Introduction
Renal cell carcinoma (RCC) is a common malignancy of
the genitourinary system, accounting for 2–3% of malignancies in
adults and 80–90% of all renal malignancies (1). Furthermore, 4–10% of RCC cases may lead
to inferior vena cava tumor embolus, particularly in patients with
right-sided RCC (1). Radical
nephrectomy and tumor embolus removal surgery are the treatments of
choice for patients with RCC and vena cava tumor embolus. However,
this surgery is challenging, involving risks and stringent
requirements for certain medical devices and conditions (2). For patients with tumor embolus level III
or worsening status (3), surgical
intervention usually requires cooperation between hepatobiliary
surgeons and cardiovascular surgeons (2).
The introduction of targeted molecular therapy has
altered the therapeutic pattern of RCC significantly, achieving
success in treating advanced RCC, and making targeted drug-based
presurgical neoadjuvant therapy an attractive approach for RCC
treatment (4). In 2008, Di Silverio
et al (5) applied preoperative
targeted molecular therapy for the first time in a patient with a
large RCC in the left kidney, renal hilum lymph node metastasis and
inferior vena cava tumor embolus. Sorafenib therapy for 24 weeks
significantly reduced the intravenous tumor embolus, and the
patient received left radical nephrectomy 5). Further studies
applied neoadjuvant targeted molecular therapy successfully in
patients with RCC and inferior vena cava tumor embolus (6–12). The
results of these studies indicated that this therapy aids the
reduction of tumor embolus size, decreases tumor embolus level and
subsequently reduces surgical risk and difficulty (6–12).
However, Cost et al (13)
demonstrated that targeted molecular therapy had minimal clinical
effects on RCC tumor thrombi. In this case, clinical regression of
the thrombus occurred only in sunitinib-treated patients, and the
authors recommended an additional prospective investigation in
order to determine the effects of targeted molecular therapy,
particularly on tumor thrombus levels (13). In the present study, it was
hypothesized that the therapeutic effects of presurgical
neoadjuvant targeted molecular therapy may improve the selection of
the surgical method, favorably impacting the timing of surgery for
even the most challenging cases, and may also reduce surgical
difficulty and complications. The present study administered
presurgical neoadjuvant targeted therapy to RCC patients with vena
cava tumor embolus. The purpose of the present study was to
investigate presurgical neoadjuvant targeted molecular therapy for
kidney cancer with vena cava tumor embolus, and to investigate the
indications and effects of this therapy in association with the
method and timing of surgery.
Patients and methods
Patient selection
A total of 12 consecutive patients with RCC (8 males
and 4 females; median age, 49.8 years) with inferior vena cava
tumor embolus, who received presurgical neoadjuvant targeted
molecular therapy at The People's Liberation Army General Hospital
(Beijing, China) between June 2009 and June 2014, were enrolled.
The inclusion criteria were as follows: Patients aged >18 years;
RCC with vena cava tumor thrombus (level II–IV); histologically
determined presence of clear cells; absence of prior systemic
therapy; Eastern Cooperative Oncology group performance status of 0
or 1; absence of brain metastasis; and no evident contraindications
to surgery. The exclusion criteria were as follows: Patients unable
to adhere to targeted therapy; patients unable to receive
follow-up; or presence of evident contraindications to surgery. The
preoperative diagnosis was RCC with concomitant vena cava tumor
embolus in all 12 patients. All patients received a renal biopsy
prior to surgery and a pathological examination demonstrated the
presence of clear-cell (cc)RCC. The Internal Review Board of The
People's Liberation Army General Hospital reviewed and approved the
study protocol. All enrolled patients provided written informed
consent for their data to be included in the study.
Neoadjuvant therapy
Prior to the administration of targeted therapy,
spiral computed tomography (CT) or magnetic resonance imaging was
performed to determine the tumor size. A mass >1 cm in diameter
was defined as a targeted tumor. If multiple foci were present, a
maximum of five foci in the same organ or ≤10 foci in the entire
body, were selected as targeted tumors, and the remaining tumors
were regarded as non-targeted tumors. In 7 patients, sunitinib
(Pfizer, Inc., New York, NY, USA; 50 mg four times a day) was
administered orally prior to surgery (4 weeks followed by an
interval of 2 weeks) for 12–18 weeks. In 5 patients, sorafenib
(Bayer AG, Leverkusen, Germany; 400 mg twice a day) was
administered orally continuously prior to surgery for 8–12 weeks.
CT, routine blood and urine tests, blood biochemical analysis, and
detection of coagulation parameters were performed once weekly.
When the tumor size had decreased to a stable size, targeted
therapy was discontinued and a comprehensive evaluation was
performed prior to surgery, aiming to exclude contraindications.
The time between discontinuing the targeted therapy and surgery was
included in the evaluation of all patients.
Surgical intervention
In total, 10/12 patients received surgical
intervention under general anesthesia. Robot-assisted laparoscopic
radical resection of the right kidney and vena cava tumor embolus
removal surgery were performed in 3 patients; radical nephrectomy
and vena cava tumor embolus removal surgery were performed in 2
patients following the percutaneous implantation of a balloon
catheter in the inferior vena cava; and open radical nephrectomy
and vena cava tumor embolus removal surgery were performed in the
remaining 5 patients. In addition, 2 patients did not receive
surgical intervention due to multiple metastases, tumor embolus or
disease progression. Following surgery, 7 patients continued to
receive targeted therapy.
