Introduction
Currently, the clinical management of metastatic
cancer of the bone or osteoporosis targets signaling pathways
associated with bone remodeling (1).
For example, bisphosphonate (BP) inhibits osteoclast function and
bone turnover to manage these diseases (1). Patients receiving BP treatment,
including zoledronate, can develop osteonecrosis of the jaw (ONJ),
specifically BP-related ONJ (BRONJ), although the relative
prevalence of this is low (1).
Denosumab, a human monoclonal antibody directed against the
receptor activator of nuclear factor-κβ ligand (RANKL), has been
approved as an antiresorptive agent (1). Denosumab inhibits the binding of RANKL
to RANK, thereby reducing osteoclast formation, function and
survival, which results in decreased bone resorption and increased
bone density (1). However, studies
have demonstrated that denosumab can induce ONJ, similarly to BP
(1,2).
There is currently insufficient evidence regarding the diagnosis
and treatment of denosumab-associated ONJ.
The present study reports a case of ONJ of the
maxilla in an 86-year-old male who had been treated with denosumab
to manage bone metastases associated with prostate cancer. To
elucidate the influence of denosumab on the development of ONJ, the
present study also reviewed the literature, including clinical
trials (3–10) and case reports (11–21), in
regards to the prevalence, clinical characteristics and management
of ONJ associated with denosumab.
Case report
An 86-year-old male was referred to the Department
of Dentistry and Oral Surgery at the University of Fukui Hospital
(Fukui, Japan) in December 2013 with bone exposure in the left
upper premolar region. The bone exposure had developed over a
period of 1 month. The patient had a prior history of endodontic
treatment of the left upper premolar teeth during 2008 (month
unknown). The patient was a non-smoker and did not drink alcohol.
In addition, the patient had no history of receiving radiation
therapy to the head and neck region, and his medical history
included tuberculosis, myocardial infarction, hypothyroidism and
prostate cancer. The patient's prostate cancer had been treated
with high-intensity focused ultrasound therapy in November 2002 and
a radical prostatectomy in January 2004. Pathological diagnosis of
the surgical specimen revealed moderately differentiated
adenocarcinoma of the prostate (pT3aN0M0, stage III), and hormone
therapy (combined androgen blockade therapy using an anti-androgen
drug and LH-RH agonist; doses unknown) was initiated in January
2004 (duration, 4 years). Due to increasing prostate-specific
antigen values, the patient was treated with chemotherapy in
February 2008 (including docetaxel 40 mg per patient triweekly and
tegafur/uracil 200 mg/day; duration, 4 years and 10 months);
skeletal metastases were identified in January 2009. The patient
had been treated once with zoledronate (4 mg per patient) in
January 2009, but it was discontinued due to hypocalcemia and renal
dysfunction. The patient underwent 6 subcutaneous injections of 120
mg denosumab between September 2012 and March 2013 and 1
subcutaneous injection of 120 mg denosumab in December 2013.
Physical examination of the patient revealed no
facial swelling or neck lymphadenopathy. No paresthesia of the left
infra-orbital region was evident. An intra-oral examination
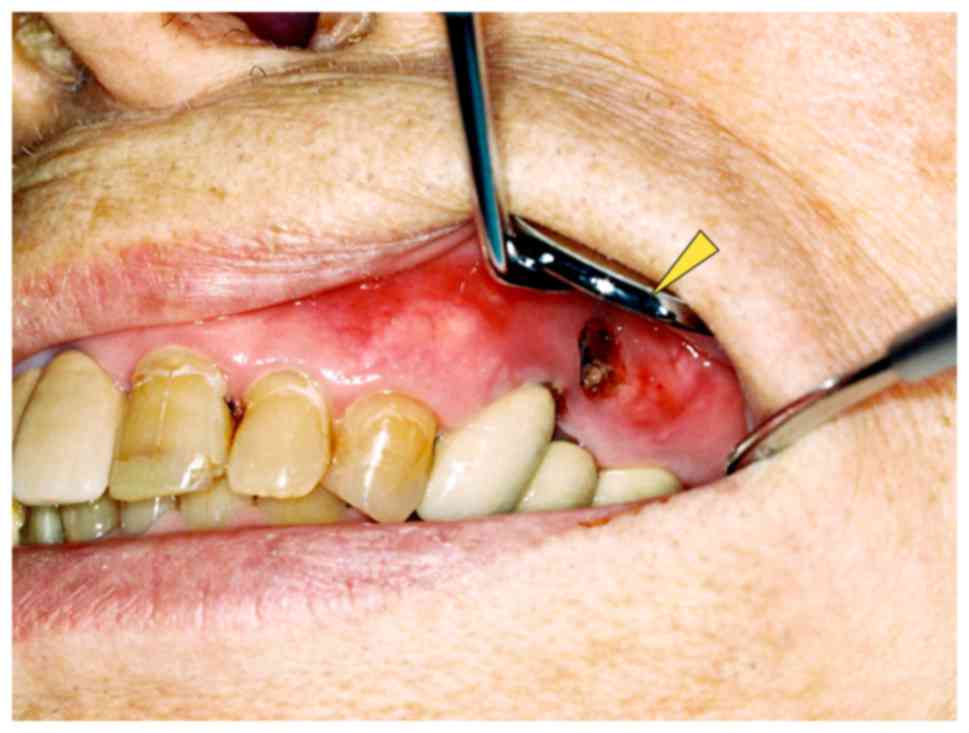
revealed a 5×10 mm dehiscence of the oral mucosa in the upper left
premolar region. Exposure of the underlying bone, redness and
slight swelling of the surrounding gingiva were also observed;
however, there was no obvious pus discharge (Fig. 1). The patient complained of tenderness
in the left infraorbital area. A panoramic radiograph revealed
radiolucencies with diffuse irregularity in the left maxilla and
around the apices of the left upper premolar teeth (Fig. 2A). An axial computed tomography image
(bone window setting) identified an area of low density in the left
posterior maxilla and the destruction of the buccal cortical bone,
indicating osteomyelitis (Fig. 2B). A
coronal image (bone window setting) demonstrated bone sequestrum in
the left maxilla, perforation in the inferior wall of the maxillary
sinus and sinusitis (Fig. 2C). Bone
scintigraphy revealed increased uptake of
99mTc-methylene diphosphonate (MDP) from the left
maxilla to part of the sinus and provided evidence of active bone
turnover (Fig. 2D). The uptake of
99mTc-MDP at the sites of the upper right humerus, left
scapula, sternum, chest and lumbar spine, ribs and pelvic bone
indicated multiple metastatic lesions of the bone (Fig. 2E). Over 8 weeks (from December 2013 to
January 2014), the patient presented no change in the area of the
exposed bone. The patient's history combined with the clinical and
radiographic findings was consistent with ONJ and the
administration of denosumab was discontinued in January 2014.
