Introduction
The prognosis of patients with pancreatic head
cancer (PHC) is poor. Curative surgical resection is the main
treatment modality contributing to good prognosis (1,2). Even
following curative pancreaticoduodenectomy, however, numerous
patients experience local recurrence around the superior mesenteric
artery (SMA) margin (3,4), adversely affecting prognosis. Curative
surgical resection requires control of tumor infiltration and lymph
node metastasis. Standard lymphadenectomy instead of extended
lymphadenectomy has been recommended as the cure for PHC (5). Lymphatic vessels form networks, allowing
lymph node metastases to spread. Standard lymphadenectomy should
therefore include identification of the primary site of lymph node
metastasis and its regional lymphatic basin.
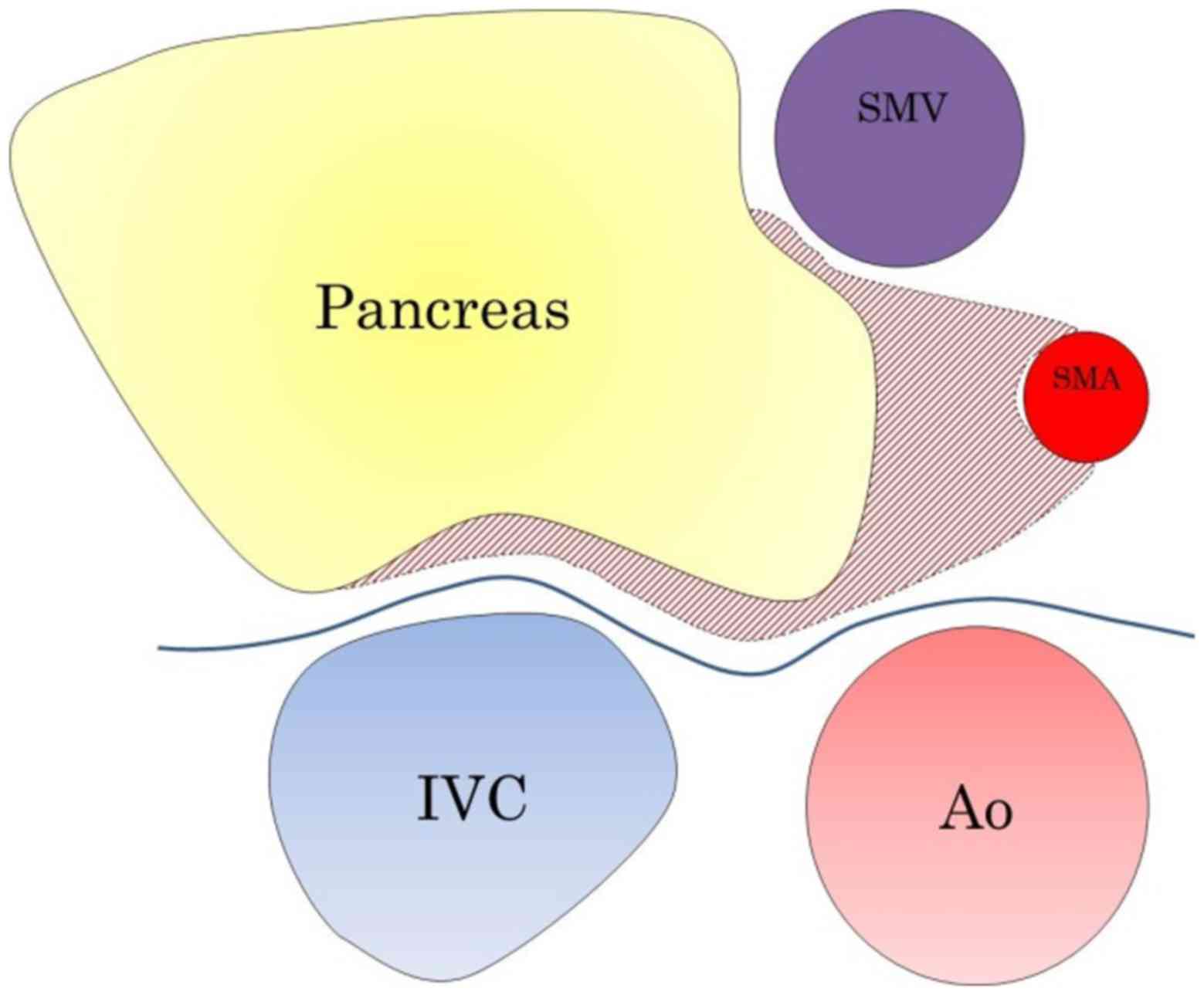
The mesopancreas, is defined as the soft connective
tissue located between the SMA and the uncinate process, or the
structure situated to the right side of the SMA, and is regarded as
the primary site of cancer cell infiltration (3,6,7). Excision of the entire mesopancreas can
result in complete clearance of peripancreatic retroperitoneal
tissue and improve the prognosis of patients with PHC. However,
numerous patients with PHC possess lymph node metastases on the
left side of the SMA (8,9), and lymphadenectomy involving the left
side of the SMA does not include the mesopancreas.
