Introduction
Esophageal cancer typically occurs in the middle
thoracic of the esophagus and is one of the leading causes of
cancer-associated mortality in China (1). There are two distinct types of
esophageal cancer, esophageal squamous cell carcinoma (ESCC), which
may occur along the entire esophagus, and esophageal
adenocarcinoma, which may develop due to short-segment
gastro-esophageal reflux disease (2).
However, the mechanisms underlying the development of ESCC have
previously not been well demonstrated. The Chaoshan region has one
of the highest incidences of esophageal cancer in China, and the
age-standardized incidence rates in the Chaoshan area for ESCC were
74/100,000, which is ~10 times greater than the worldwide rate in
2014 (3–5). These findings indicate that there is a
unique environment and/or genetic factors involved in the
pathogenesis of ESCC.
Oxidative stress is an imbalance between the
production of reactive oxygen species (ROS) and antioxidant
defenses (6). The major mechanisms
underlying the oxidative stress-mediated promotion of cancer have
been previously demonstrated (7,8). A certain
mechanism involved oxidative stress-damaged DNA and led to cell
transformation, which promoted cancer development (8). Another mechanism was induced oxidative
stress, which activated numerous cell signaling pathways and may
promote carcinogenesis (8). However,
there are specific mechanisms that require further investigation.
Chronic inflammation is another important risk factor associated
with a number of human cancer subtypes, including colon
adenocarcinoma and pancreatic cancer (9,10). The
association between inflammation and cancer has been clearly
demonstrated in previous studies (6,9,10). However, further pathological evidence
is required in order to support the results of these studies.
The present study investigated the hypothesis that
oxidative stress is one of the driving forces of ESCC. The
expression of 4-hydroxynonenal (4-HNE) in a large cohort of ESCC
tissues obtained from patients living in Chaoshan was evaluated by
immunohistochemistry (IHC) in order to determine the clinical and
prognostic significance of 4-HNE in ESCC. Finally, the association
of 4-HNE expression level and severity of inflammation in ESCC was
determined.
Materials and methods
ESCC tumor samples
The present study obtained 57 consecutive ESCC
tumors from surgery, randomly selected from the First Affiliated
Hospital of Shantou University Medical College (Shantou, China)
from July 2000 to June 2012. All patients underwent potentially
curative surgery without preoperative chemotherapy or radiotherapy.
In this cohort, 46 patients were male and 11 were female; the age
range was 40–75 years, with a median of 58 years. Follow-up data
were available for all patients. The majority of patients (48,
84.2%) succumbed to the disease during the follow-up period
(median, 33.6 months). The present study was approved by the Ethics
Committee of the Medical College of Shantou University (Shantou,
China). Written informed consent was obtained from all patients
prior to enrollment in the present study.
IHC
IHC staining was performed using the EnVision
Labeled Peroxidase System (Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA), according to manufacturers protocol. Consecutive
sections of tissue (4-µm thick) were obtained from each sample and
deparaffinized in dimethyl benzene, rehydrated through a graded
ethanol series and incubated with fresh 3%
H2O2 for 10 min in order to inhibit
endogenous peroxidase activity. The tissues were subsequently
washed in PBS, and antigen retrieval was performed by microwave
heating at 100°C for 20 min. Following incubation with 10 mmol/l
citrate buffer (pH 6.0) for 20 min, primary antibodies against
4-HNE (#ab48506; dilution, 1:75; Abcam, Cambridge, UK) were
incubated at 4°C overnight. Subsequent to washing in PBS three
times, immunostaining was visualized with a labeled
streptavidin-biotin method using the Dako REAL™ EnVision™ Detection
system, Peroxidase/3,3′-diaminobenzidine+, Rabbit/Mouse (1:200;
catalog no. K5007; Dako; Agilent Technologies, Inc.) and included
20 min incubation with the biotinylated antibody and 20 min in
streptavidin horseradish peroxidase, followed by counterstaining
with 10% hematoxylin, all performed at room temperature. Images
were captured using an IM50 microscope (Leica Microsystems GmbH,
Wetzlar, Germany) at ×400 magnification. IHC staining was evaluated
by two pathologists who were blinded to the clinical outcome, and
concordance between the two pathologies was guaranteed.
