Introduction
Bladder cancer is a common malignancy of the urinary
system. According to the American Cancer Society, bladder cancer
was the fourth most common cancer and the eighth leading cause of
cancer-associated mortality in men in 2015, and its incidence and
mortality accounted for 7 and 4% of female patients with tumors,
respectively (1). The morbidity and
mortality of bladder cancer is lower in women than men, and is
ranked greater than tenth, for mortality, of all types of female
cancer (2,3). In China, the incidence of bladder cancer
is slightly lower, and it ranks the eighth most common malignancy
in males (4). The majority of types
of bladder cancer are of epithelial origin, of which ~90% of cases
are bladder transitional cell carcinoma (BTCC) with a papillary
appearance (5).
The carcinogenesis of bladder cancer is very
complex, in which genetic mutations and epigenetic alterations
serve important roles (2,3,6,7). Furthermore, inflammation and oxidative
damage contribute to the occurrence and progression of bladder
cancer (6,8,9). Cellular
oxidative damage is primarily caused by reactive oxygen species
(ROS) (10), which have been
implicated in the pathogenesis of a number of diseases (11,12).
Oxidative stress (OxS) is caused by the excessive production of
oxidants (including ROS and free radicals) and/or a reduction of
antioxidants in the target cells and tissues (13). OxS may cause damage to proteins,
lipids and DNA, which is a critical pathophysiological event
implicated in a number of human pathologies, including cancer
(12,13). Increased OxS has been demonstrated in
patients with bladder cancer (14–18).
However, previous studies have primarily focused on a single or
several oxidants/antioxidants (9,19–22), whereas the overall OxS status has not
been widely studied. Thus, it is difficult to fully elucidate the
association between the pathogenesis of bladder cancer and overall
serum OxS (10–12).
The molecular and genetic changes that occur during
the pathogenesis of BTCC can be divided into three steps: The first
step is chromosomal alteration, which triggers the initial
carcinogenic event; the second is cancer cell proliferation due to
loss of cell-cycle regulation and dysregulation of normal apoptotic
turnover; the third is cancer metastasis, which involves cell
migration, angiogenesis and loss of cell adhesion (23). It has been confirmed that there are
associations between genetic mutations and BTCC, and some genes
associated with BTCC have been extensively investigated in previous
studies (24–27). There is evidence that the
cyclin-dependent kinase inhibitors p21 and p16 are associated with
the increased recurrence and progression of cancer (23). Additionally, the pathogenesis and/or
progression of BTCC have been demonstrated to be a consequence of
genetic instability, and chromosomes 3, 7, 9 and 17 are involved in
uroepithelial oncogenesis (28–30).
Fluorescence in situ hybridization (FISH) is a sensitive
method that can be used to detect chromosomal abnormalities in
exfoliated bladder cells and to evaluate of BTCC malignancy.
In the present study, FISH was performed to detect
levels of CSP3, CSP7, CSP17 and GLPp16 of exfoliated bladder cells
in the urine. In addition, serum OxS was determined in patients
with BTCC, with the aim of evaluating the association between
genetic changes and OxS in these patients.
Materials and methods
Subjects
A total of 246 patients were recruited from the
Department of Urology of Mianyang Central Hospital (Mianyang,
China) between June 2008 and May 2014. All patients were initially
diagnosed with BTCC or had recurrent BTCC and their diagnoses were
based on cystoscopy with urinary cytology. There were 192 males and
54 females with a mean age of 61.3±11.7 years (range, 35–87 years).
According to the International Union Against Cancer TNM
classification system (31) and World
Health Organization criteria (32),
patients were classified as stage Ta (24 cases), T1 (75 cases), T2
(74 cases), T3 (48 cases) and T4 (25 cases), and as G0 (35 cases),
G1 (57 cases), G2A (72 cases), G2B (52 cases) and G3 (30 cases). In
addition, 40 healthy volunteers (33 males and 7 females; mean age
58.8±15.4 years; age range, 31–73 years) were also recruited as
controls. These volunteers from the same region were confirmed to
be healthy and without history of cardiovascular, kidney, hepatic,
pulmonary, hematological, gastrointestinal, metabolic, endocrine,
immunological, neurological and/or psychiatric diseases. Healthy
subjects had no history of drug and food hypersensitivity and they
had not undergone any drug treatment. Furthermore, the pregnancy
and lactation individuals also were excluded. No significant
differences were identified in the age (t=−1.195, P=0.233) and
gender (χ2=0.406, P=0.524) between patients with BTCC
and controls. The present study was approved by the Medical Ethics
Committee of Mianyang Central Hospital (Mianyang, China) and
written informed consent was obtained from all subjects.
Sample collection
Urine collection
The urine from the first urination of healthy
control subjects was collected in the morning for three consecutive
days (1,500 ml daily). The first washing buffer (200–400 ml) was
collected from patients with BTCC at cystoscopy. Both urine and
washing buffer were used as urine samples for FISH.
Blood collection
Following cystoscopy in patients with BTCC and the
last urination in healthy controls, venous blood (~5 ml) was
collected into BD Vacutainer® Serum Tubes (BD
Biosciences, Franklin Lakes, NJ, USA). Serum was separated by
centrifugation at 1,600 × g at room temperature for 15 min within 2
h after sample collection and then stored at −30°C; experiments
were carried out within 48 h. Serum was used for measuring total
oxidant status (TOS) and total antioxidant status (TAS).
Measurement of OxS parameters
TAS
TAS was determined colorimetrically using the Total
Antioxidant Status® kit (Randox Laboratories, Ltd.,
Crumlin, UK). In this assay, 2,2′-azino-di-3-ethylbenz-thiazoline
sulfonate was incubated with a peroxidase (metmyoglobin) and
hydrogen peroxide to produce the radical cation
2,2′-azino-di-3-ethylbenz-thiazoline sulfonate+. This
forms a relatively stable blue-green color solution, which was
measured using a 7600-020 automatic biochemical analyzer (Hitachi,
Ltd., Tokyo, Japan) at 600 nm. The assay was calibrated with a 1.65
mmol/l Trolox standard. TAS results were expressed as mmol Trolox
equivalent/l (mmol Trolox Eq./l).
TOS
The TOS level in serum was measured using a
modification of automated colorimetric method with the 7600-020
automatic biochemical analyzer (33).