The meso-pancreatoduodenum (meso-pd), consisting of
a cluster of soft connective tissue situated along the inferior
pancreaticoduodenal artery (IPDA) and the first jejunal artery
(FJA), is thought to be a site of lymphatic spread, and total
excision of the meso-pancreatoduodenum (tMPDe) is regarded as
necessary for pathological cure (10). Although tMPDe includes the left side
of the SMA, arterial branches from the SMA to the head of the
pancreas exhibit various patterns. The present study examined the
patterns of the arteries feeding the pancreatic head and the
distribution of the meso-pd.
Patients and methods
Between January 2003 and December 2013, 123 patients
with pancreatic cancer underwent preoperative
64-multidetector-computed tomography (CT). Written informed consent
was obtained from each patient for the use of their data in the
present study. Iodinated contrast material (350 mg/ml) was
delivered at a dose of 1.8 ml/kg over 30 sec. Early and late
arterial phase images were captured at 25 and 40 sec, respectively.
These images were examined to determine the routes of the IPDA and
the FJA.
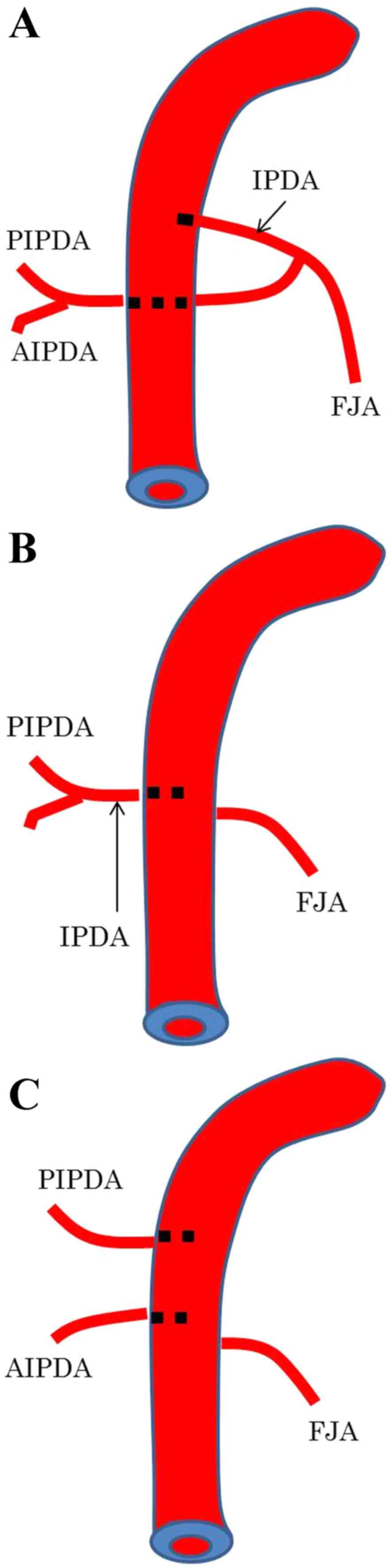
The branches of the pancreatic head were categorized
into three types as described (11)
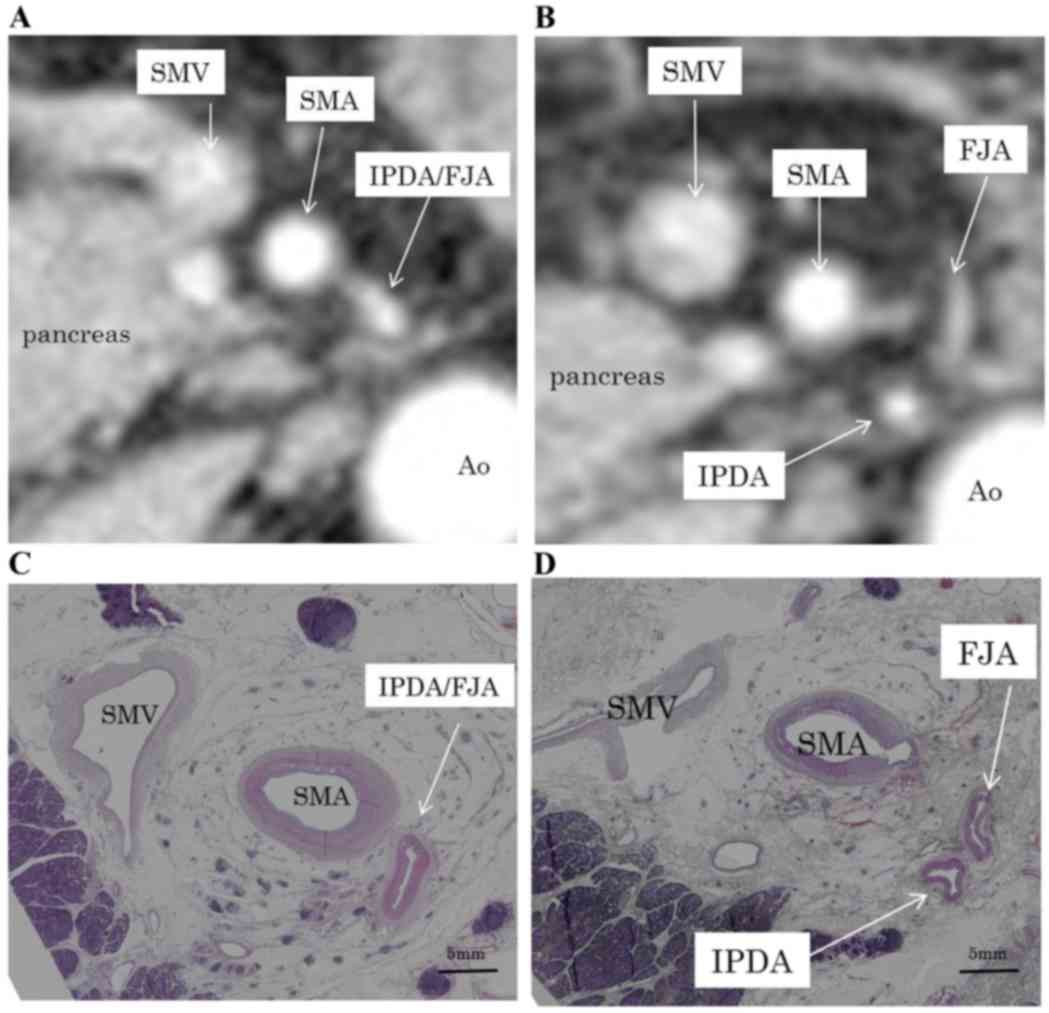
(Fig. 1). In type A, the IPDA formed
a common vessel with the FJA; in type B, the IPDA branched directly