Data collection
Therapeutic efficacy was determined according to the
Response Evaluation Criteria in Solid Tumors (14), and evaluated once during every course
of therapy. Adverse effects were evaluated and graded once during
every course of therapy according to the National Cancer Institute
Common Terminology Criteria for Adverse Events version 3.0 (2006)
(15). The following information was
collected for analysis: Clinical characteristics, CT or magnetic
resonance imaging results prior to and following therapy, dose of
targeted drugs, duration of targeted therapy, therapeutic efficacy,
adverse effects, time of surgery, intraoperative findings,
intraoperative blood loss, postoperative drainage volume,
perioperative complications, postoperative pathological findings
and therapies administered following surgery. All patient data were
reviewed and analyzed retrospectively.
Histochemical staining
For histochemical analysis, embryos were fixed in
10% formalin. Sections 4-µm thick were obtained. The tissue was
dewaxed, rehydrated with a descending alcohol series
(concentration, 90–80%) and stained with hematoxylin and eosin.
Sections were deparaffinized three times by xylene for 5 min each
time in 80°C and then re-hydrated twice in absolute alcohol for 5
min each, followed by 3.95% alcohol for 2 min and 70% alcohol for 2
min. Sections were then washed briefly in distilled water.
Subsequently, sections were stained with Harris hematoxylin
solution for 8 min, washed under a running tap water for 5 min,
differentiated in 1% acid alcohol for 30 sec, and washed again
under a running tap water for 1 min. This was followed by bluing in
0.2% ammonia water or saturated lithium carbonate solution for 30
sec to 1 min. Sections were then washed under a running tap water
for 5 min, rinsed in 95% alcohol (10 dips), counterstained in
eosin-phloxine solution for 30 sec to 1 min and dehydrated through
95% alcohol, 2 changes of absolute alcohol for 5 min each. Sections
were then cleared in 2 changes of xylene for 5 min each. Finally,
sections were mounted with xylene-based mounting medium. All tissue
specimens were examined using bright-field microscopy (Axiovert
200; Carl Zeiss AG, Oberkochen, Germany) at a magnification of ×200
or ×400 (16).
Statistical analysis
Non-parametric statistical analyses were applied due
to the small sample size. Continuous data are presented as the
median and interquartile ranges, together with Mann-Whitney U tests
for intergroup comparisons and Wilcoxon signed-rank tests for
comparisons of differences between pre- and postoperative
variables. Categorical data are presented as counts and
percentages, together with Fisher's exact tests for group
comparisons. For assessments of therapy efficacy, the objective
response rate (ORR) and disease control rate (DCR) were calculated
based on the complete response (CR), partial respremission (PR) and
stable disease (SD) as follows: ORR=CR+PR and DCR=CR+PR+SD. All
statistical analyses were performed using IBM SPSS statistical
software version 22 (IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients' baseline demographic and
clinical characteristics
Table I presents the
baseline characteristics of all patients enrolled in the present
study, grouped according to the type of neoadjuvant targeted
molecular therapy (sorafenib vs. sunitinib) received. RCC was right
sided in 9 patients and left sided in 3 patients. The median
diameter of the primary RCC tumor was 8.4 cm (range, 5.4–10.6 cm).
Tumor embolus levels II, III and IV were observed in 2, 6 and 4
patients, respectively. The median length of tumor emboli was 9.7
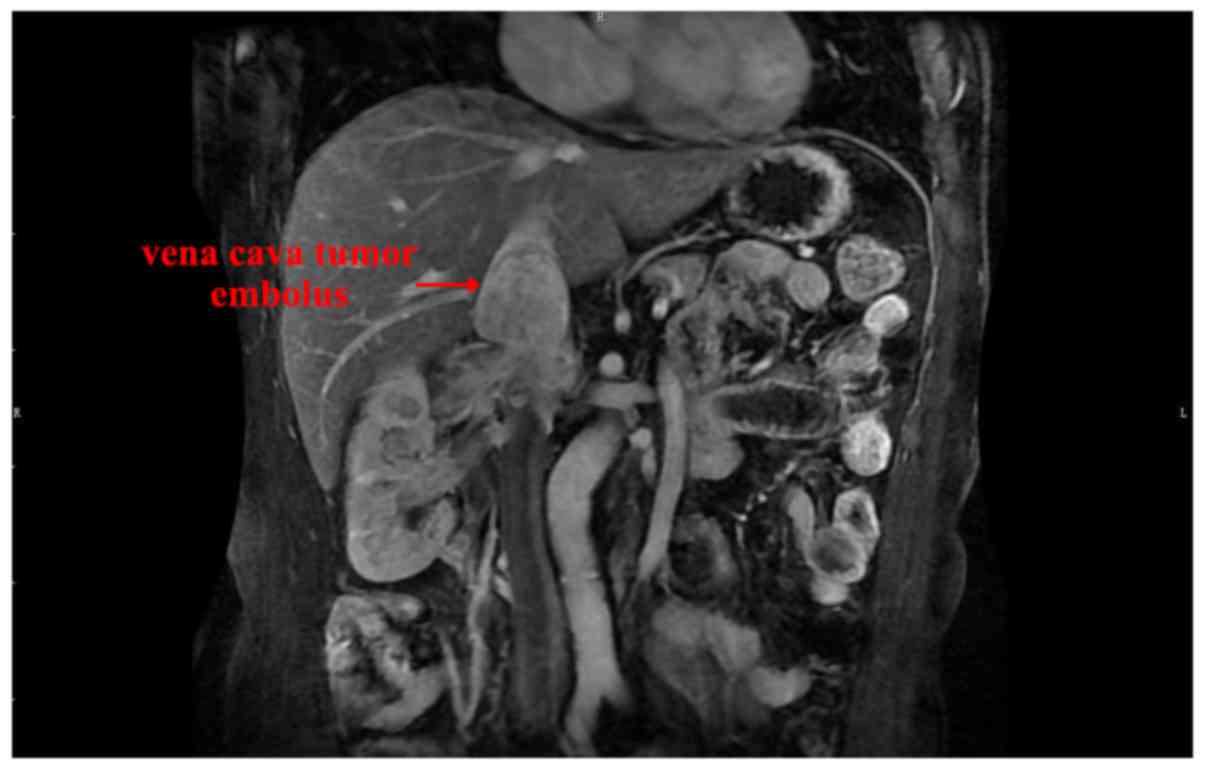
cm (range, 6.5–14.0 cm), and a representative RCC with tumor
embolus is presented in Fig. 1. No
significant differences were observed between the two therapy
groups. A total of 4 patients developed perioperative
complications, including delayed wound healing, hypertension,
kidney dysfunction and anemia, all of which were resolved following
symptomatic therapy. Preoperative examinations revealed
hypertension in 3 patients, lower extremity edema in 1 patient,
ascites in 1 patient and coagulation dysfunction in 1 patient,
whilst the remaining patients had no contraindications to surgery.