In this method, the ferrous ions were oxidized to ferric ions in
the presence of various oxidants in an acidic medium. Ferric ion
concentrations were determined using xylenol orange. TOS
measurements were performed using the following instrument
settings: Method, end-point measurement; serum, 10 µl; reagent 1,
200 µl; reagent 2, 50 µl; reaction time, 10 min; temperature, 37°C;
primary wavelength, 560 nm; secondary wavelength, 800 nm; and
reading point, 34. A known concentration hydrogen peroxide of 39.16
µmol/l was used as the standard to calculate oxidant levels in the
samples. TOS values were expressed in µmol
H2O2 equivalent/l (µmol
H2O2Eq./l).
Oxidative stress index (OSI)
The ratio percentage of TOS-to-TAS potential gave
OSI (34,35), which was calculated as follows: OSI
(arbitrary unit, AU) = [(TOS, µmol
H2O2Eq./l)/(TAS, µmol TroloxEq./l)]x100
(36).
Interphase FISH analysis
Slide preparation
A total of ~40 ml freshly voided urine (the first
urination of the day) was added to 50 ml Falcon centrifuge tubes.
Cells of voided urine were separated by centrifugation at 1,500 × g
for 10 min at room temperature. A fraction of supernatant was
removed and ~1 ml of supernatant and cell sediment were left in the
tube. Glacial acetic acid (2–3 ml) was added, and the mixture was
subjected to constant agitation for ~10 sec at room temperature and
then diluted with normal saline twice. The diluted mixture was
centrifuged at 1,500 × g for 5 min at room temperature, the
supernatant was removed, and the cells were re-suspended and added
onto a clean slide at 3 locations. To ensure appropriate cell
density, 3, 10, and 30 µl of cell suspension were added to the
slide. Fresh Carnoy's fixative (methanol-to-glacial acetic acid
ratio, 3:1; v/v) was added to cell sediment, which was mixed and
resuspended. Fresh fixative was added again and the cells were
mixed and stored at −20°C for 30 min. The slides were air-dried at
room temperature.
The slides used for FISH analysis were treated with
2x saline sodium citrate (SSC) buffer for 2 min at 73°C and then
with 0.075 mol/l protease for 10 min at 37°C, washed in PBS for 5
min at room temperature, fixed in 1% formaldehyde for 5 min at room
temperature, and washed again in PBS for 5 min at room temperature.
The slides were dehydrated in a series of ethanol solutions (70,
85, and 100%; 1 min for each) at room temperature and then
air-dried completely.
Denaturation and hybridization
A specific probe kit (F01008-00; Beijing GP Medical
Technologies, Ltd., Beijing, China) was used in the study and
included CEP3, CEP7 (both labeled with rhodamine), CEP17 and GLPp16
(both labeled with fluorescein isothiocyanate) probes. The probe
mixtures were composed of 7.0 µl hybrid buffer, 1.0 µl deionized
water and 2.0 µl of probe; thus, each antibody probe was diluted
5X. In brief, 10 µl of probe mixture was added to each cell
sediment on the slide, followed by mounting with a small glass
coverslip and sealing with rubber cement. The target DNA and probe
were placed in the StatSpin® ThermoBrite Slide
Denaturation and Hybridization System (Iris Sample Processing,
Inc., Westwood, MA, USA) for denaturation at 73°C for 5 min and
then hybridization at 37°C for 16 h.
The rubber cement and coverslip were removed
following hybridization. The slides were washed with 50% formamide,
2X SSC and 0.1% NP-40 in 2X SSC for 2 min at 46°C to remove unbound
probes. The slides were transferred to room temperature and washed
in 70% ethyl alcohol for 1 min to remove NP-40 and subsequently
air-dried. After 1 min, 10 µl of DAPI was added to the target area,
and coverslips were mounted. The slides were stored in the dark at
−20°C until signal quantification.
Analysis of signals
Interphase nuclei were analyzed with a fluorescence
microscopy imaging system (Imstar S.A., Paris, France) to determine
the numbers of each chromosome. The following criteria were used to
select 100 nuclei for each probe: Cells with large nuclei; nuclear
shape irregularity, patchy DAPI staining, and clustering. Cell
nuclei were identified using the DAPI filter. Squamous cells,
neutrophils, umbrella cells and inflammatory cells were not
counted. Only non-overlapping cells with distinct signals were
scored. The number of signals was determined and recorded for all 4
probes. If chromosomes 3, 7, or 17 exhibited the loss of the two
signals (red and green), the cell was considered to be
un-interpretable owing to hybridization failure. If the cell
exhibited abnormal signals in ≥2 chromosomes, the cell was
considered abnormal.
A total of 40 voided urine samples from healthy
subjects were used to establish the cut-off values that were
defined as mean and 3 standard deviations (mean + 3SD) of the
percentage of nuclei with abnormal signals (Table I). Specimens were considered FISH
positive if they had abnormalities that include daneusomy of
locus-specific probes, chromosome monosomy and polysomy, and only
when the percentage of cells with 1 or ≥3 FISH signals for each
chromosome were higher than the cut-off values.
 | Table I.Optimal cut-off values for
fluorescence in situ hybridization-positive voided urine
specimens (n=30). |
Table I.
Optimal cut-off values for
fluorescence in situ hybridization-positive voided urine
specimens (n=30).
| Probe | 0 signal (%) | 1 signal (%) | ≥3 signals (%) |
|---|
| CSP3 | – | 0.37±0.72,
2.53 | 1.07±1.82,
6.53 |
| CSP7 | – | 0.40±0.81,
2.83 | 1.10±1.39,
5.27 |
| GLPp16 | 1.27±1.11,
4.60 | 1.03±1.61,
5.86 | 0.73±0.83,
3.22 |
| CSP17 | – | 0.67±0.92,
3.43 | 1.60±1.52,
6.16 |
Statistical analysis
Count data are expressed as percentage, and rates
were compared using a χ2 test. Quantitative data are
expressed as the mean ± SD. A Spearman's rank correlation analysis
was performed to evaluate the association between FISH data and
OxS, and Gamma rank correlation analysis (for count data) or
Spearman's rank correlation analysis (for quantitative data) was
performed to assess the association of FISH data and OxS with
clinical stage and grade. Statistical analyses were performed with
PASW Statistics software (version 18.0; IBM SPSS, Armonk, NY, USA)
and MedCalc statistical software (version 11.5; MedCalc Software,
Mariakerke, Belgium). P<0.05 was considered to indicate a
statistically significant difference.