from the SMA; and in type C, the anterior inferior
pancreaticoduodenal artery and the posterior inferior
pancreaticoduodenal artery (PIPDA) branched out separately from the
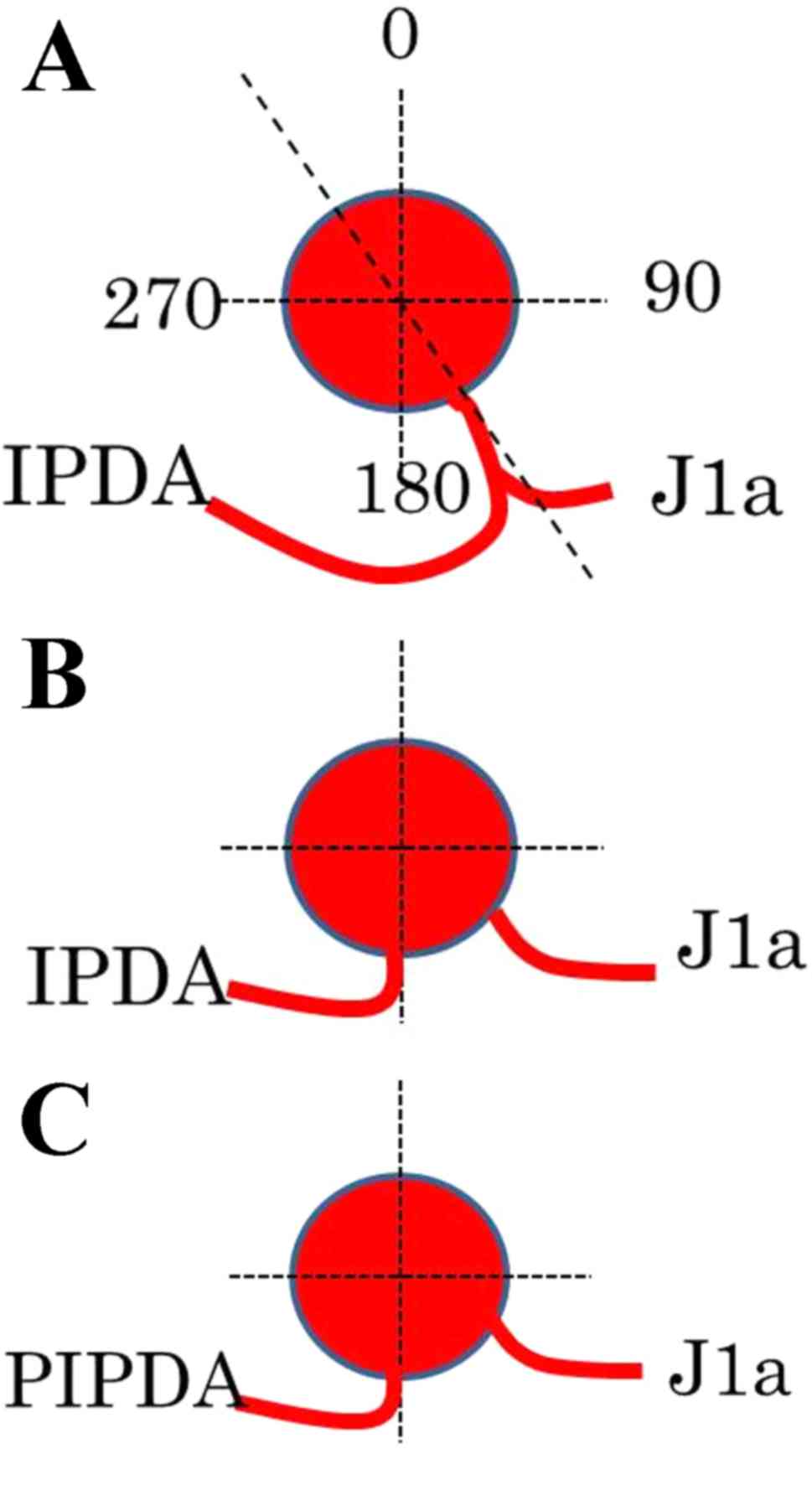
SMA. The angles formed at the point of emergence from the SMA of
these branches were measured using horizontal CT; using the SMA as
the axis, the ventral side was considered 0°, the left side 90° and
the dorsal side 180°.
The surgical specimens and cadavers were assessed
histologically to determine the distribution of meso-pd. The
cadaveric study was conducted at the Department of Functional
Anatomy, Kanazawa University (Kanazawa, Japan). All cadavers were
donated to the university for medical education and study purposes.
Cadavers with any intra-abdominal injury or gross intra-abdominal
pathology were excluded. Dissection was started with a midline
vertical abdominal incision. All the tissues between the pancreas
and aorta, including the SMA, inferior vena cava, superior
mesenteric vein and posterior abdominal wall, were preserved. The
aorta and inferior vena cava were ligated and divided from the
level of the celiac trunk to the level of the inferior mesenteric
artery. The SMA was preserved at the level of the third duodenal
portion. Surgical and cadaveric specimens were fixed in 10%
formalin, cut horizontally into 5-mm tissue blocks corresponding