In addition, 2 patients developed lung metastases and one exhibited
multiple bone metastases.
 | Table I.Baseline demographic and clinical
characteristics of patients according to therapy group. |
Table I.
Baseline demographic and clinical
characteristics of patients according to therapy group.
| Characteristics | Total, n (%) | Sorafenib, n (%) | Sunitinib, n (%) | P-value |
|---|
| Total | 12 | 5 | 7 |
|
| Gender |
|
|
| 1.000 |
| Male | 8 (66.7) | 3 (60.0) | 5 (71.4) |
|
|
Female | 4 (33.3) | 2 (40.0) | 2 (28.6) |
|
| Age, years
(range) | 51.5 (42.2–58.5) | 52.1 (44.1–58.3) | 51.0 (37.0–59.2) | 0.755 |
| Tumor location |
|
|
| 1.000 |
| Right
side | 9 (75.0) | 4 (80.0) | 5 (71.4) |
|
| Left
side | 3 (25.0) | 1 (20.0) | 2 (28.6) |
|
| Tumor thrombus
level |
|
|
| 0.419 |
| II | 2 (16.7) | 0 (0.0) | 2 (28.6) |
|
| III | 6 (50.0) | 4 (80.0) | 2 (28.6) |
|
| IV | 4 (33.3) | 1 (20.0) | 3 (42.9) |
|
| Targeted therapy |
|
|
|
|
|
| Treatment
time, weeks (range) | 12 (12–18) | 12 (8–12) | 18 (12–18) | 0.030a |
|
Preoperative average target
therapy termination time, days (range) | 14 (12–14) | 12 (12–13) | 14 (14–15) | 0.048a |
| Curative effect |
|
|
| 0.773 |
| PR | 4 (33.3) | 1 (20.0) | 3 (42.9) |
|
| SD | 6 (50.0) | 3 (60.0) | 3 (42.9) |
|
| PD | 2 (16.7) | 1 (20.0) | 1 (14.3) |
|
| Surgery results |
|
|
|
|
|
| Surgery
time, min (range) | 280 (240–317) | 250 (230–290) | 300 (240–320) | 0.202 |
| Blood
loss volume, ml (range) | 1,600
(800–2,150) | 1,600
(700–2,100) | 1,600
(800–2,300) | 1.000 |
| Blood
transfusion volume, ml (range) | 800 (0–1,450) | 800 (0–1,400) | 800 (0–1,600) | 0.876 |
|
Drainage volume, ml
(range) | 370 (330–417) | 350 (325–395) | 410 (330–450) | 0.343 |
| Length