Results
Chromosomal aberrations and OxS
The chromosomal aberrations of exfoliated bladder
cells and blood OxS are illustrated in Table II. The proportions of abnormal CSP3,
CSP7, CSP17 and GLPp16, and FISH positive rates were 50.4, 53.3,
44.7, 60.2 and 54.5%, respectively, in patients with BTCC, which
were significantly higher compared with healthy controls
(χ2 test, P<0.01). The serum TOS and OSI were
18.56±3.72 and 1.35±0.43 µmol H2O2Eq./l,
respectively, in patients with BTCC, which were significantly
higher compared with those of healthy controls (P<0.001).
However, the serum TAS in patients with BTCC (1.44±0.23
mmolTroloxEq./l) was significantly lower compared with that of
healthy controls (P<0.001).
 | Table II.FISH of exfoliated bladder cells, and
oxidative stress of patients with BTCC and healthy controls. |
Table II.
FISH of exfoliated bladder cells, and
oxidative stress of patients with BTCC and healthy controls.
| Parameter | BTCC group | Control group | χ2 or
t-test value | P-value |
|---|
| Total, n | 246 | 40 |
|
|
| CSP3, n (%) | 124 (50.4) | 4 (10.0) | 21.115a | <0.001 |
| CSP7, n (%) | 131 (53.3) | 5 (12.5) | 21.305a | <0.001 |
| CSP17, n (%) | 110 (44.7) | 3 (7.5) | 18.411a | <0.001 |
| GLPp16, n (%) | 148 (60.2) | 7 (17.5) | 23.537a | <0.001 |
| FISH, n (%) | 134 (54.5) | 0 (0.0) | 38.839a | <0.001 |
| Total oxidant
status, µmol H2O2 Eq./l | 18.56±3.72 | 14.64±1.29 | 6.604b | <0.001 |
| Total antioxidant
status, mmol Trolox Eq./l | 1.44±0.23 | 1.65±0.17 | −5.623b | <0.001 |
| Oxidative stress
index | 1.35±0.43 | 0.89±0.11 | 6.669b | <0.001 |
Hybridization signals of exfoliated
bladder cells of BTCC patients
The typical hybridization signals of CSP3, CSP7,
CSP17 and GLPp16 in exfoliated bladder cells of patients with BTCC
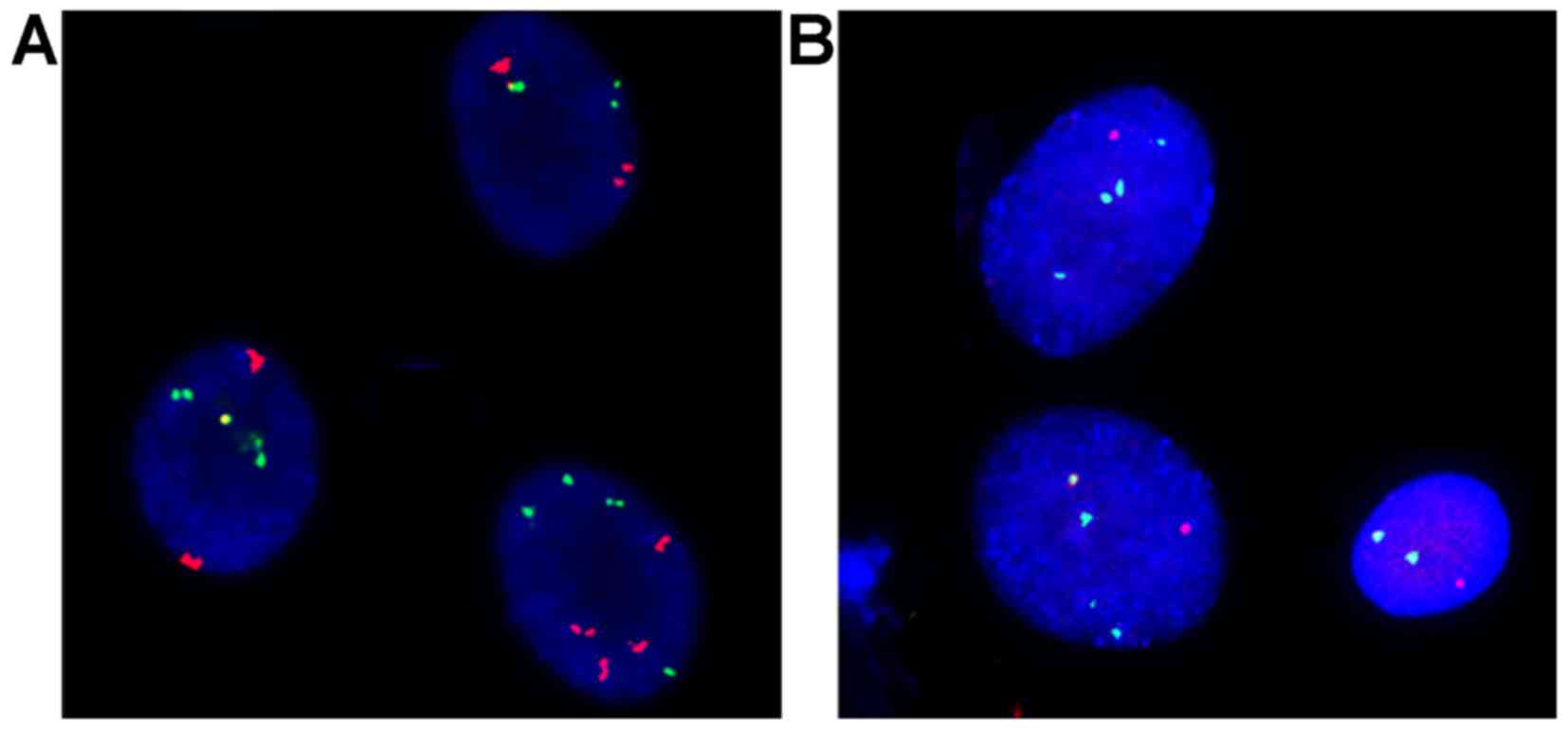
are presented in Fig. 1. Of 246
patients, 217 (88.2%) patients had at least one abnormal
hybridization signals, exhibiting mixed missing, haploid and
polyploidy cells (Table III). In
the present study, abnormal CSP3, CSP7 and CSP17 signals were
typically associated with polyploidy, whereas abnormal GLPp16
signals were typically associated with haploid cells.
 | Table III.Hybridization signals in exfoliated
bladder cells of 246 patients with bladder transitional cell
carcinoma. |
Table III.