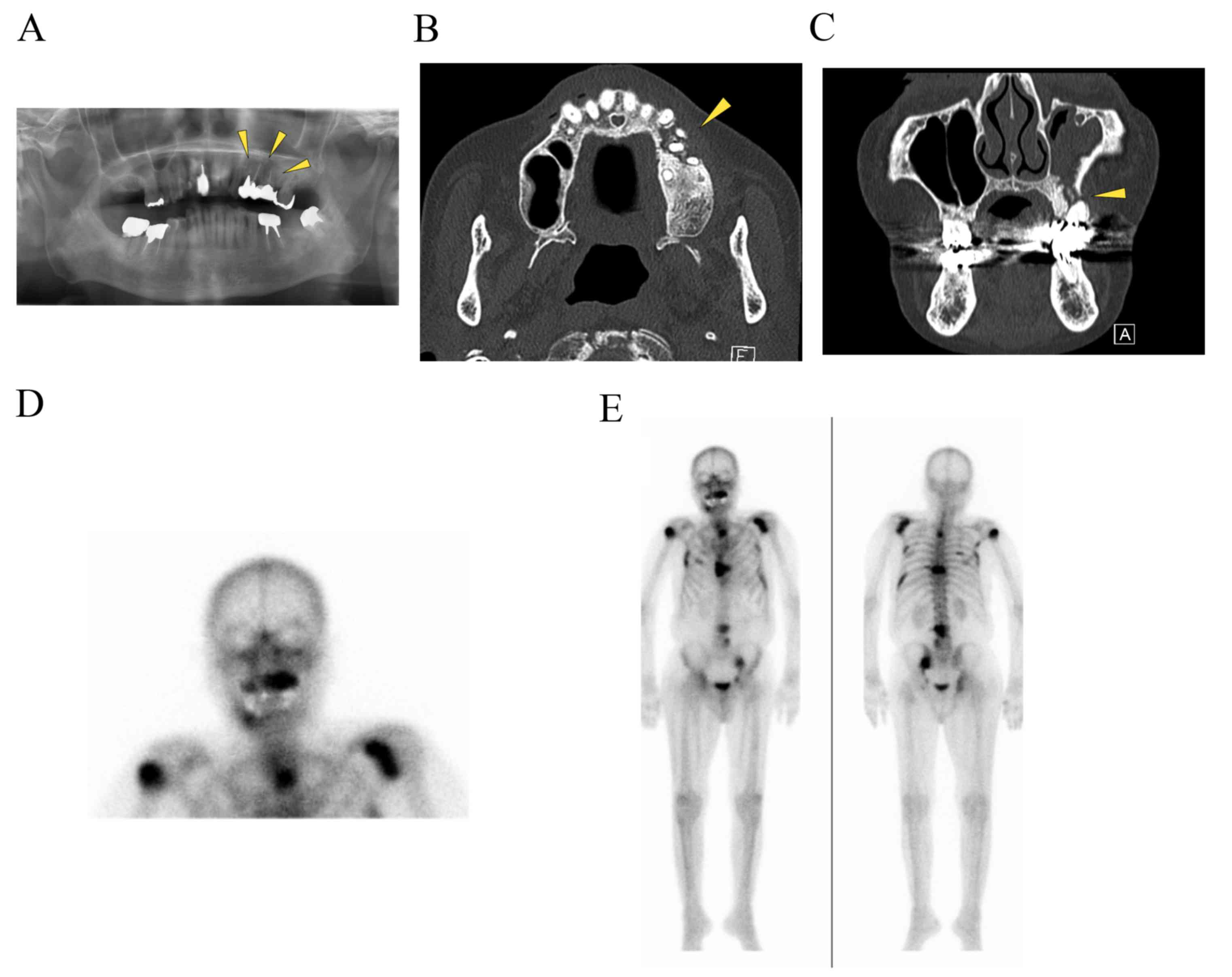 | Figure 2.Imaging findings. (A) A panoramic
radiograph revealed radiolucencies with diffuse irregularity in the
left maxilla and around the apices of the left upper premolar teeth
(indicated by the arrowheads). (B) An axial CT (bone window
setting) identified an area of low density in the left posterior
maxilla and the destruction of the buccal cortical bone (indicated
by the arrowhead). (C) A coronal CT (bone window setting)
demonstrated bone sequestrum in the left maxilla (indicated by the
arrowhead), a perforation in the inferior wall of the maxillary
sinus and sinusitis. Bone scintigraphy revealed (D) increased
uptake of 99mTc-MDP from the left maxilla to part of the
sinus, providing evidence of active bone turnover, and (E) uptake
of 99mTc-mMDP at the upper right humerus, left scapula,
sternum, chest and lumbar spine, ribs, pelvic bone, indicating
multiple metastatic lesions in the bone. CT, computed
tomography. |
The patient refused radical surgical treatment under
general anesthesia, so the exposed bone was partially removed under
local anesthesia. Histological examination of 5-µm sections of the
surgical sample, using light microscopy and hematoxylin and eosin
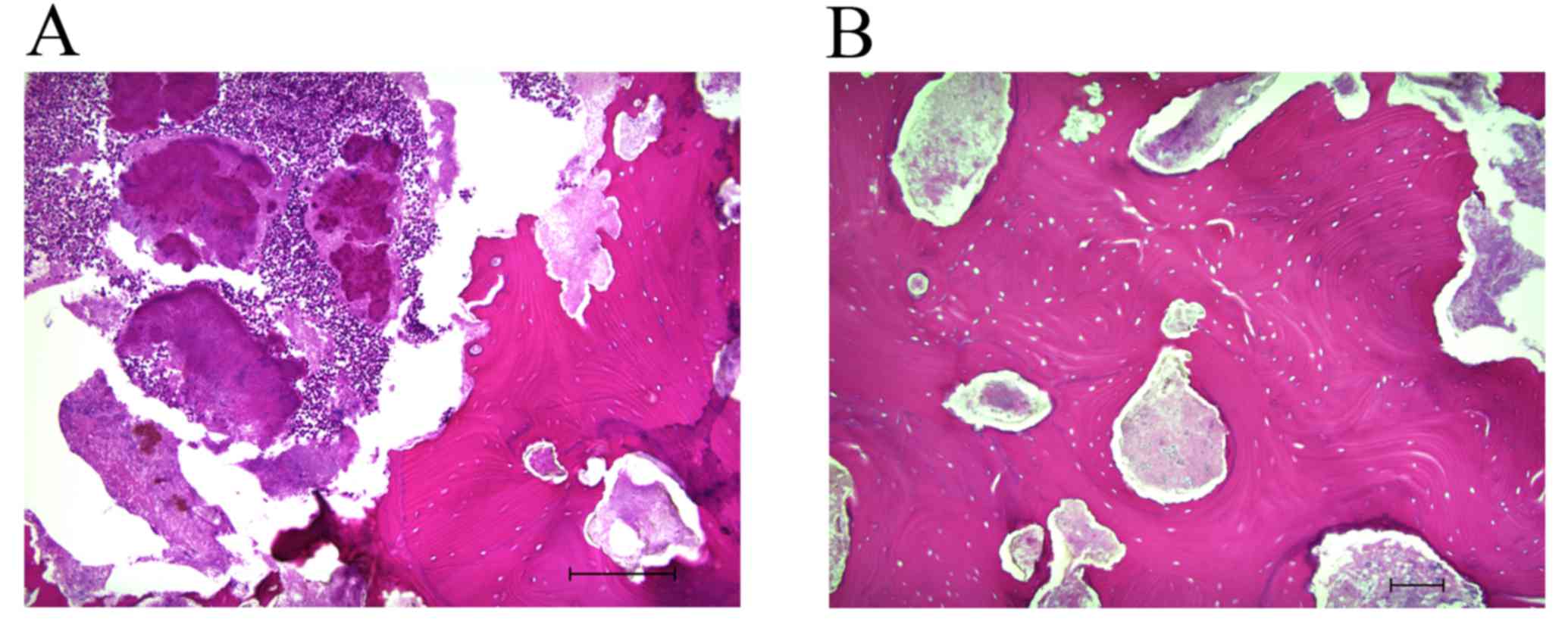
staining, revealed complete osteonecrosis with empty osteocytic
lacunae throughout the extent of the bone fragment (Fig. 3A and B). Actinomyces colonies
were observed, but no osteocytes, osteoblasts or osteoclasts were
present in the histologically examined sample; there was also no
sign of prostate cancer metastasis. Microbiological analysis
revealed a mixed normal oral flora, including Streptococcus,
Neisseria and Corynebacterium species, but
Actinomyces was not identified in the microbiologically
examined sample. A final diagnosis of ONJ of the maxilla was
confirmed. For >30 months (December 2013 to May 2016, the date
of writing), the patient was maintained on daily chlorhexidine
mouth rinses, and an antibiotic (amoxicillin 750 mg/day for 7 days)
was used during periods of exacerbation (increased pain, redness
and pus-containing discharge). The intraoral dehiscence remained,
but the patient did not complain of pain. The patient provided
written informed consent for the use of their data in the present
study.