For the analysis of 4-HNE immunostaining, the
intensity and percentage of immunostained cells was determined, and
scoring of 4-HNE immunostaining was performed. The percentage of
positive cells in each case was categorized as: 0 (<5% positive
cells), 1+ (6–25% positive cells), 2+ (26–50% positive cells), 3+
(51–75% positive cells) or 4+ (>75% positive cells). The
staining intensity was categorized as 0–3+ as follows: 3+, intense
positive stain; 2+, moderate positive stain; 1+, weak positive
stain; 0, negative stain. The final scores were based on the
multiplication of the percentage score and the intensity score,
which ranged from 0 to 12. Tumors were considered to have low 4-HNE
expression levels when they were assigned a score of 0–4, whereas
tumors were considered to have high 4-HNE expression levels if they
had a final score of ≥6.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The prognostic significance of the expression of various markers
was analyzed using the Kaplan-Meier test. The association between
4-HNE and clinical parameters was evaluated using the χ2
or Student's t-test. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of 4-HNE in ESCC
patient tissue samples
In order to determine the role of oxidative stress
in esophageal tissues, the present study investigated the status of
oxidative stress in esophageal epithelial tissues exhibiting
various degrees of chronic inflammation. For identifying oxidative
stress, 4-HNE is one of most bioactive and studied lipid
peroxidation biomarkers (11). For
the evaluation of the expression levels of 4-HNE in the cohort of
ESCC tissue samples, the present study performed IHC on
paraffin-embedded tissues. The immunoreactivity of 4-HNE was
determined based on the presence of cytoplasmic staining, and was
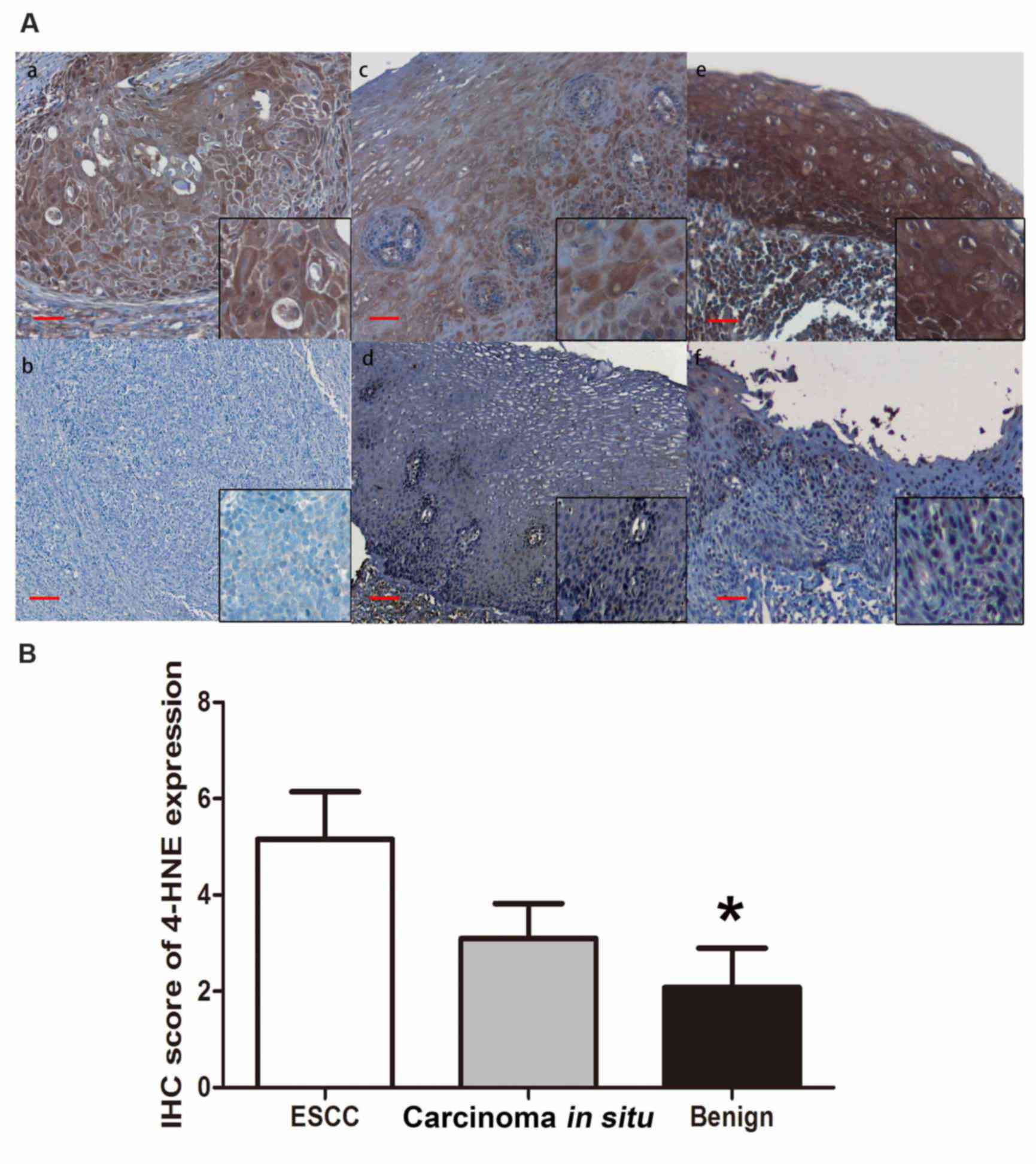
identified in the majority of ESCC tissue samples (40/57, 70.1%;