of stay, days (range) | 9 (8–11) | 9 (8–11) | 9 (8–12) | 0.876 |
|
Complications | 4 (33.3) | 2 (40.0) | 2 (28.6) | 1.000 |
|
Follow-up time, months
(range) | 19 (10–23) | 22 (14–26) | 12 (10–24) | 0.530 |
| Adverse
events |
|
|
|
|
|
|
Hand-foot skin reaction | 8 (66.7) | 3 (60.0) | 5 (71.4) | 1.000 |
|
Hypertension | 4 (33.3) | 2 (40.0) | 2 (28.6) | 1.000 |
|
Diarrhea | 8 (66.7) | 4 (80.0) | 4 (57.1) | 0.576 |
|
Mucositis | 2 (16.7) | 2 (40.0) | 0 (0.0) | 0.152 |
|
Fatigue | 3 (25.0) | 0 (0.0) | 3 (42.9) | 0.205 |
| Loss of
appetite | 3 (25.0) | 0 (0.0) | 3 (42.9) | 0.205 |
|
Neutropenia | 3 (25.0) | 0 (0.0) | 3 (42.9) | 0.205 |
|
Thrombocytopenia | 2 (16.7) | 0 (0.0) | 2 (28.6) | 0.470 |
| Skin
stained yellow | 2 (16.7) | 0 (0.0) | 2 (28.6) | 0.470 |
|
Hypothyroidism | 1 (8.3) | 0 (0.0) | 1 (14.3) | 1.000 |
|
Nausea | 1 (8.3) | 0 (0.0) | 1 (14.3) | 1.000 |
Neoadjuvant therapy
A total of 5 patients received sorafenib therapy and
7 patients received sunitinib therapy. The median duration of
targeted molecular therapy was 13.3 weeks (range, 8–18 weeks) prior
to surgery. In overall assessments of therapeutic efficacy, PR was
observed in 4 patients, SD in 6 patients and PD in 2 patients. The
ORR was 33.3% and DCR was 83.3%. For primary RCC, PR was noted in 2
patients, SD in 8 patients and PD in 2 patients; for tumor embolus,
PR was observed in 4 patients and SD in 8 patients. No patients in
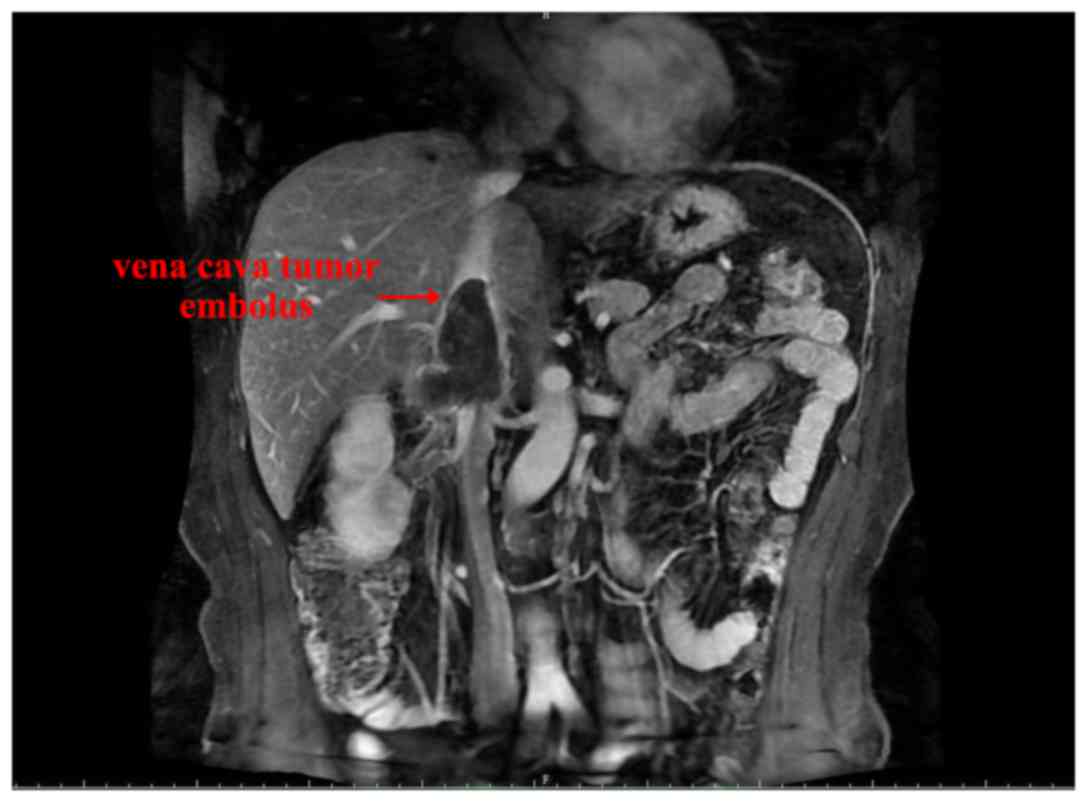
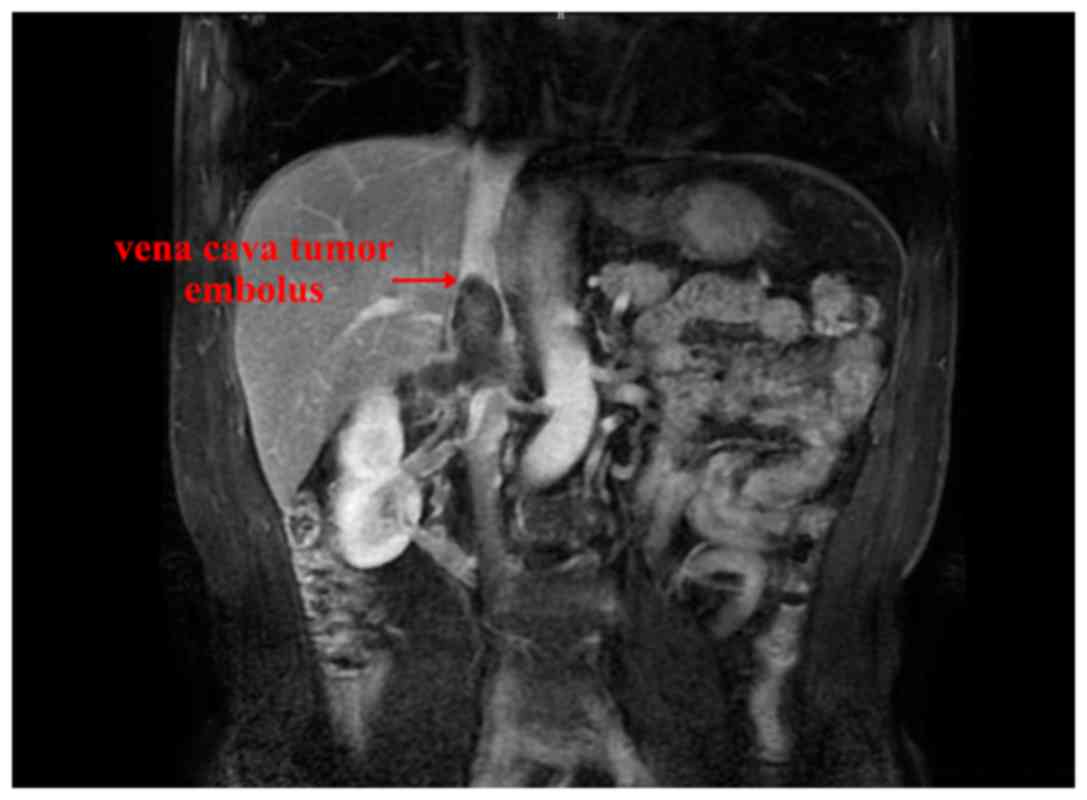
the present study exhibited complete remission. Representative
images revealing the reduced size of the vena cava tumor emboli at
4- and 8-week time points following neoadjuvant therapy are
presented in Figs. 2 and 3, respectively.