Discussion
A relatively recent development for the treatment of
metastatic cancer of the bone or osteoporosis has been the
inhibition of RANKL, a member of the tumor necrosis factor
superfamily of ligands (1). Denosumab
is a human monoclonal antibody directed against RANKL, which
inhibits the binding of RANKL to RANK, thereby inhibiting
osteoclast differentiation and function (1).
ONJ is defined as exposed bone in the maxillofacial
region that does not heal for ≥8 weeks in patients with no history
of craniofacial radiation (2,22,23). ONJ
was first described in patients receiving BP therapy (24), and an increasing number of patients
with BRONJ have been reported. There has been a previous report
that the use of denosumab can also lead to ONJ (11). The American Association of Oral and
Maxillofacial Surgeons has proposed that ONJ caused by BP or
denosumab is called medication-related ONJ (MRONJ) (2). Although the etiology and pathophysiology
of MRONJ remain unclear, several potential mechanisms affecting the
risk of developing ONJ have been proposed. Hypotheses include the
inhibition of osteoclastic bone resorption and remodeling,
inflammation and infection, inhibition of angiogenesis, soft tissue
toxicity and immune dysfunction (2).
To elucidate the relationship between denosumab use
and the development of ONJ, the results of clinical trials
presenting denosumab-related ONJ were reviewed. Typically, the
patients with metastatic bone cancer received monthly 120 mg
denosumab injections (for 6–36 months) and ONJ was observed at a
rate of 0–5% (Table I) (3–10). By
contrast, the patients with osteoporosis typically received 60 mg
denosumab injections every 6 months (for 12–36 months) and ONJ was
observed at the rate of 0% (25–27). In a
previous report, the prevalence of BRONJ was ≤10% following
intravenous BP treatment for malignancy-associated bone disease and
<0.05% following oral BP treatment for osteoporosis (28). The greater prevalence of BRONJ in
patients with malignancy was thought to be associated with the
greater and more frequent dosing of BP (29). Thus, the high risk of ONJ in patients
with cancer receiving denosumab may also be attributable to the
dose and frequency of administration.
 | Table I.Clinical trials presenting
denosumab-related ONJ. |
Table I.