Fig. 1A, a and b). The staining was
categorized as negative (n=17, 29.8%) if the staining score was 0,
and positive if the staining score was >0 (n=40, 70.2%).
Furthermore, the expression level of 4-HNE was positive in 8/23
(34.8%) benign esophageal epithelial tissue samples. The remaining
15/23 (65.2%) tissue samples displayed no detectable cytoplasmic
expression level of 4-HNE (Fig. 1A, c and
d). The esophageal carcinoma in situ tissues revealed
relatively negative 4-HNE immunostaining in 6/11 cases (54.5%) and
positive staining in 5/11 cases (45.5%; Fig. 1A, e and f). The IHC score of 4-HNE is
presented in Fig. 1B. The final IHC
score of ESCC tissues is significantly higher compared with that of
benign esophageal epithelial tissues (P<0.05); however, the
score of 4-HNE in carcinoma in situ tissue samples is higher
compared with that in benign tissues, but no significant difference
was identified (P=0.067).
Clinical significance of 4-HNE
expression level in ESCC tissues
The present study investigated whether the
expression level of 4-HNE determined by IHC was associated with
various clinicopathological parameters, including gender, location
and size of the tumor, lymph node metastasis, histologic grade,
depth of tumor invasion and overall clinical stage. As summarized
in Table I, the results revealed that
the expression level of 4-HNE was associated with the clinical
stage (P=0.043). Tissue samples with positive 4-HNE expression
levels also exhibited an association with a late degree of clinical
stage. The expression level of 4-HNE revealed no significant
association with other clinicopathological parameters.
 | Table I.Association of 4-HNE expression level
with various clinicopathological parameters in ESCC. |
Table I.
Association of 4-HNE expression level
with various clinicopathological parameters in ESCC.