The preoperative average targeted therapy stop time
for all 12 patients was 14 days. The median treatment time and
preoperative average targeted therapy termination time were
significantly higher in patients receiving sunitinib than in those
receiving sorafenib (treatment time, 18 vs. 12 weeks, P=0.03;
preoperative average target therapy termination time, 14 vs. 12
weeks, P=0.048). In sunitinib-treated patients, the observed
primary adverse effects included hypertension, skin reactions of
the hands and feet, fatigue, diarrhea, and loss of appetite. In
sorafenib-treated patients, the primary adverse effects were skin
reactions of the hands and feet, hypertension, and diarrhea. All
adverse effects were graded as 1–2 (15), and no patients received intermittent
administration of the targeted therapy or a reduction in drug
dosage due to adverse effects.
Surgical results
The median duration from therapy discontinuation to
surgery was 14.1 days (range, 10–20 days). The median operative
time was 274 min (range, 210–420 days). Median intraoperative blood
loss was 1,520 ml (range, 600–2,960 ml). A total of 8 patients
received blood transfusion, and the median volume of transfused
blood was 1,080 ml (range, 400–2,000 ml). The median drainage
volume following surgery was 380 ml (range, 270–470 ml). The median
postoperative hospital stay was 9.5 days (range, 8–14 days). The
median follow-up time was 18.5 months (range, 3–50 months).
Patient follow-up
For all 12 patients, the median duration of patient
follow-up was 18.5 months (range, 3–50 months). The final follow-up
was conducted on 1 September 2014, and no patients had succumbed to
the disease; however, 2 patients developed novel lung metastases
(Table I).
Postoperative pathology results
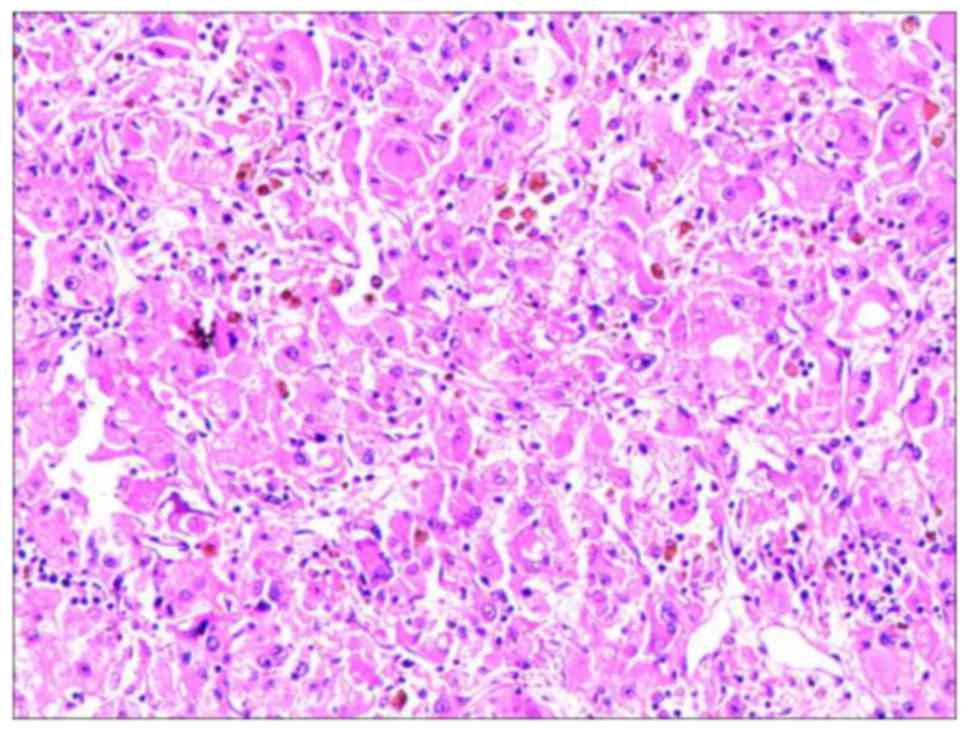
Postoperative pathology revealed ccRCC. Observed
using hematoxylin and eosin staining, the principal pathological
findings were as follows: Significant atrophy of the capillary
sinus in the renal parenchyma; the tumor became fibrotic and
necrotic; and the tumor cells exhibited nuclear condensation and
degeneration (Fig. 4). Following
surgery, 7 patients continued to receive targeted therapy.