Hybridization signals in exfoliated
bladder cells of 246 patients with bladder transitional cell
carcinoma.
| Probe | Mixed missing (0 or
1 signal) | Haploid (1
signal) | Polyploid (>3
signals) | Total |
|---|
| CSP3, n (%) | 6 (2.4) | 12 (4.9) | 106 (43.1) | 124 (50.4) |
| CSP7, n (%) | 12 (4.9) | 15 (6.1) | 104 (42.3) | 131 (53.3) |
| CSP17, n (%) | 10 (4.1) | 25 (10.2) | 75 (30.5) | 110 (44.7) |
| GLPp16, n (%) | 27 (11.0) | 110 (44.7) | 11 (4.5) | 148 (60.2) |
OxS status of BTCC patients
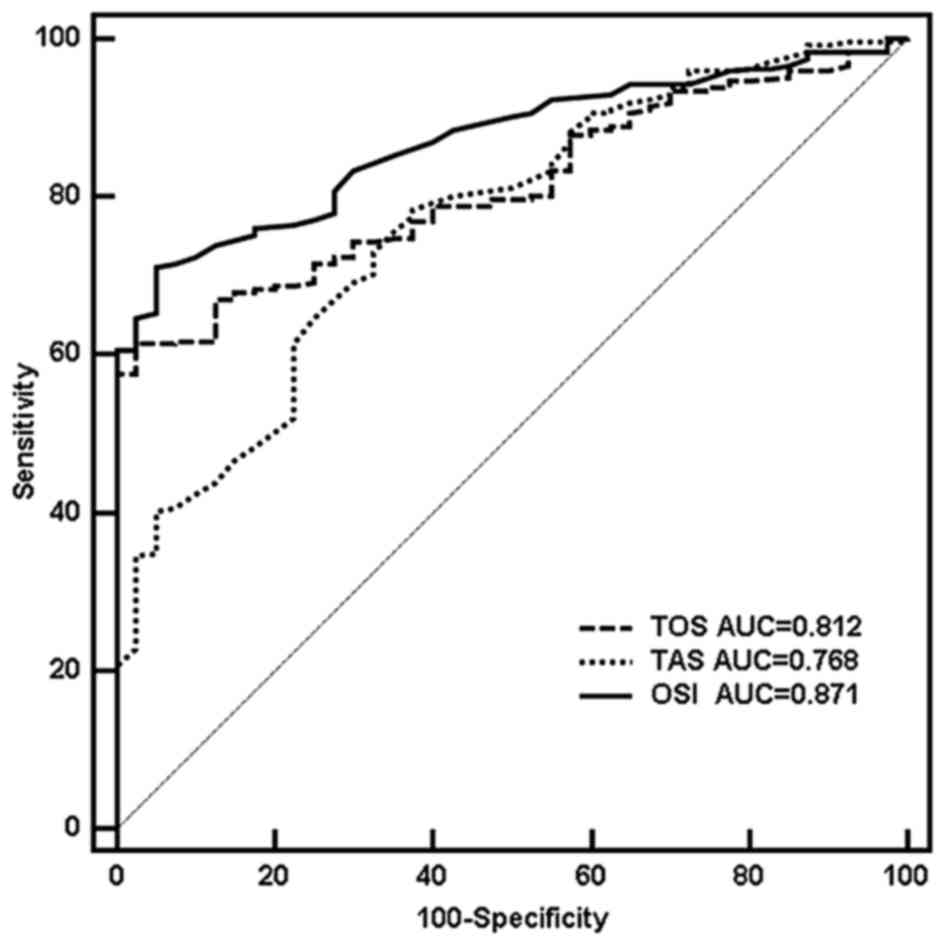
OxS parameters were analyzed using a receiver
operating characteristic curve. The results demonstrated that when
the cut-off values of TOS, TAS and ISO were 16.82 µmol
H2O2 Eq./l, 1.60 mmol Trolox Eq./l and 1.04
AU, respectively. The diagnostic performance reached the highest
level of diagnostic accuracy: The sensitivity was 61.4, 78.5 and
71.1%, respectively; the specificity was 97.5, 62.5 and 95.0%,
respectively; area under curve (AUC) was 0.812, 0.768 and 0.871,
respectively (z=11.555, 7.103 and 16.715, respectively; all
P<0.001; Fig. 2). No significant
differences were identified between AUCTOS and
AUCTAS (z=1.067, P=0.286), but significant differences
were observed between AUCOSI and AUCTOS
(z=2.671, P=0.008) or between AUCOSI and
AUCTAS (z= 3.566, P<0.001).
Correlation between chromosomal
aberrations and OxS inpatients with BTCC
The FISH data and OxS parameters of patients with
BTCC at different clinical stages and pathological grades are
illustrated in Table IV. The
correlation of proportions of different chromosomal aberrations and
FISH positive rate with the clinical stage and grade of BTCC were
evaluated with Gamma rank correlation analysis. The proportions of
abnormal CSP3, CSP7 and CSP17 and FISH positive rate were not
correlated with the clinical stage of BTCC (r=0.131, 0.118, 0.1384
and −0.014, respectively; all P>0.05), but were positively
correlated with pathological grade (r=0.515, 0.639, 0.584 and
0.413, respectively, all P<0.001); the proportion of abnormal
GLPp16 was not correlated with clinical stage (r=−0.026, P=0.792)
or pathological grade (r=0.063, P=0.497).
 | Table IV.Correlation between chromosomal
aberrations and oxidative stress parameters in patients with
bladder transitional cell carcinoma. |
Table IV.