|
|
| 4-HNE expression
level determined by IHC |
|
|---|
|
|
|
|
|
|---|
| Parameter | No. of cases | Negative | Positive | P-value |
|---|
| Age (years) |
|
|
|
|
| ≤57 | 28 | 6 | 22 | 0.248 |
|
>58 | 29 | 11 | 18 |
|
| Gender |
|
|
|
|
| Male | 46 | 12 | 34 | 0.275 |
|
Female | 11 | 5 | 6 |
|
| Tumor site |
|
|
|
|
|
Upper | 6 | 4 | 2 | 0.770 |
|
Middle | 45 | 23 | 22 |
|
|
Lower | 6 | 3 | 3 |
|
| Differentiation |
|
|
|
|
| Poor | 4 | 3 | 1 | 0.555 |
|
Intermediate | 31 | 15 | 16 |
|
| Well | 22 | 10 | 12 |
| Tumor size (cm) |
|
|
|
|
| ≥5 | 38 | 13 | 25 | 0.370 |
|
<5 | 19 | 4 | 15 |
|
| Depth of
invasion |
|
|
|
|
|
T1-T2 | 48 | 13 | 35 | 0.428 |
|
T3-T4 | 9 | 4 | 5 |
|
| Lymph metastasis |
|
|
|
|
| Yes | 30 | 8 | 22 | 0.773 |
| No | 27 | 9 | 18 |
|
| Clinical stage |
|
|
|
|
| 1 | 2 | 1 | 1 | 0.043 |
| 2 | 26 | 18 | 8 |
|
| 3 | 27 | 8 | 19 |
|
| 4 | 2 | 1 | 1 |
|
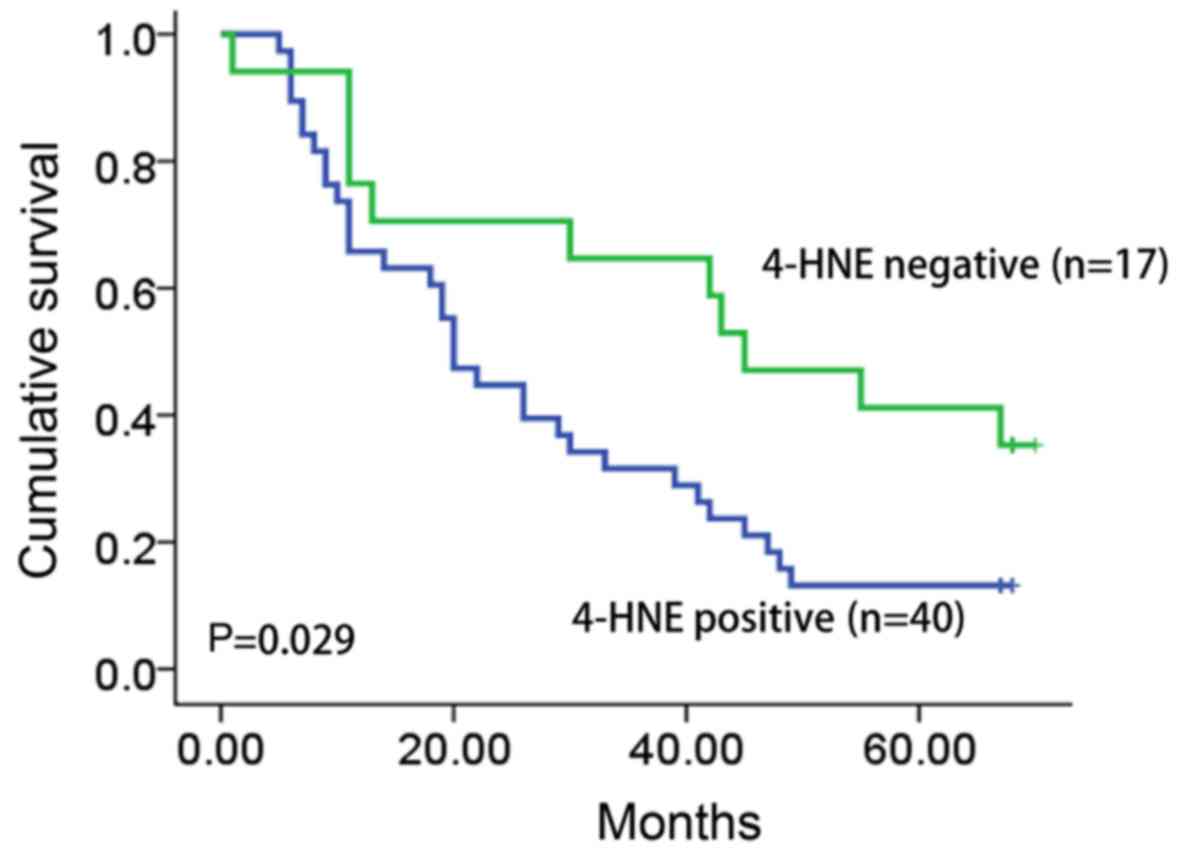
Clinical follow-up data were available for all
patients included in the present study (median follow-up time, 33.6
months; range, 1–68 months). The survival data was analyzed by the
Kaplan-Meier test. The overall survival time of patients with
4-HNE-positive tumors was significantly longer compared with that
of patients with 4-HNE-negative tumors (30.7 vs. 10.9 months;
P=0.029; Fig. 2).