Clinical trials presenting
denosumab-related ONJ.
|
| Denosumab
regimen | Prior treatment
with BP and route of administration |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Study | Dose | Duration | IV | Oral | No. of
patients | No. of ONJ
cases | ONJ rate (%) | Oral adverse events
evaluationa |
|---|
| Breast cancer with
bone metastases (3) | 30, 120, or 180 mg
QM, 60 or 180 mg Q3M | 24 weeks | No | ND |
211 | 0 | 0.0 | ND |
| Prostate cancer,
breast cancer or other neoplasms with bone metastases (4) | 180 mg QM or
Q3M | 25 weeks | ND | ND | 73 | 0 | 0.0 | ND |
| Non-metastatic
prostate cancer (5) | 60 mg Q6M | 36 months | No pretreatment
within 5 years was acceptable | No pretreatment of
>3 years was acceptable |
731 | 0 | 0.0 | Yes |
| Bone metastases
with breast cancer (6) | 120 mg QM | 34 months | No | No | 1,020 | 20 | 2.0 | Yes |
| Bone metastases of
advanced cancer (excluding breast and prostate cancer) or multiple
myeloma (7) | 120 mg QM | 34 months | No | ND |
878 | 10 | 1.1 | Yes |
| Bone metastases in
males with castration-resistant prostate cancer (8) | 120 mg QM | ND | No | Previous oral BP
use for osteoporosis was allowed |
943 | 22 | 2.0 | Yes |
| Males with
non-metastatic castration-resistant prostate cancer (9) | 120 mg QM | 24 months | No | Less than 3 years
was acceptable |
720 | 33 | 5.0 | Yes |
| Lung cancer and
bone metastases (10) | 120 mg QM | ND | No | ND |
406 | 3 | 0.7 | ND |
The risk of ONJ following treatment with denosumab
or BP (including zoledronate, parmidronate or ibandronate) in
clinical trials was reviewed (Table
II) (3,4,6–8,10). In
patients with cancer, the prevalence of denosumab-related ONJ was
0–2% compared with 0–1.4% for BRONJ. These reports demonstrated
that the prevalence of ONJ in patients treated with denosumab
compared with BP is not statistically significant. Although
denosumab and BP have different mechanisms of action (30), the prevalence of ONJ was similar in
patients treated with either. Qi et al (31) reported the risk of denosumab-related
ONJ in patients with cancer in a meta-analysis of seven randomized
control trials. The overall prevalence of ONJ in patients with
cancer receiving denosumab was 1.7% (31). This analysis demonstrated that the use
of denosumab was associated with an increased risk of developing
ONJ when compared with BP treatment or a placebo; however, the
increased risk was not statistically significant between denosumab
and BP treatment (31).
 | Table II.Comparison of risk of developing ONJ
between patients treated with denosumab and BPs in clinical
trials. |
Table II.
Comparison of risk of developing ONJ
between patients treated with denosumab and BPs in clinical
trials.
| Study | Drug | No. of
patients | No. ONJ cases | ONJ rate (%) | Statistical
evaluation |
|---|
| Breast cancer with
bone metastasis (3) | Denosumab |
211 | 0 | 0.0 | ND |
|
| Zoledronate,
Parmidronate or Ibandronate | 43 | 0 | 0.0 |
|
| Prostate cancer,
breast cancer or other neoplasms with bone metastases (4) | Denosumab | 73 | 0 | 0.0 | ND |
|
| Zoledronate,
Parmidronate | 35 | 0 | 0.0 |
|
| Bone metastases
with breast cancer (6) | Denosumab, | 1,020 | 20 | 2.0 | Not significant
(P=0.39) |
|
| Zoledronate | 1,013 | 14 | 1.4 |
|
| Bone metastases of
advanced cancer (excluding breast and prostate cancer) or multiple
myeloma (7) | Denosumab, |
878 | 10 | 1.1 | Not significant
(P=1.00) |
|
| Zoledronate |
878 | 11 | 1.3 |
|
| Bone metastases in
males with castration-resistant prostate cancer (8) | Denosumab, |
943 | 22 | 2.0 | Not significant
(P=0.09) |
|
| Zoledronate |
945 | 12 | 1.0 |
|
| Lung cancer and
bone metastases (10) | Denosumab, |
406 | 3 | 0.7 | ND |
|
| Zoledronate |
395 | 3 | 0.8 |
|
In the review of the clinical trials conducted in
the present study, certain problems in the evaluation of the
prevalence of denosumab-related ONJ were concerning. Firstly,
several studies included patients that were currently receiving or
had previously received oral BP (5,8,9). BP is an etiopathological factor for
BRONJ and the effect of BP on denosumab-related ONJ was not
sufficiently discussed. Secondly, in the majority of the trials
reviewed, oral adverse events were adjudicated by an independent
blinded committee of dental experts, but it was unclear how the
oral evaluations were performed prior to and after denosumab
treatment (3,4,10).
Thirdly, it has been estimated that ~30% of patients with ONJ do
not demonstrate bone exposure (32)
and are defined as having stage 0 ONJ (2). These patients may be undiagnosed and not
included in the prevalence statistics. Therefore, the actual
prevalence of denosumab-related ONJ may be higher compared with
that reported in the clinical trials.
To gain a better understanding of the clinical
characteristics and management of denosumab-related ONJ, case
reports were reviewed (Table III)
(11–21). Since 2010, 26 well-documented cases of
denosumab-related ONJ have been reported in the English language
literature. The reported data includes characteristics of the
patients (age, gender, disease), denosumab regimen (dose,
administration times), risk factors (systemic factors, medications,
experience of BP treatment, local factors) (1,2,22,23,33,34),
clinical manifestations (site, symptoms, duration), treatments
(conservative and surgical treatment, cessation of denosumab) and
prognosis.
 | Table III.Reported cases of denosumab-related
ONJ in the English language literature (n=26). |
Table III.
Reported cases of denosumab-related
ONJ in the English language literature (n=26).
|
Characteristics | Value |
|---|
| Age, range (mean),
years | 49–86 (65) |
| Gender ratio,
M:F | 1.4:1 |
| Type of disease,
no. of patients |
|
|
Prostatic cancer | 15 |
| Breast
cancer | 9 |
|
Colorectal cancer | 1 |
| Lung
cancer | 1 |
| Denosumab
regimen |
|
| Dose,
mg, no. of patients (n=25a) |
|
|
120 | 23 |
| 60 | 2 |
|
Administration time, range
(mean) months (n=22a) | 2–36 (13) |
| Risk factors for
ONJ, no. of patients |
|
|
Systemic factors
(n=23a) |
|
|
Diabetes | 2 |
|
Obesity | 1 |
|
Smoking | 1 |
| Medications
(n=23a) |
|
|
Chemotherapy | 8 |
|
Corticosteroid therapy | 6 |
| Prior BP treatment
(n=9a) | 2 |
| Local factors
(n=25a) |
|
| Tooth
extraction | 14 |
| Apical
periodontitis | 2 |
| Clinical
manifestations, no. of patients (n=26a) |
|
|
Mandible | 17 |
|
Maxilla | 6 |
| Maxilla
and mandible | 3 |
| Symptom, no. of
patients (n=26a) |
|
| Bone
exposure | 25 |
|
Pain | 9 |
|
Redness | 5 |
| Pus
discharge | 4 |
|
Swelling | 4 |
|
Tenderness | 2 |
|
Fistula | 2 |
| Duration of
symptoms, range (mean) months (n=19a) | 0.25–12 (5) |
| Treatment, no. of
patients |
|
|
Conservative treatment
(n=23a) |
|
|
Antibiotics | 22 |
|
Mouth rinse | 21 |
| Surgical treatment
(n=16a) |
|
| Removal
of necrotic bone | 12 |
|
Debridement | 5 |
|
Incision and drainage | 1 |
| Tooth
extraction | 1 |
| Cessation of
denosumab (n=22a) |
|
|
Yes | 19 |
| No | 3 |
| Prognosis, no. of
patients (n=24a) |
|
Healed | 12 |
|
Unhealed | 12 |
In regards to the characteristics of the patients in
these case reports, the mean age of the patients with
denosumab-related ONJ was 65 years (range, 49–86 years). Among the
patients, 24 (92%) were aged >55 years and only 2 patients were
aged <55 years. Denosumab-related ONJ was observed with a
predilection for males (male: female ratio, 1.4:1.0).