Correlation between chromosomal
aberrations and oxidative stress parameters in patients with
bladder transitional cell carcinoma.
|
| Clinical stage |
|---|
|
|
|
|---|
| A, Group | Ta | T1 | T2 | T3 | T4 | R-value | P-value |
|---|
| Total, n | 24 | 75 | 74 | 48 | 25 |
|
|
| CSP3a, n (%) | 8 (33.3) | 39 (52.0) | 33 (44.6) | 34 (70.8) | 10 (40.0) | 0.131 | 0.155 |
| CSP7a, n (%) | 9 (37.5) | 41 (54.7) | 36 (48.6) | 34 (70.8) | 11 (44.0) | 0.118 | 0.207 |
| CSP17a, n (%) | 5 (20.8) | 31 (41.3) | 43 (58.1) | 20 (41.7) | 11 (44.0) | 0.138 | 0.133 |
| GLPp16a, n (%) | 9 (37.5) | 49 (65.3) | 50 (67.6) | 33 (68.8) | 7 (28.0) | −0.026 | 0.792 |
| FISHa, n (%) | 6 (25.0) | 49 (65.3) | 44 (59.5) | 23 (47.9) | 12 (48.0) | −0.014 | 0.885 |
| TOSb, µmol H2O2
Eq./l | 16.68±3.28 | 18.98±3.96 | 18.56±3.32 | 17.52±3.94 | 20.61±3.02 | 0.087 | 0.175 |
| TASb, mmol Trolox Eq./l | 1.45±0.26 | 1.45±0.23 | 1.42±0.21 | 1.49±0.18 | 1.34±0.32 | −0.043 | 0.502 |
| OSIb, AU | 1.22±0.49 | 1.36±0.42 | 1.34±0.36 | 1.19±0.32 | 1.66±0.57 | 0.093 | 0.146 |
|
|
| Patdological
grade |
|
|
|
| B, Group | G0 | G1 | G2A | G2B | G3 | R-value | P-value |
|
| Total, n | 35 | 57 | 72 | 52 | 30 |
|
|
| CSP3a, n (%) | 4 (11.4) | 27 (47.4) | 33 (45.8) | 36 (69.2) | 24 (80.0) | 0.515 | <0.001 |
| CSP7a, n (%) | 8 (22.9) | 16 (28.1) | 41 (56.9) | 39 (75.0) | 27 (90.0) | 0.639 | <0.001 |
| CSP17a, n (%) | 8 (22.9) | 12 (21.1) | 32 (44.4) | 32 (61.5) | 26 (86.7) | 0.584 | <0.001 |
| GLPp16a, n (%) | 19 (54.3) | 37 (64.9) | 37 (51.4) | 38 (73.1) | 17 (56.7) | 0.063 | 0.497 |
| FISHa, n (%) | 10 (28.6) | 28 (49.1) | 38 (52.8) | 31 (59.6) | 27 (90.0) | 0.413 | <0.001 |
| TOSb, µmol H2O2
Eq./l | 14.58±1.33 | 16.16±2.47 | 19.35±3.15 | 20.79±3.34 | 22.00±2.42 | 0.671 | <0.001 |
| TASb, mmol Trolox Eq./l | 1.52±0.23 | 1.54±0.19 | 1.43±0.19 | 1.37±0.24 | 1.27±0.28 | −0.326 | <0.001 |
| OSIb, AU | 0.98±0.17 | 1.07±0.24 | 1.38±0.29 | 1.55±0.36 | 1.84±0.54 | 0.66 | <0.001 |
A Spearman's rank correlation analysis was employed
to evaluate the correlation of OxS parameters with clinical stage
and pathological grade of BTCC. Serum TOS, TAS and OSI were not
correlated with clinical stage (P>0.05), but the pathological
grade was positively correlated with serum TOS (r=0.671,
P<0.001) and OSI (r= 0.660, P<0.001) and negatively to serum
TAS (r=−0.326, P<0.001).
Correlation between chromosomal
aberrations and serum OxS parameters
A Spearman's rank correlation analysis revealed that
abnormal CSP3, CSP7 and CSP17 signals were positively correlated
with TOS and OSI (all P<0.001), and abnormal CSP7 and CSP17
signals were negatively correlated with TAS (both P<0.001).
However, GLPp16 signals were not correlated with TOS, TAS or OSI
(all P>0.05; Table V).
 | Table V.Spearman's rank correlation analysis
of fluorescent in situ hybridization data and serum
oxidative stress parameters. |
Table V.
Spearman's rank correlation analysis
of fluorescent in situ hybridization data and serum
oxidative stress parameters.
| Parameter | CSP3 | CSP7 | CSP17 | GLPp16 |
|---|
| Total oxidant
status | 0.248,
<0.001 | 0.351,
<0.001 | 0.304,
<0.001 | 0.047, 0.461 |
| Total antioxidant
status | −0.122, 0.057 | −0.297,
<0.001 | −0.182,
<0.001 | −0.015, 0.821 |
| Oxidative stress
index | 0.237,
<0.001 | 0.435,
<0.001 | 0.317,
<0.001 | 0.018, 0.781 |
Discussion
Bladder cancer is a common malignancy of the urinary
system that affects individuals worldwide. The pathogenesis of
bladder cancer is complex and involves a number of factors at
multiple steps, including intrinsic genetic factors and extrinsic
environmental factors (2,37). Thus, evaluating the correlation
between genetic variation at chromosomes and oxidative stress is
crucial for the elucidation of the molecular mechanisms underlying
the occurrence and development of bladder cancer.
Abnormal chromosomal structure and number are
typical characteristics of cancer cells, which have been confirmed
in numerous genetic studies (27–29). Thus,
these abnormal characteristics may be used for the diagnosis of
cancers. FISH of bladder tissues or exfoliated bladder cells is
effective for identifying chromosomal aberrations, including
structural aberration and/or number aberration (28,29,37–40).
The US Food and Drug Administration approved
UroVysion®multicolor-FISH (Abbott Laboratories, Abbott
Park, IL, USA) for the detection of exfoliated cells in the urine,
which is a non-invasive tool used for the adjunctive diagnosis of
bladder cancer (39,40). In the present study, FISH similar to
UroVysion® was performed to detect chromosomal
aberrations in exfoliated bladder cells of patients with BTCC, and
blood oxidative stress was also evaluated. The results demonstrated
that the proportions of abnormal CSP3, CSP7, CSP17 and GLPp16
signals in exfoliated bladder cells were significantly increased
compared with in healthy controls (P<0.001), and all of them
were positively associated with the pathological grade of BTCC
(P<0.001). This suggests that the specific probes used in the
present study can be used for the non-invasive early diagnosis of
BTCC and the monitoring of post-operative recurrence of BTCC. In
addition, hyperdiploidy of exfoliated bladder cells was positively
associated with the progression of BTCC, indicating that this tool
may be used to determine the severity of BTCC.