Expression level of 4-HNE is
associated with the severity of chronic inflammation in ESCC
tissues
Oxidative stress has previously been reported to be
an important factor in the promotion of ESCC carcinogenesis
(12). Therefore, the present study
hypothesized that the expression level of 4-HNE was associated with
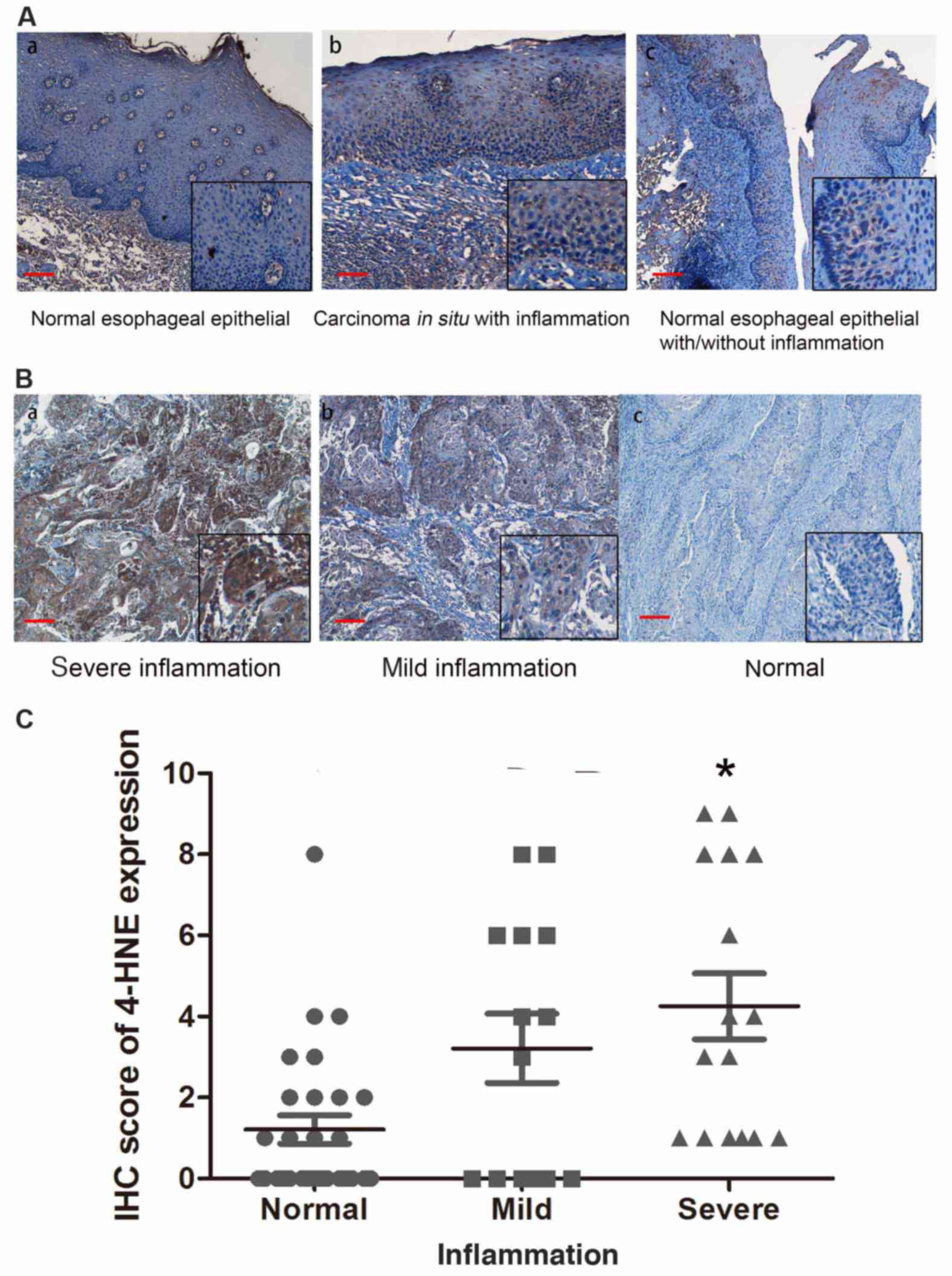
the stage of ESCC chronic inflammation of ESCC tissue samples. In
order to support this hypothesis, the present study evaluated the
status of chronic inflammation in esophageal tissues and revealed
that chronic inflammation was positively associated with the level
of dysplasia in esophageal epithelial tissues, indicating that
inflammation is a potential factor promoting esophageal
carcinogenesis (Fig. 3A).