Denosumab-related ONJ was reported in 15 patients (58%) with
prostatic cancer, 9 patients (34%) with breast cancer, 1 patient
with colorectal cancer (4%) and 1 patient with lung cancer (4%).
The regimen of denosumab treatment was reported in 25 of these
patients and was typically 120 mg monthly injections.
The risk factors for ONJ in these case reports were
examined in the present study, including systemic factors,
medications, prior BP treatment and local factors. In the systemic
factors, diabetes (n=2), obesity (n=1) and smoking (n=1) were
reported. In regards to medication, 8 patients were treated with
chemotherapy and 6 were treated with corticosteroid therapy. A
prior history of BP treatment was specified for 2 patients, and the
2 patients additionally had past zoledronate treatment. For local
factors, a history of dental procedures or conditions was reported
in 25 patients. Of these patients, ONJ developed following tooth
extraction in 14 patients (54%) and apical periodontitis in 2
patients (8%). ONJ most frequently occurred at the site of the
dental procedure, but 6 cases occurred spontaneously without a
dental procedure. In the review of clinical manifestations, the
mandible was the most commonly affected site (n=17), followed by
the maxilla (n=6), and 3 patients developed ONJ of the maxilla and
mandible. Symptoms of ONJ were reported in 26 patients, including
bone exposure (n=25), pain (n=9), redness (n=5), pus-containing
discharge (n=4), swelling (n=4), tenderness (n=2) and fistula
(n=2). The duration of symptoms was reported in 19 cases, with a
mean duration of 5 months (range, 0.25–12 months).
In regards to the treatments for ONJ that these
patients received, conservative treatments were reported in 23
patients [administration of antibiotics in 22 patients (96%) and a
mouth rinse in 21 patients (91%]. A total of 14 (54%) were also
treated surgically. The surgical treatments for ONJ included the
removal of necrotic bone (n=12), debridement (n=5), incision and
drainage (n=1) and tooth extraction (n=1). Descriptions about the
cessation of denosumab were found for 22 patients; 19 patients
(86%) had their denosumab administration discontinued and 3
patients (14%) preferred to continue denosumab therapy. The
prognosis was described in 24 patients; 12 patients healed, but the
other 12 patients did not.
In the present review of the reported cases,
denosumab-related ONJ had a similar clinical presentation to BRONJ
(22,23). There was a tendency for ONJ to develop
in the mandible in elderly patients. In addition, dental extraction
or inflammatory dental disease was an important initiating factor.
A complex medical history was also suspected to affect the
prevalence of ONJ.
Denosumab-related ONJ was primarily observed in
patients with prostate cancer. Qi et al (31) reported an analysis of ONJ based on
tumor type, demonstrating that the prevalence of denosumab-related
ONJ in patients with prostate cancer was higher compared with that
in patients with non-prostate cancers. In their analysis, the
median follow-up period for prostate cancer was longer compared
with that for non-prostate cancer (31). The authors suggested that the
variability in the prevalence of ONJ in the different cancer types
may be due to this variation, as it is probable that the prevalence
of ONJ would have increased with a longer follow-up period
(31). A similar phenomenon has been
observed in clinical trials, which demonstrated a trend toward
cumulative increases in ONJ over time in patients with cancer
(6,7).
In addition, the relatively long clinical course of prostate cancer
(35) may affect the prevalence of
denosumab-related ONJ. In the review conducted in the present
study, ONJ was also observed prevalently in males. This is likely
due to the fact that denosumab-related ONJ was most frequently
observed in patients with prostate cancer.
Denosumab has produced excellent clinical results
compared with BP for the treatment of patients with bone-associated
cancer and osteoporosis, causing great increases in bone mineral
density and the suppression of bone turnover markers (3,26), in
addition to efficacy even in patients who were previously resistant
to BP treatment (36). In the review
of case reports conducted in the present study, 2 patients
transitioned from BP and developed denosumab-related ONJ. Thus, the
effect of prior BP treatment should be considered as an
etiopathological factor for denosumab-related ONJ. In the case
reported in the present study, the patient received a single
zoledronate treatment followed by denosumab therapy. Although the
short duration of BP treatment in this patient was unlikely to have
contributed to the development of ONJ, the possibility that BP
treatment may synergistically enhance the denosumab-related
inhibition of osteoclastic activity and development of ONJ should
be considered.
In the literature review performed in the present
study, no clear relationship between the duration of denosumab
treatment and the development of denosumab-related ONJ was
observed. In one case, a patient developed ONJ after receiving two
60 mg doses of denosumab (14), while
in another, a patient developed ONJ after 36 monthly
administrations of 120 mg denosumab (21). Due to the limited number of patients
included in the present review, the accurate assessment of the
association between the dose/duration of denosumab treatment and
the onset of ONJ was difficult to determine. This association
should be examined in a larger patient cohort.
The concept of a ‘drug holiday’ in patients
receiving denosumab who require invasive dental treatments is an
ongoing area of controversy (2).