Comparison of long-axis diameters of
the embolus and tumor pre- and post-targeted molecular therapy
Table II presents the
median long-axis diameters of the embolus and tumor for all 12
patients, which were significantly shorter prior to, compared with
following, targeted molecular therapy (long-axis diameter of
embolus, 8.5 vs. 9.3 cm, P=0.003; long-axis diameter of tumor, 7.8
vs. 8.5 cm, P=0.018, respectively). For patients receiving
sorafenib, no significant differences were observed between the
pre- and post-targeted molecular therapy long-axis diameters of
emboli and tumors (P>0.05). For patients receiving sunitinib,
the median long-axis diameters of the emboli and tumors were
significantly shorter prior to targeted molecular therapy, compared
with those following targeted molecular therapy (long-axis diameter
of embolus, 8.5 vs. 10.1 cm, P=0.018; long-axis diameter of tumor,
7.5 vs. 8.5 cm, P=0.028, respectively). The length of the tumor
emboli was reduced by a median value of 18.7% (range, 0.0–42%) or
1.8 cm (range, 0.1–5.2 cm), and the tumor diameter was reduced by a
median value of 8.6% (range, 0.0–38.9%) or 0.7 cm (range, 0.0–3.5
cm; Table II). The level of the
tumor thrombus, classified by the Mayo Clinic standard, was
observed to decrease following sunitinib treatment, including two
cases that were downgraded from tumor thrombus level IV to II, one
case from level IV to III and two cases from level III to II.
 | Table II.Comparison of long-axis diameters of
embolus and tumor pre- and post-targeted molecular therapy. |
Table II.
Comparison of long-axis diameters of
embolus and tumor pre- and post-targeted molecular therapy.
|
Characteristics | Pre-targeted
molecular therapy, median (range) | Post-targeted
molecular therapy, median (range) | P-value |
|---|
| Total (n=12) |
|
|
|
|
Long-axis diameter of embolus,
cm | 9.3 (8.6–11.9) | 8.5 (7.2–9.0) | 0.003a |
|
Long-axis diameter of tumor,
cm | 8.5 (7.4–9.4) | 7.8 (6.3–8.9) | 0.018a |
| Sorafenib therapy
(n=5) |
|
|
|
|
Long-axis diameter of embolus,
cm | 9.0 (8.6–10.5) | 8.4 (7.9–9.0) | 0.066 |
|
Long-axis diameter of tumor,
cm | 8.5 (7.1–9.5) | 8.5 (5.8–9.5) | 0.317 |
| Sunitinib therapy
(n=7) |
|
|
|
|
Long-axis diameter of embolus,
cm | 10.0
(6.7–12.0) | 8.5 (5.1–10.0) | 0.018a |
|
Long-axis diameter of tumor,
cm | 8.5 (7.8–9.5) | 7.5 (6.0–8.2) | 0.028a |
Discussion
In total, 12 patients with RCC and concomitant vena
cava tumor embolus were administered targeted molecular therapy
consisting of sorafenib or sunitinib for a median duration of 13.3
weeks prior to surgery. Overall assessment of therapeutic efficacy
demonstrated that 4 patients exhibited a PR, whilst 6 patients had
SD and 2 patients had progressive disease. For the tumor emboli, PR
was observed in 4 patients and SD in 8 patients. None of the
patients exhibited complete remission. Similar adverse effects were
observed between patients treated with sorafenib and sunitinib. All
adverse effects were grades 1–2, including primarily hand or foot
skin reactions, hypertension, and diarrhea. No patients had
intermittent administration of the targeted therapy or a dose
reduction due to adverse effects. Median long-axis diameters of
emboli and tumors in all 12 patients were significantly shorter
following targeted molecular therapy, compared with those prior to
targeted molecular therapy. However, the median long-axis diameter
of the embolus and tumor were only significantly shorter in
sunitinib-treated patients compared with sorafenib-treated
patients. Pre-surgical downsizing of the tumor embolus may
potentially have a clinically significant impact on surgical
treatment (12). This effect was
observed in the present study, during which, the level of the tumor
thrombus was decreased following sunitinib treatment, including two
cases that were downgraded from tumor thrombus level IV to II, one
case from level IV to III and two cases from level III to II.
The majority of previous studies have demonstrated
that targeted therapy is able to downsize RCC tumors in order to
allow organ-sparing surgeries to be performed (11,17),
including a partial nephrectomy for patients with localized and
advanced RCC (18). Downstaging may
also decrease the risk of recurrence (10). Previous studies have demonstrated that
targeted therapy may result in progression-free survival of ≤15
months and overall survival of ~26 months, and continuing therapy
has resulted in overall survival of ≤4 years (19,20).