The present study determined the association of
4-HNE with the inflammation level in ESCC tissues. Based on the
severity of chronic inflammation, 57 ESCC tissue samples were
divided into negative, mild and severe subgroups. An increased
level of staining of 4-HNE was identified in esophageal tissues
that had an increased level of chronic inflammation (Fig. 3B). The strongest immunostaining of
4-HNE was observed in severe dysplastic tissues accompanied with
severe chronic inflammation (Fig.
3C), indicating that oxidative stress is a potential factor
promoting ESCC inflammation.
Discussion
The present study evaluated the expression levels of
4-HNE in a cohort of ESCC patients and demonstrated that 4-HNE
expression is significantly higher in ESCC tissues, compared with
that in benign esophageal epithelial tissues. Furthermore, the
4-HNE expression level was significantly associated with the
poorest clinical stage of ESCC. Patients with positive staining of
4-HNE revealed poorer clinical outcomes compared with patients with
a negative 4-HNE expression.
ESCC is one of the most common cancer subtypes in
China, particularly in the Chaoshan region (13). The rate of incidence of ESCC in
Chaoshan is amongst the highest in China, indicating that there may
be certain genetic and/or environmental factors contributing to
ESCC carcinogenesis (1). A previous
study revealed that tobacco smoking, and consumption of hot drinks
and alcohol are established risk factors for ESCC (14). The potential mechanisms underlying
these risk factors may induce endogenous metabolic reactions in
cells and produce ROS, including superoxide anions
(O2−), hydroxyl radicals (OH•) and
organic peroxides (15). ROS serve
various roles in cellular processes, and low concentrations of ROS
have beneficial effect on cells including the promotion of wound
healing and tissue repair, in addition to pathogen destruction
(16).
However, the accumulation of ROS may induce
oxidative stress to damaged cells and contribute to
inflammation-induced tissue damage, carcinogenesis, diabetes,
cardiovascular disease, Alzheimer's disease, Parkinson's disease
and autoimmune diseases (17).
Furthermore, the association between oxidative stress and
inflammation has previously been demonstrated in numerous studies
(9,10). A previous study demonstrated that
oxidative stress induces a number of diseases, including chronic
inflammation and various cancer subtypes (9). Oxidative stress may induce DNA damage
and increase the mutation rate of cells, and promote cell
transformation (8). However,
oxidative stress may also activate certain cell signaling pathways
and contribute to cell survival, angiogenesis and metastasis
(9). Oxidative stress may be involved
in various phases of tumorigenesis, including cell survival,
proliferation, invasion and chemoresistance (9). 4-HNE is considered to be a product of
lipid peroxidation and an inducer of oxidative stress (12).
4-HNE is a major product of lipid peroxidation that
forms covalent adducts with nucleophilic functional groups in
macromolecules, including proteins, DNA and lipids (18). In previous studies, 4-HNE was
identified to be involved in a number of degenerative diseases,
including Alzheimer's disease, atherosclerosis and cancer (19–21). For
example, the expression level of 4-HNE was increased in the blood
and muscles of obese patients, compared with patients of a healthy
weight (22,23). However, the expression level of 4-HNE
has not been extensively investigated in cancer tissues. The
present study demonstrated that the expression level of 4-HNE is
higher in ESCC tissues compared with that in benign esophageal
epithelial and esophageal carcinoma in situ tissues,
indicating that oxidative stress may be a driving force of ESCC
carcinogenesis. In conclusion, the results of the present study
suggested that 4-HNE may be highly expressed in ESCC tissues and
that a high expression level of 4-HNE was associated with the
severity of inflammation in ESCC. The present study provided
evidence that oxidative stress may promote the tumorigenicity and
inflammation of ESCC tissues.