There are no previous studies to support or refute the strategy of
stopping denosumab therapy for the prevention or treatment of MRONJ
(2). In the review conducted in the
present study, 3 patients continued on denosumab and administration
was stopped in 19 patients following MRONJ. Otto et al
(37) reported 2 successful cases of
surgical treatment of denosumab-related ONJ. The authors
recommended that any surgical intervention should be withheld until
≥4 months after cessation of denosumab treatment (37). By contrast, Diz et al (12) reported a case of denosumab-related ONJ
due to tooth extraction 6 months after cessation of denosumab
therapy. Notably, Vyas et al (38) reported a case of denosumab-related ONJ
that healed within 1 month of cessation of denosumab. Thus, it is
difficult to determine the appropriate period of a drug holiday for
denosumab.
BP is incorporated into the mineral matrix of the
bone, but denosumab has no binding affinity for the bone matrix
(14). In terms of drug half-life,
denosumab has an advantage compared with BP, with a shorter
half-life of 25.4 days compared with 10–12 years, respectively
(39). Thus, ONJ may resolve more
rapidly after a drug holiday in patients taking denosumab compared
with patients taking BP. Based on the pharmacokinetics of
denosumab, it may be possible to place patients on an effective
drug holiday prior to surgical interventions to promote bone
healing. In the evaluation of bone metabolism, the measurement of
bone turnover markers aids in the assessment of the patient's
condition (40). Further studies
using bone turnover markers are required to verify the rate of bone
turnover after cessation of denosumab in patients who develop, or
are at significant risk of developing, denosumab-related ONJ.
Discontinuing bone antiresorptive agent therapy is an important
part of the management of bone conditions (41). The injudicious discontinuation of
denosumab therapy can lead to an increased risk of the progression
of bony lesions in patients with cancer. Thus, sufficient medical
consultation and discussion are appropriate prior to the cessation
of denosumab treatment.
In patients with cancer that develop ONJ, the
current recommendations are conservative treatment and to avoid
additional invasive surgeries (2). In
a previous report, conservative treatment (oral monitoring, oral
rinses, antibiotics) led to an overall ONJ resolution rate of 36%
(42). The resolution rate was
greater in the denosumab-treated group (40.4%) compared with the
zoledronate-treated group (29.7%) (42). This more rapid recovery is likely due
to the more rapidly reversible effects of denosumab on bone
turnover compared with BP (42).
Conservative therapy may not always lead to the
complete resolution of lesions, but it can provide long-term
symptomatic relief (43). In the case
reported in the present study, the patient refused radical surgical
treatment under general anesthesia and was treated with a minimal
debridement of the necrotic bone followed by chlorhexidine mouth
rinses and antibiotic therapy. Although the lesion did not heal,
the symptoms disappeared or were manageable. The patient was
satisfied with the results of the treatment, and their condition
was maintained throughout the long follow-up period.
Although treatment guidelines supports the
conservative management of ONJ (2,23), in the
review of the literature conducted in the present study, certain
lesions did not respond well to conservative treatment and were
thus managed surgically. Ristow et al (44) reported treatment outcomes for MRONJ,
with the success rates of conservative treatment regimens ranging
from between <20% and >50%, which is significantly lower
compared with the success rates of >85% reported for surgical
approaches (44). The authors
suggested that surgical interventions should be considered for all
stages of MRONJ (44). Although
surgery is an option for the resolution of non-responsive ONJ
lesions, certain cases that were reviewed in the present study did
not heal despite surgical treatments (14,15). These
results indicate that the appropriate treatment plan for ONJ must
be decided in accordance with the situation and condition of the
individual patient.
In the present case report, Actinomyces
colonies were observed in the histological examination, but not in
the microbiological analysis. Recent studies have revealed the
presence of Actinomyces colonies in biopsy material from
patients with MRONJ, suggesting that these bacteria contribute to
MRONJ-associated infections (2,45,46). Members of the Actinomyces genus
are filamentous, gram-positive and anaerobic bacteria that
typically colonize the mouth, colon and vagina. The most common
disease caused by these bacteria is cervicofacial actinomycosis
(47), which presents as a soft
tissue induration with abscesses and multiple fistula.
Histopathologically, Actinomyces is identified primarily by
its characteristic formation of sulfur granules (45). The diagnosis of actinomycosis is based
on microbiological and/or histological analysis (48,49). The
results of the two examinations often differ from one another
(49). The microbiological
identification of Actinomyces is difficult because the
anaerobic processing of the specimens is strict (48), requiring careful culture, preferably
when the patient has received no antibiotics for 7–10 days
(50). Furthermore, the growth rate
of Actinomyces bacteria is slow (48). Thus, false negative results are
frequently caused by the taking or transporting of specimens for
studies (49). By contrast, the
tissue samples for histological examination do not require any
special treatment and the diagnosis of actinomycosis relies only on
the detection of sulfur granules (48). Therefore, the final diagnosis has a
tendency to depend on histopathological confirmation (51). This explains the discrepancy in the
results of the case reported in the current study. It remains
unclear whether Actinomyces organisms trigger MRONJ or
whether MRONJ triggers secondary infection with these bacteria. The
role of these organisms in the development MRONJ requires further
investigation.
In conclusion, the prevalence of denosumab-related
ONJ is low, but remains potentially severe complication of
denosumab treatment. Thus, the management of denosumab-related ONJ
represents a challenge for clinicians. Since denosumab may offer
superior results compared with BP for the treatment of metastatic
cancer to the bone or osteoporosis, the use of denosumab is
expected to increase in the near future. Clinicians should also be
aware of the recognized risk factors for denosumab-related ONJ, in
order to aid in its diagnosis. The results of the current study
indicate that patients treated with denosumab should receive
prophylactic treatment to maintain their oral health prior to,
during and after denosumab treatment. Invasive dental procedures
should also be avoided during and after denosumab use. If a patient
develops denosumab-related ONJ, conservative therapy is the
mainstay; however, surgery is an option for non-responsive ONJ
lesions. The contribution of denosumab to the development of ONJ
remains unclear; further studies specifically designed to assess
the prevalence of ONJ in larger cohorts over long observation
periods are required. Further research on animal models is also
needed to elucidate the underlying mechanism of denosumab-related
ONJ. Analyses of the required length of drug holiday and the timing
of surgical intervention in denosumab-related ONJ by measuring the
levels of bone turnover markers are warranted. Further studies
remain necessary to establish guidelines for the prevention and
effective treatment of denosumab-related ONJ.