Oxidative stress is caused by the excess production
of ROS and/or increased consumption of antioxidants in target cells
and tissues. Previous studies have demonstrated that oxidative
stress serves important roles in the occurrence, development and
metastasis of bladder cancer (14–18).
However, these studies primarily focused on one or several
oxidative products, antioxidants or their metabolites (9,19–22), and the oxidative stress levels were
predicted according to the change in these products. This
evaluation is not comprehensive, as the changes in one or several
oxidative products or antioxidants may not represent the overall
status of oxidative stress. In addition, oxidants/antioxidants may
interact with each other, leading to overlapping effects (41,42), and
there are unknown oxidants/antioxidants that were not detectable in
these studies. Thus, findings in these studies may not
comprehensively and systematically evaluate the oxidative stress.
TAS represents a sum of enzyme and non-enzyme antioxidants, and TOS
reflects the overall level of oxidants (43). In the present study, TAS and TOS were
measured and OSI was calculated, which may reflect the overall
oxidative stress status. The results from the present study
demonstrated that the serum TOS (t=6.604, P<0.001) and OSI
(t=6.669, P<0.001) in patients with BTCC were significantly
higher compared with that of healthy controls. However, serum TAS
(t=−5.623, P<0.001) was significantly lower compared with that
of healthy controls, suggesting the oxidative stress induced injury
in patients with BTCC. Analysis using a receiver operating
characteristic curve demonstrated that these OxS parameters were
able to distinguish patients with BTCC from healthy controls
(AUC=0.812, 0.768 and 0.871, respectively; all P<0.001). Further
analysis revealed that the AUC of OSI was higher than that of TOS
and TAS, indicating that OSI is superior to TOS and TAS in the
diagnosis of BTCC. Correlation analysis demonstrated that these OxS
parameters were associated with the pathological grade of BTCC
(R=0.671 for TOS, −0.326 for TAS and 0.660 for OSI; all
P<0.001), indicating that OxS parameters may be used to evaluate
the clinical progression of BTCC. Thus, serum OxS parameters used
in the present study may accurately reflect the oxidative stress
status, and may be used to differentiate between BTCC and healthy
status in addition to evaluating the disease condition. Notably,
serum TAS was inferior to TOS and OSI in the diagnosis of BTCC, and
its cut-off value (1.60 mmol Trolox Eq./l) was located in the
reference range of healthy control (1.48–1.82 mmol Trolox Eq./l),
which resulted in poor sensitivity (78.5%) and poor specificity
(62.5%). These findings indicate that detecting antioxidant levels
alone fails to comprehensively evaluate the oxidative stress
status, and that it is necessary to detect TAS and TOS in addition
to calculating OSI for the scientific and rational evaluation of
oxidative stress status.
Previous studies have revealed that radiation,
smoking, long-lasting chronic infection and stimulation of foreign
bodies are factors that are associated with bladder cancer
(44,45). These factors may change the oxidative
stress status, cause DNA oxidation, activate oncogenes (46,47)
inactivate tumor suppressor genes, induce the occurrence of bladder
cancer and promote its progression (48,49). A
number of FISH techniques have been developed for the investigation
of DNA oxidative damage in tissues and cells in patients with
bladder cancer (39,50,51). In
the present study, the correlation between blood OxS parameters and
chromosomal aberrations of exfoliated bladder cells was evaluated.
The results demonstrated that the serum TOS and OSI were positively
associated with abnormal CSP3, CSP7 and CSP17 (P<0.001) and
parameters reflecting the proliferation of cancer cells. However,
serum TAS was negatively associated with abnormal CSP7 and CSP17
(P<0.001). This suggests that the oxidative stress status in
patients with BTCC is associated with chromosomal aberrations of
bladder cells. Notably, the OxS parameters detected in the present
study were not associated with abnormal GLPp16 (P>0.05). p16 is
a tumor suppressor gene and may act on p16-INK4a to regulate the
p38-mitogen activated protein kinase signaling pathway, which then
regulates the expression of downstream genes, regulates the ROS
production in cells, inhibits excess proliferation and mediates the
apoptosis of cells, avoiding carcinogenesis (52,53).
Dysregulated p16 during carcinogenesis fails to control the
intracellular production of ROS and is unable to effectively
regulate oxidative stress, which explains the absence of
correlation between abnormal GLPp16 and blood oxidative stress.
In conclusion, chromosomal alterations are typical
in patients with bladder cancer, which can be detected via FISH.
Oxidative stress may cause damage to protein, lipid, and DNA and
has been revealed to be a critical pathophysiological event
implicated in numerous human diseases, including cancer. Thus, in
the present study, the correlation between chromosomal alterations
and oxidative stress status was explored, which may aid with the
elucidation of mechanisms underlying the occurrence and development
of bladder cancer and the determination of the severity of BTCC.
However, the molecular mechanisms underlying the association
between chromosomal alterations and oxidative stress status remain
unclear, and it is probable that the two facilitate the occurrence
and development of cancer. Therefore, studies on the association
between the two may provide reliable theoretical evidence for the
prevention and treatment of cancer.
Acknowledgements
The present study was supported by the Ministry of
Science and Technology of the People's Republic of China (grant no.
2006AA020905) and the Ministry of Health of the People's Republic
of China (grant no. WKJ2007-3-001).