Although nephrectomy and tumor embolus removal is
still the first-line therapy for RCC with tumor thrombi, targeted
molecular agents are among the recommendations for first-line
systemic therapy in international guidelines, including those of
the European Society of Medical Oncology (ESMO) (21). The ESMO Clinical Practice Guidelines
indicate that ccRCC is the most common (70–85%) subtype of RCC in
adults, and it has subsequently been the focus of the majority of
trials on ccRCC. Therefore, recommendations within the guidelines
are primarily associated with this histological subtype (22). Drugs that have demonstrated efficacy
as systemic treatments in early RCC include sunitinib, pazopanib
and sorafenib (22–24). In the present study, pathological
examination of renal cell biopsies demonstrated that all patients
involved had ccRCC, and that sorafenib and sunitinib demonstrated
efficacy and safety when used as presurgical neoadjuvant
therapies.
Currently, no established protocol for neoadjuvant
targeted therapy exists, and the duration of targeted therapy may
range from 23 to 262 days (12). This
discrepancy may be attributed to variations in the responses of
primary RCC and metastatic foci to this type of therapy (23). In the present study, patients who
received targeted therapy had been diagnosed with advanced-stage
cancer (localized extensive infiltration and/or distant
metastasis), and only when the primary cancer and/or metastatic
foci are controlled, are the patients able to receive radical
surgery. The various responses to targeted therapy may
significantly impact the duration of targeted therapy that is
selected.
Targeted drugs may also affect wound healing; thus,
surgery is typically performed several days or weeks following the
discontinuation of therapy (12).
Generally, the interval between therapy discontinuation and surgery
is 2–3 half-lives of the targeted drug (sorafenib, 8–12 days;
sunitinib, 12–18 days). Sorafenib may be advantageous due to its
shorter half-life compared with sunitinib; however, the optimum
therapeutic agent remains to be determined as the preoperative use
of these drugs continues to be evaluated (9). Shuch et al (3) reported that the time between therapy
discontinuation and surgery by sunitinib ranged from 2–4 weeks.
Cowey et al (11) administered
sorafenib for 33 days, with a 3-day interval prior to surgery
(described as ‘synchronously with surgery’), and identified that
sorafenib therapy reduced the size of the primary tumor and had a
positive impact on the surgical outcome. Thomas et al
(25) administered neoadjuvant
targeted therapy to 19 patients, observing that the incidence of
complications was ~16% (wound complications in 2 patients). Chapin
et al (26) compared
postoperative complications between patients who received immediate
cytoreductive surgery and patients who received neoadjuvant therapy
and cytoreductive surgery, concluding that, even though the risk
for wound complications was relatively high, there were no marked
differences in the overall or severe complications between the two
groups (26). Therefore, patients who
received preoperative neoadjuvant therapy were not at greater risk
for complications than those undergoing surgery without
preoperative therapy (26).
In the present study, if surgery was indicated to
remove target tumors, then presurgical neoadjuvant therapy was
considered. The ESMO guidelines recommend a period of early
observation following diagnosis (21). Bex et al (17) reported that the indications for
neoadjuvant targeted molecular therapy include RCC with vena cava
tumor embolus level III/IV and RCC at the tumor-node-metastasis
system stage T1b or T2 as suitable for partial nephrectomy
(bilateral RCC or solitary kidney) or as tentative therapy for
advanced RCC prior to cytoreductive surgery (18,19). For
patients with tumor embolus level III or lower, presurgical
neoadjuvant targeted molecular therapy may be considered to reduce
tumor size if the imaging examinations suggest that resection is
impossible due to the tumor embolus being adhered to the vena cava
wall (12). Preoperative evaluation
of the disease condition is necessary in all cases, and the
dysfunction of vital organs (heart, lung, brain and kidney) or the
presence of coagulation disorders are major contraindications to
surgical intervention (12).
The present study possessed several limitations,
including that the data were reviewed retrospectively. Although the
12 patients involved in the present study represent the largest
sample size to date for studies on targeted molecular therapy for
RCC with vena cava tumor embolus in China, the results are limited
by the small sample size. In addition, follow-up was limited to a
median value of 18.5 months (range, 3.0–50.0 months), and all
patients survived, meaning that overall survival time was not
reached. Long-term follow-up is required to fully evaluate
progression-free survival following neoadjuvant targeted molecular
therapy and surgery for patients with RCC and vena cava tumor
embolus. Additional prospective studies including a larger sample
size of this patient population are required to investigate the
results of the present study, particularly with regard to the
long-term efficacy of specific targeted molecular therapy.
In conclusion, presurgical neoadjuvant targeted
molecular therapy for RCC with vena cava tumor embolus reduces the
size of the tumor and thrombus, in turn reducing the surgical
complexity associated with performing a radical nephrectomy. Based
on the aforementioned 12 cases, presurgical neoadjuvant targeted
molecular therapy may form a strategic component of comprehensive
RCC treatment. Additional studies are required to further elucidate
the long-term efficacy of presurgical neoadjuvant targeted
molecular therapy for RCC with vena cava tumor thrombus.
References
|
1
|
Bhatt JR and Finelli A: Landmarks in the
diagnosis and treatment of renal cell carcinoma. Nat Rev Urol.
11:517–525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
González J, Gorin MA and Ciancio G:
Long-term survival after radical surgery for renal cell carcinoma
with tumour thrombus. BJU Int. 111:E8–E9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Novick AC and Campbell SC: Renal
TumorsCampbell's Urology. Walsh PC, Retik AB, Vaughan ED and Wein
AJ: WB Saunders; Philadephia: pp. 2672–2731. 2002
|
|
4
|
Shuch B, Riggs SB, LaRochelle JC,
Kabbinavar FF, Avakian R, Pantuck AJ, Patard JJ and Belldegrun AS:
Neoadjuvant targeted therapy and advanced kidney cancer:
Observations and implications for a new treatment paradigm. BJU
Int. 102:692–696. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Silverio F, Sciarra A, Parente U,
Andrea A, Von Heland M, Panebianco V and Passariello R: Neoadjuvant
therapy with sorafenib in advanced renal cell carcinoma with vena
cava extension submitted to radical nephrectomy. Urol Int.