References
|
1
|
Su M, Liu M, Tian DP, Li XY, Zhang GH,
Yang HL, Fan X, Huang HH and Gao YX: Temporal trends of esophageal
cancer during 1995–2004 in Nanao Island, an extremely high-risk
area in China. Eur J Epidemiol. 22:43–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang WR, Chen ZJ, Lin K, Su M and Au WW:
Development of esophageal cancer in Chaoshan region, China:
Association with environmental, genetic and cultural factors. Int J
Hyg Environ Health. 218:12–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li K and Yu P: Food groups and risk of
esophageal cancer in Chaoshan region of China: A high-risk area of
esophageal cancer. Cancer Invest. 21:237–240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu S, Huang B, Huang H, Li X, Chen G,
Zhang G, Lin W, Guo D, Wang J, Yu Z, et al: Patrilineal background
of esophageal cancer and gastric cardia cancer patients in a
Chaoshan high-risk area in China. PLoS One. 8:e816702013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: How are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and LLeonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Georgakilas AG: Oxidative stress, DNA
damage and repair in carcinogenesis: Have we established a
connection? Cancer Lett. 327:3–4. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shacter E and Weitzman SA: Chronic
inflammation and cancer. Oncology (Williston Park). 16:217–226,
229; discussion 230–232. 2002.PubMed/NCBI
|
|
10
|
Rakoff-Nahoum S: Why cancer and
inflammation? Yale J Biol Med. 79:123–130. 2006.PubMed/NCBI
|
|
11
|
Chen ZH and Niki E: 4-hydroxynonenal
(4-HNE) has been widely accepted as an inducer of oxidative stress.
Is this the whole truth about it or can 4-HNE also exert protective
effects? IUBMB Life. 58:372–373. 2006.PubMed/NCBI
|
|
12
|
Sehitogullar A, Aslan M, Sayr F, Kahraman
A and Demir H: Serum paraoxonase-1 enzyme activities and oxidative
stress levels in patients with esophageal squamous cell carcinoma.
Redox Rep. 9:199–205. 2014. View Article : Google Scholar
|
|
13
|
Zhang Y, Zhang Y, Yun H, Lai R and Su M:
Correlation of STAT1 with apoptosis and cell-cycle markers in
esophageal squamous cell carcinoma. PLoS One. 9:e1139282014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Islami F, Cao Y, Kamangar F,
Nasrollahzadeh D, Marjani HA, Shakeri R, Fahimi S, Sotoudeh M,
Dawsey SM, Abnet CC, et al: Reproductive factors and risk of
esophageal squamous cell carcinoma in northern Iran: A case-control
study in a high-risk area and literature review. Eur J Cancer Prev.
22:461–466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hardbower DM, de Sablet T, Chaturvedi R
and Wilson KT: Chronic inflammation and oxidative stress: The
smoking gun for helicobacter pylori-induced gastric cancer? Gut
Microbes. 4:475–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ray PD, Huang BW and Tsuji Y: Reactive
oxygen species (ROS) homeostasis and redox regulation in cellular
signaling. Cell Signal. 24:981–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uttara B, Singh AV, Zamboni P and Mahajan
RT: Oxidative stress and neurodegenerative diseases: A review of
upstream and downstream antioxidanttherapeutic options. Curr
Neuropharmacol. 7:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dalleau S, Baradat M, Guéraud F and Huc L:
Cell death and diseases related to oxidative stress:
4-hydroxynonenal (HNE) in the balance. Cell Death Differ.
20:1615–1630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siegel SJ, Bieschke J, Powers ET and Kelly
JW: The oxidative stress metabolite 4-hydroxynonenal promotes
Alzheimer protofibril formation. Biochemistry. 46:1503–1510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leonarduzzi G, Chiarpotto E, Biasi F and
Poli G: 4-Hydroxynonenal and cholesterol oxidation products in
atherosclerosis. Mol Nutr Food Res. 49:1044–1049. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhong H and Yin H: Role of lipid
peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing
on mitochondria. Redox Biol. 4:193–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mattson MP: Roles of the lipid
peroxidation product 4-hydroxynonenal in obesity, the metabolic
syndrome, andassociated vascular and neurodegenerative disorders.
Exp Gerontol. 44:625–633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Samjoo IA, Safdar A, Hamadeh MJ, Raha S
and Tarnopolsky MA: The effect of endurance exercise on both
skeletal muscle and systemic oxidative stress in previously
sedentary obese men. Nutr Diabetes. 3:e882013. View Article : Google Scholar : PubMed/NCBI
|