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under curve
|
|
BTCC
|
bladder transitional cell
carcinoma
|
|
DAPI
|
4,6-diamidino-2-phenylindole
|
|
FISH
|
fluorescence in situ
hybridization
|
|
OSI
|
oxidative stress index
|
|
OxS
|
oxidative stress
|
|
ROS
|
reactive oxygen species
|
|
SSC
|
saline sodium citrate
|
|
TAS
|
total antioxidant status
|
|
TOS
|
total oxidant status
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Figueroa JD, Ye Y, Siddiq A, Garcia-Closas
M, Chatterjee N, Prokunina-Olsson L, Cortessis VK, Kooperberg C,
Cussenot O, Benhamou S, et al: Genome-wide association study
identifies multiple loci associated with bladder cancer risk. Hum
Mol Genet. 23:1387–1398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang Z, Lu T, Du L, Hu Z, Zhuang Q, Li Y,
Wang CY, Zhu H and Ye Z: Plasmacytoid urothelial carcinoma of the
urinary bladder: A clinical pathological study and literature
review. Int J Clin Exp Pathol. 5:601–608. 2012.PubMed/NCBI
|
|
5
|
Kanojia D, Garg M, Saini S, Agarwal S,
Parashar D, Jagadish N, Seth A, Bhatnagar A, Gupta A, Kumar R, et
al: Sperm associated antigen 9 plays an important role in bladder
transitional cell carcinoma. PLoS One. 8:e813482013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patchsung M, Boonla C, Amnattrakul P,
Dissayabutra T, Mutirangura A and Tosukhowong P: Long interspersed
nuclear element-1 hypomethylation and oxidative stress: Correlation
and bladder cancer diagnostic potential. PLoS One. 7:e370092012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Redondo-Gonzalez E, de Castro LN,
Moreno-Sierra J, de las Casas ML Maestro, Vera-Gonzalez V, Ferrari
DG and Corchado JM: Bladder carcinoma data with clinical risk
factors and molecular markers: A cluster analysis. Biomed Res Int.
2015:1686822015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gakis G: The role of inflammation in
bladder cancer. Adv Exp Med Biol. 816:183–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gecit I, Aslan M, Gunes M, Pirincci N,
Esen R, Demir H and Ceylan K: Serum prolidase activity, oxidative
stress, and nitric oxide levels in patients with bladder cancer. J
Cancer Res Clin Oncol. 138:739–743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Li S, Liu Y and Ma C: Redox
regulated peroxisome homeostasis. Redox Biol. 4:104–108. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Lay S, Simard G, Martinez MC and
Andriantsitohaina R: Oxidative stress and metabolic pathologies:
From an adipocentric point of view. Oxid Med Cell Longev.
2014:9085392014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thanan R, Oikawa S, Hiraku Y, Ohnishi S,
Ma N, Pinlaor S, Yongvanit P, Kawanishi S and Murata M: Oxidative
stress and its significant roles in neurodegenerative diseases and
cancer. Int J Mol Sci. 16:193–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaudhari N, Talwar P, Parimisetty A,
d'Hellencourt C Lefebvre and Ravanan P: A molecular web:
Endoplasmic reticulum stress, inflammation, and oxidative stress.
Front Cell Neurosci. 8:2132014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng H, Lv L, Li Y, Zhang C, Meng F, Pu Y,
Xiao J, Qian L, Zhao W, Liu Q, et al: miR-193a-3p regulates the
multi-drug resistance of bladder cancer by targeting the LOXL4 gene
and the oxidative stress pathway. Mol Cancer. 13:2342014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higgins JA, Zainol M, Brown K and Jones
GD: Anthocyans as tertiary chemopreventive agents in bladder
cancer: Anti-oxidant mechanisms and interaction with mitomycin C.
Mutagenesis. 29:227–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ellidag HY, Eren E, Aydin O, Akgol E,
Yalcinkaya S, Sezer C and Yilmaz N: Ischemia modified albumin
levels and oxidative stress in patients with bladder cancer. Asian
Pac J Cancer Prev. 14:2759–2763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Savic-Radojevic A, Djukic T, Simic T,
Pljesa-Ercegovac M, Dragicevic D, Pekmezovic T, Cekerevac M,
Santric V and Matic M: GSTM1-null and GSTA1-low activity genotypes
are associated with enhanced oxidative damage in bladder cancer.
Redox Rep. 18:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amasyali AS, Kucukgergin C, Erdem S, Sanli
O, Seckin S and Nane I: Nitric oxide synthase (eNOS4a/b) gene
polymorphism is associated with tumor recurrence and progression in
superficial bladder cancer cases. J Urol. 188:2398–2403. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soini Y, Haapasaari KM, Vaarala MH,
Turpeenniemi-Hujanen T, Kärjä V and Karihtala P:
8-hydroxydeguanosine and nitrotyrosine are prognostic factors in
urinary bladder carcinoma. Int J Clin Exp Pathol. 4:267–275.
2011.PubMed/NCBI
|
|
20
|
Yilmaz IA, Akçay T, Cakatay U, Telci A,
Ataus S and Yalçin V: Relation between bladder cancer and protein
oxidation. Int Urol Nephrol. 35:345–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Badjatia N, Satyam A, Singh P, Seth A and
Sharma A: Altered antioxidant status and lipid peroxidation in
Indian patients with urothelial bladder carcinoma. Urol Oncol.
28:360–367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yalçin O, Karataş F, Erulaş FA and Ozdemir
E: The levels of glutathione peroxidase, vitamin AE, C and lipid
peroxidation in patients with transitional cell carcinoma of the
bladder. BJU Int. 93:863–866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abat D, Demirhan O, Inandiklioglu N, Tunc
E, Erdogan S, Tastemir D, Uslu IN and Tansug Z: Genetic alterations
of chromosomes, p53 and p16 genes in low- and high-grade bladder
cancer. Oncol Lett. 8:25–32. 2014.PubMed/NCBI
|
|
24
|
Lopez-Beltran A, Santoni M, Massari F,
Ciccarese C, Tortora G, Cheng L, Moch H, Scarpelli M, Reymundo C
and Montironi R: Bladder cancer: Molecular determinants of
personalized therapy. Curr Drug Targets. 16:115–124. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drayton RM, Peter S, Myers K, Miah S,
Dudziec E, Bryant HE and Catto JW: MicroRNA-99a and 100 mediated
upregulation of FOXA1 in bladder cancer. Oncotarget. 5:6375–6386.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wieczorek E, Wasowicz W, Gromadzinska J
and Reszka E: Functional polymorphisms in the matrix
metalloproteinase genes and their association with bladder cancer
risk and recurrence: A mini-review. Int J Urol. 21:744–752. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morrison CD, Liu P, Woloszynska-Read A,
Zhang J, Luo W, Qin M, Bshara W, Conroy JM, Sabatini L, Vedell P,
et al: Whole-genome sequencing identifies genomic heterogeneity at
a nucleotide and chromosomal level in bladder cancer. Proc Natl
Acad Sci USA. 111:pp. E672–E681. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Campos SR, Melo TC, Assaf S, Araldi RP,
Mazzuchelli-de-Souza J, Sircili MP, Carvalho RF, Roperto F, Beçak W
and Stocco RC: Chromosome aberrations in cells infected with bovine
papillomavirus: Comparing cutaneous papilloma, esophagus papilloma,
and urinary bladder lesion cells. ISRN Oncol.