References
|
1
|
Yamashita J and McCauley LK:
Antiresorptives and osteonecrosis of the jaw. J Evid Based Dent
Pract. 12 3 Suppl:S233–S247. 2012. View Article : Google Scholar
|
|
2
|
Ruggiero SL, Dodson TB, Fantasia J,
Goodday R, Aghaloo T, Mehrotra B and O'Ryan F: American Association
of Oral and Maxillofacial Surgeons: American Association of Oral
and Maxillofacial Surgeons position paper on medication-related
osteonecrosis of the jaw-2014 update. J Oral Maxillofac Surg.
72:1938–1956. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lipton A, Steger GG, Figueroa J, Alvarado
C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin
KS, et al: Randomized active-controlled phase II study of denosumab
efficacy and safety in patients with breast cancer-related bone
metastases. J Clin Oncol. 25:4431–4437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fizazi K, Lipton A, Mariette X, Body JJ,
Rahim Y, Gralow JR, Gao G, Wu L, Sohn W and Jun S: Randomized phase
II trial of denosumab in patients with bone metastases from
prostate cancer, breast cancer, or other neoplasms after
intravenous bisphosphonates. J Clin Oncol. 27:1564–1571. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith MR, Egerdie B, Hernández Toriz N,
Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic
A, et al: Denosumab in men receiving androgen-deprivation therapy
for prostate cancer. N Engl J Med. 361:745–755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stopeck AT, Lipton A, Body JJ, Steger GG,
Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA,
Viniegra M, et al: Denosumab compared with zoledronic acid for the
treatment of bone metastases in patients with advanced breast
cancer: A randomized, double-blind study. J Clin Oncol.
28:5132–5139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Henry DH, Costa L, Goldwasser F, Hirsh V,
Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A,
Vadhan-Raj S, et al: Randomized, double-blind study of denosumab
versus zoledronic acid in the treatment of bone metastases in
patients with advanced cancer (excluding breast and prostate
cancer) or multiple myeloma. J Clin Oncol. 29:1125–1132. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fizazi K, Carducci M, Smith M, Damião R,
Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al:
Denosumab versus zoledronic acid for treatment of bone metastases
in men with castration-resistant prostate cancer: A randomised,
double-blind study. Lancet. 377:813–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith MR, Saad F, Coleman R, Shore N,
Fizazi K, Tombal B, Miller K, Sieber P, Karsh L, Damião R, et al:
Denosumab and bone-metastasis-free survival in men with
castration-resistant prostate cancer: Results of a phase 3,
randomised, placebo-controlled trial. Lancet. 379:39–46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scagliotti GV, Hirsh V, Siena S, Henry DH,
Woll PJ, Manegold C, Solal-Celigny P, Rodriguez G, Krzakowski M,
Mehta ND, et al: Overall survival improvement in patients with lung
cancer and bone metastases treated with denosumab versus zoledronic
acid: Subgroup analysis from a randomized phase 3 study. J Thorac
Oncol. 7:1823–1829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taylor KH, Middlefell LS and Mizen KD:
Osteonecrosis of the jaws induced by anti-RANK ligand therapy. Br J
Oral Maxillofac Surg. 48:221–223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diz P, López-Cedrún JL, Arenaz J and
Scully C: Denosumab-related osteonecrosis of the jaw. J Am Dent
Assoc. 143:981–984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Malan J, Ettinger K, Naumann E and Beirne
OR: The relationship of denosumab pharmacology and osteonecrosis of
the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol. 114:671–676.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pichardo SE, Kuypers SC and van Merkesteyn
JP: Denosumab osteonecrosis of the mandible: A new entity? A case
report. J Craniomaxillofac Surg. 41:e65–e69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olate S, Uribe F, Martinez F, Almeida A
and Unibazo A: Osteonecrosis of the jaw in patient with denosumab
therapy. Int J Clin Exp Med. 7:3707–3709. 2014.PubMed/NCBI
|
|
16
|
O'Halloran M, Boyd NM and Smith A:
Denosumab and osteonecrosis of the jaws-the pharmacology,
pathogenesis and a report of two cases. Aust Dent J. 59:516–519.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohga N, Yamazaki Y, Tsuboi K and Kitagawa
Y: Healing of osteonecrosis of the jaw (ONJ) after discontinuation
of denosumab in a patient with bone metastases of colorectal
cancer: A case report and hypothesis. Quintessence Int. 46:621–626.
2015.PubMed/NCBI
|
|
18
|
You Tm, Lee KH, Lee SH and Park W:
Denosumab-related osteonecrosis of the jaw: A case report and
management based on pharmacokinetics. Oral Surg Oral Med Oral
Pathol Oral Radiol. 120:548–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsushita Y, Hayashida S, Morishita K,
Sakamoto H, Naruse T, Sakamoto Y, Yamada SI, Yanamoto S, Fujita S,
Ikeda T and Umeda M: Denosumab-associated osteonecrosis of the jaw
affects osteoclast formation and differentiation: Pathological
features of two cases. Mol Clin Oncol. 4:191–194. 2016.PubMed/NCBI
|
|
20
|
Yamagata K, Nagai H, Baba O, Uchida F,
Kanno N, Hasegawa S, Yanagawa T and Bukawa H: A case of brain
abscess caused by medication-related osteonecrosis of the jaw. Case
Rep Dent. 2016:70386182016.PubMed/NCBI
|
|
21
|
Owosho AA, Blanchard A, Levi L, Kadempour
A, Rosenberg H, Yom SK, Farooki A, Fornier M, Huryn JM and Estilo
CL: Osteonecrosis of the jaw in patients treated with denosumab for
metastatic tumors to the bone: A series of thirteen patients. J
Craniomaxillofac Surg. 44:265–270. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Advisory Task Force on
Bisphosphonate-Related Ostenonecrosis of the Jaws, American
Association of Oral and Maxillofacial Surgeons: American
Association of Oral and Maxillofacial Surgeons position paper on
bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac
Surg. 65:369–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruggiero SL, Dodson TB, Assael LA,
Landesberg R, Marx RE and Mehrotra B: American Association of Oral
and Maxillofacial Surgeons: American Association of Oral and
Maxillofacial Surgeons position paper on bisphosphonate-related
osteonecrosis of the jaws-2009 update. J Oral Maxillofac Surg. 67 5
Suppl:S2–S12. 2009. View Article : Google Scholar
|
|
24
|
Marx RE: Pamidronate (Aredia) and
zoledronate (Zometa) induced avascular necrosis of the jaws: A
growing epidemic. J Oral Maxillofac Surg. 61:1115–1117. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cummings SR, San Martin J, McClung MR,
Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A,
et al: Denosumab for prevention of fractures in postmenopausal
women with osteoporosis. N Engl J Med. 361:756–765. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roux C, Hofbauer LC, Ho PR, Wark JD,
Zillikens MC, Fahrleitner-Pammer A, Hawkins F, Micaelo M, Minisola
S, Papaioannou N, et al: Denosumab compared with risedronate in
postmenopausal women suboptimally adherent to alendronate therapy:
Efficacy and safety results from a randomized open-label study.