80:451–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karakiewicz PI, Suardi N, Jeldres C, Audet
P, Ghosn P, Patard JJ and Perrotte P: Neoadjuvant sutent induction
therapy may effectively down-stage renal cell carcinoma atrial
thrombi. Eur Urol. 53:845–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peters I, Winkler M, Jüttner B, Teebken
OE, Herrmann TR, von Klot C, Kramer M, Reichelt A, Abbas M, Kuczyk
MA and Merseburger AS: Neoadjuvant targeted therapy in a primary
metastasized renal cell cancer patient leads to down-staging of
inferior vena cava thrombus (IVC) enabling a cardiopulmonary
bypass-free tumor nephrectomy: A case report. World J Urol.
32:245–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horn T, Thalgott MK, Maurer T, Hauner K,
Schulz S, Fingerle A, Retz M, Gschwend JE and Kübler HR:
Presurgical treatment with sunitinib for renal cell carcinoma with
a level III/IV vena cava tumour thrombus. Anticancer Res.
32:1729–1735. 2012.PubMed/NCBI
|
|
9
|
Amin C, Wallen E, Pruthi RS, Calvo BF,
Godley PA and Rathmell WK: Preoperative tyrosine kinase inhibition
as an adjunct to debulking nephrectomy. Urology. 72:864–868. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kondo T, Hashimoto Y, Kobayashi H, Iizuka
J, Nishikawa T, Nakano M and Tanabe K: Presurgical targeted therapy
with tyrosine kinase inhibitors for advanced renal cell carcinoma:
Clinical results and histopathological therapeutic effects. Jpn J
Clin Oncol. 40:1173–1179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cowey CL, Amin C, Pruthi RS, Wallen EM,
Nielsen ME, Grigson G, Watkins C, Nance KV, Crane J, Jalkut M, et
al: Neoadjuvant clinical trial with sorafenib for patients with
stage II or higher renal cell carcinoma. J Clin Oncol.
28:1502–1507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rini BI, Garcia J, Elson P, Wood L, Shah
S, Stephenson A, Salem M, Gong M, Fergany A, Rabets J, et al: The
effect of sunitinib on primary renal cell carcinoma and
facilitation of subsequent surgery. J Urol. 187:1548–1554. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cost NG, Delacroix SE Jr, Sleeper JP,
Smith PJ, Youssef RF, Chapin BF, Karam JA, Culp S, Abel EJ,
Brugarolas J, et al: The impact of targeted molecular therapies on
the level of renal cell carcinoma vena caval tumor thrombus. Eur
Urol. 59:912–918. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. J Natl Cancer Inst.
92:205–216. 2000. View Article : Google Scholar
|
|
15
|
National Cancer Institute, . Cancer
Therapy Evaluation Program, Common Terminology Criteria for Adverse
Events v3.0 (CTCAE). http://ctep.cancer.gov
|
|
16
|
Kiernan JA: Histological and Histochemical
Methods: Theory and Practice. 4th. Scion; Bloxham, UK: 2008
|
|
17
|
Bex A, Kroon BK and de Bruijn R: Is there
a role for neoadjuvant targeted therapy to downsize primary tumors
for organ sparing strategies in renal cell carcinoma? Int J Surg
Oncol. 2012:2504792012.PubMed/NCBI
|
|
18
|
Kroon BK, de Bruijn R, Prevoo W, Horenblas
S, Powles T and Bex A: Probability of downsizing primary tumors of
renal cell carcinoma by targeted therapies is related to size at
presentation. Urology. 81:111–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vogelzang NJ, Samlowski W and Weissman A:
Long-term response in primary renal cancer to sequential
antiangiogenic therapy. J Clin Oncol. 27:e106–e107. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hutson TE, Bukowski RM, Cowey CL, Figlin
R, Escudier B and Sternberg CN: Sequential use of targeted agents
in the treatment of renal cell carcinoma. Crit Rev Oncol Hematol.
77:48–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Escudier B, Eisen T, Porta C, Patard JJ,
Khoo V, Algaba F, Mulders P and Kataja V; ESMO Guidelines Working
Group, : Renal cell carcinoma: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 23 Suppl
7:vii65–vii71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al: Bevacizumab plus interferon alfa2a for
treatment of metastatic renal cell carcinoma: A randomised,
double-blind phase III trial. Lancet. 370:2103–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Escudier R, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatis renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas AA, Rini BI, Stephenson AJ, Garcia
JA, Fergany A, Krishnamurthi V, Novick AC, Gill IS, Klein EA, Zhou
M, et al: Surgical resection of renal cell carcinoma after targeted
therapy. J Urol. 182:881–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chapin BF, Delacroix SE Jr, Culp SH,
Gonzalez GM Nogueras, Tannir NM, Jonasch E, Tamboli P and Wood CG:
Safety of presurgical targeted therapy in the setting of metastatic
renal cell carcinoma. Eur Urol. 60:964–971. 2011. View Article : Google Scholar : PubMed/NCBI
|