2013:9108492013.PubMed/NCBI
|
|
29
|
Köhler CU, Martin L, Bonberg N, Behrens T,
Deix T, Braun K, Noldus J, Jöckel KH, Erbel R, Sommerer F, et al:
Automated quantification of FISH signals in urinary cells enables
the assessment of chromosomal aberration patterns characteristic
for bladder cancer. Biochem Biophys Res Commun. 448:467–472. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bonberg N, Taeger D, Gawrych K, Johnen G,
Banek S, Schwentner C, Sievert KD, Wellhäußer H, Kluckert M, Leng
G, et al: Chromosomal instability and bladder cancer: The
UroVysion(TM) test in the UroScreen study. BJU Int. 112:E372–E382.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumours. Urological TumoursRenal
Pelvis and Ureter. Wiley-Blackwell; 2009
|
|
32
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: World Health Organization classification of tumours:
Pathology and genetics of head and neck tumours. Lyon: IARC; pp.
168–175. 2005
|
|
33
|
Erel O: A new automated colorimetric
method for measuring total oxidant status. Clin Biochem.
38:1103–1111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harma M, Harma M and Erel O: Oxidative
stress in women with preeclampsia. Am J Obstet Gynecol.
192:656–657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aycicek A, Erel O and Kocyigit A:
Decreased total antioxidant capacity and increased oxidative stress
in passive smoker infants and their mothers. Pediatr Int.
47:635–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aycicek A and Erel O: Total
oxidant/antioxidant status in jaundiced newborns before and after
phototherapy. J Pediatr (Rio J). 83:319–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dwivedi AN, Jain S and Dixit R: Gall
bladder carcinoma: Aggressive malignancy with protean loco-regional
and distant spread. World J Clin Cases. 3:231–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwak KW, Kim SH and Lee HM: The utility of
fluorescence in situ hybridization for detection of bladder
urothelial carcinoma in routine clinical practice. J Korean Med
Sci. 24:1139–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Caraway NP, Khanna A, Fernandez RL, Payne
L, Bassett RL Jr, Zhang HZ, Kamat A and Katz RL: Fluorescence in
situ hybridization for detecting urothelial carcinoma: A
clinicopathologic study. Cancer Cytopathol. 118:259–268. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Caraway NP and Katz RL: A review on the
current state of urine cytology emphasizing the role of
fluorescence in situ hybridization as an adjunct to diagnosis.
Cancer Cytopathol. 118:175–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feng JF, Lu L, Zeng P, Yang YH, Luo J,
Yang YW and Wang D: Serum total oxidant/antioxidant status and
trace element levels in breast cancer patients. Int J Clin Oncol.
17:575–583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang D, Feng JF, Zeng P, Yang YH, Luo J
and Yang YW: Total oxidant/antioxidant status in sera of patients
with thyroid cancers. Endocr Relat Cancer. 18:773–782. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cumurcu BE, Ozyurt H, Etikan I, Demir S
and Karlidag R: Total antioxidant capacity and total oxidant status
in patients with major depression: Impact of antidepressant
treatment. Psychiatry Clin Neurosci. 63:639–645. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Salim EI, Morimura K, Menesi A, El-Lity M,
Fukushima S and Wanibuchi H: Elevated oxidative stress and DNA
damage and repair levels in urinary bladder carcinomas associated
with schistosomiasis. Int J Cancer. 123:601–608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vadhanam MV, Thaiparambil J, Gairola CG
and Gupta RC: Oxidative DNA adducts detected in vitro from redox
activity of cigarette smoke constituents. Chem Res Toxicol.
25:2499–2504. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bellavia M, Gioviale MC, Damiano G,
Palumbo VD, Spinelli G, Buscemi G and Lo Monte AI: Dissecting the
different biological effects of oncogenic Ras isoforms in cancer
cell lines: Could stimulation of oxidative stress be the one more
weapon of H-Ras? Regulation of oxidative stress and Ras biological
effects. Med Hypotheses. 79:731–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo S, Mao X, Chen J, Huang B, Jin C, Xu Z
and Qiu S: Overexpression of Pim-1 in bladder cancer. J Exp Clin
Cancer Res. 29:1612010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li S, Peng Q, Chen Y, You J, Chen Z, Deng
Y, Lao X, Wu H, Qin X and Zeng Z: DNA repair gene XRCC1
polymorphisms, smoking, and bladder cancer risk: A meta-analysis.
PLoS One. 8:e734482013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang D, Liu C, Shi J, Wang N, Du X, Yin Q
and Wang Y: Association of XRCC1 Arg399Gln polymorphism with
bladder cancer susceptibility: A meta-analysis. Gene. 534:17–23.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
McKenna DJ, Doherty BA, Downes CS, McKeown
SR and McKelvey-Martin VJ: Use of the comet-FISH assay to compare
DNA damage and repair in p53 and hTERT genes following ionizing
radiation. PLoS One. 7:e493642012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ke Z, Lai Y, Ma X, Lil S and Huang W:
Diagnosis of bladder cancer from the voided urine specimens using
multi-target fluorescence hybridization. Oncol Lett. 7:325–330.
2014.PubMed/NCBI
|
|
52
|
Jenkins NC, Liu T, Cassidy P, Leachman SA,
Boucher KM, Goodson AG, Samadashwily G and Grossman D: The p16
(INK4A) tumor suppressor regulates cellular oxidative stress.
Oncogene. 30:265–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rayess H, Wang MB and Srivatsan ES:
Cellular senescence and tumor suppressor gene p16. Int J Cancer.
130:1715–1725. 2012. View Article : Google Scholar : PubMed/NCBI
|