Bone. 58:48–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura T, Matsumoto T, Sugimoto T, Hosoi
T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Sone T, et al:
Clinical trials express: Fracture risk reduction with denosumab in
Japanese postmenopausal women and men with osteoporosis: Denosumab
fracture intervention randomized placebo controlled trial (DIRECT).
J Clin Endocrinol Metab. 99:2599–2607. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dinca O, Bucur MB, Bodnar D, Vladan C and
Bucur A: Extensive osteonecrosis of the mandible after therapy with
denosumab following bisphosphoantes therapy. Acta Endo. 10:457–462.
2014. View Article : Google Scholar
|
|
29
|
Capozzi A, Lello S and Pontecorvi A: The
inhibition of RANK-ligand in the management of postmenopausal
osteoporosis and related fractures: The role of denosumab. Gynecol
Endocrinol. 30:403–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baron R, Ferrari S and Russell RG:
Denosumab and bisphosphonates: Different mechanisms of action and
effects. Bone. 48:677–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi WX, Tang LN, He AN, Yao Y and Shen Z:
Risk of osteonecrosis of the jaw in cancer patients receiving
denosumab: A meta-analysis of seven randomized controlled trials.
Int J Clin Oncol. 19:403–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fedele S, Porter SR, D'Aiuto F, Aljohani
S, Vescovi P, Manfredi M, Arduino PG, Broccoletti R, Musciotto A,
Di Fede O, et al: Nonexposed variant of bisphosphonate-associated
osteonecrosis of the jaw: A case series. Am J Med. 123:1060–1064.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Campisi G, Fedele S, Fusco V, Pizzo G, Di
Fede O and Bedogni A: Epidemiology, clinical manifestations, risk
reduction and treatment strategies of jaw osteonecrosis in cancer
patients exposed to antiresorptive agents. Future Oncol.
10:257–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Patel V, McLeod NM, Rogers SN and Brennan
PA: Bisphosphonate osteonecrosis of the jaw-a literature review of
UK policies versus international policies on bisphosphonates, risk
factors and prevention. Br J Oral Maxillofac Surg. 49:251–257.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Body JJ, Lipton A, Gralow J, Steger GG,
Gao G, Yeh H and Fizazi K: Effects of denosumab in patients with
bone metastases with and without previous bisphosphonate exposure.
J Bone Miner Res. 25:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Otto S, Baumann S, Ehrenfeld M and Pautke
C: Successful surgical management of osteonecrosis of the jaw due
to RANK-ligand inhibitor treatment using fluorescence guided bone
resection. J Craniomaxillofac Surg. 41:694–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vyas S, Hameed S and Murugaraj V:
Denosumab-associated osteonecrosis of the jaw-a case report. Dent
Update. 41:449–450. 2014.PubMed/NCBI
|
|
39
|
Uyanne J, Calhoun CC and Le AD:
Antiresorptive drug-related osteonecrosis of the jaw. Dent Clin
North Am. 58:369–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Scott LJ and Muir VJ: Denosumab: In the
prevention of skeletal-related events in patients with bone
metastases from solid tumours. Drugs. 71:1059–1069. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Epstein MS, Ephros HD and Epstein JB:
Review of current literature and implications of RANKL inhibitors
for oral health care providers. Oral Surg Oral Med Oral Pathol Oral
Radiol. 116:e437–e442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Saad F, Brown JE, van Poznak C, Ibrahim T,
Stemmer SM, Stopeck AT, Diel IJ, Takahashi S, Shore N, Henry DH, et
al: Incidence, risk factors, and outcomes of osteonecrosis of the
jaw: Integrated analysis from three blinded active-controlled phase
III trials in cancer patients with bone metastases. Ann Oncol.
23:1341–1347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Khan AA, Morrison A, Hanley DA, Felsenberg
D, McCauley LK, O'Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis
S, et al: Diagnosis and management of osteonecrosis of the jaw: A
systematic review and international consensus. J Bone Miner Res.
30:3–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ristow O, Otto S, Troeltzsch M,
Hohlweg-Majert B and Pautke C: Treatment perspectives for
medication-related osteonecrosis of the jaw (MRONJ). J
Craniomaxillofac Surg. 43:290–293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schipmann S, Metzler P, Rössle M, Zemann
W, von Jackowski J, Obwegeser JA, Grätz KW and Jacobsen C:
Osteopathology associated with bone resorption inhibitors-which
role does Actinomyces play? A presentation of 51 cases with
systematic review of the literature. J Oral Pathol Med. 42:587–593.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Katsarelis H, Shah NP, Dhariwal DK and
Pazianas M: Infection and medication-related osteonecrosis of the
jaw. J Dent Res. 94:534–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miller M and Haddad AJ: Cervicofacial
actinomycosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
85:496–508. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hansen T, Kunkel M, Kirkpatrick CJ and
Weber A: Actinomyces in infected osteoradionecrosis-underestimated?
Hum Pathol. 37:61–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bartkowski SB, Zapala J, Heczko P and
Szuta M: Actinomycotic osteomyelitis of the mandible: Review of 15
cases. J Craniomaxillofac Surg. 26:63–67. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Goldberg MH: Diagnosis and treatment of
cervicofacial actinomycosis. Oral Maxillofac Surg Clin North Am.
15:51–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kuyama K, Sun Y, Fukui K, Maruyama S,
Ochiai E, Fukumoto M, Ikeda N, Kondoh T, Iwadate K, Takagi R, et
al: Tumor mimicking actinomycosis of the upper lip: Report of two
cases. Oral Med Pathol. 15:95–99. 2011. View Article : Google Scholar
|