatani T, Tomii C,
et al: Prognostic significance of PAK4 expression in gastric
cancer. J Clin Pathol. 69:580–585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giroux V, Iovanna J and Dagorn JC: Probing
the human kinome for kinases involved in pancreatic cancer cell
survival and gemcitabine resistance. FASEB J. 20:1982–1991. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong W, An Z, Wang Y, Pan X, Fang W, Jiang
B and Zhang H: P21-activated kinase 5 is overexpressed during
colorectal cancer progression and regulates colorectal carcinoma
cell adhesion and migration. Int J Cancer. 125:548–555. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu J, Li K, Li M, Wu X, Zhang L, Ding Q,
Wu W, Yang J, Mu J, Wen H, et al: A role for p21-activated kinase 7
in the development of gastric cancer. FEBS J. 280:46–55. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu X, Wang C, Wang X, Ma G, Li Y, Cui L,
Chen Y, Zhao B and Li K: Efficient inhibition of human glioma
development by RNA interference-mediated silencing of PAK5. Int J
Biol Sci. 11:230–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma. (3rd English).
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
International Union Against Cancer (UICC),
. TNM classification of malignant tumors. Sobin LH, Gospodarowicz
MK and Wittekind C: 7th. Wiley-Blackwell; Oxford: 2009
|
|
17
|
Dan C, Nath N, Liberto M and Minden A:
PAK5, a new brain-specific kinase, promotes neurite outgrowth in
N1E-115 cells. Mol Cell Biol. 22:567–577. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Timm T, Matenia D, Li XY, Griesshaber B
and Mandelkow EM: Signaling from MARK to tau: Regulation,
cytoskeletal crosstalk, and pathological phosphorylation.
Neurodegener Dis. 3:207–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu X and Frost JA: Multiple Rho proteins
regulate the subcellular targeting of PAK5. Biochem Biophys Res
Commun. 351:328–335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cotteret S, Jaffer ZM, Beeser A and
Chernoff J: p21-activated kinase 5 (Pak5) localizes to mitochondria
and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol.
23:5526–5539. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cotteret S and Chernoff J:
Nucleocytoplasmic shuttling of Pak5 regulates its antiapoptotic
properties. Mol Cell Biol. 26:3215–3230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bang YJ, van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hofmann M, Stoss O, Shi D, Büttner R, van
de Vijver M, Kim W, Ochiai A, Rüschoff J and Henkel T: Assessment
of a HER2 scoring system for gastric cancer: Results from a
validation study. Histopathology. 52:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Radu M, Semenova G, Kosoff R and Chernoff
J: PAK signalling during the development and progression of cancer.
Nat Rev Cancer. 14:13–25. 2014. View
Article : Google Scholar : PubMed/NCBI
|