with the CT scan images, dehydrated and embedded in paraffin.
Sections 5 µm in thickness were cut and stained with hematoxylin
and eosin, elastica van Gieson and D2-40, the latter for detection
of lymphatic vessels.
Immunohistochemical assays were performed on
formalin-fixed paraffin-embedded tissues. Sections were cut 5 µm
thick, deparaffinized in xylene, rehydrated in graded alcohols
(100%) and washed in PBS. Slides were boiled in citrate buffer (pH
6.0) at 120°C for 5 min and allowed to cool for 20 min. Endogenous
peroxidases were blocked by incubation in 0.3% hydrogen peroxide in
distilled water, followed by washing in PBS three times. Sections
were incubated with D2-40 monoclonal antibody (cat. no. M3619;
dilution, 1:50, Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA) for 90 min at room temperature, with immunohistochemical
staining performed using an EnVision + horseradish peroxidase DAB
system (Dako; Agilent Technologies, Inc.). The tissue sections were
subsequently counterstained with hematoxylin and dehydrated through
a graded ethanol series (100%) and xylene three times.
Results
The characteristics of patients enrolled in this
study are summarized in Table I. All
three types of branching of the IPDA and FJA in the pancreatic head
were observed in our patients, with 79 (64.2%) having type A, 35
(28.5%) having type B and 9 (7.3%) having type C branches (Table II). In type A, the vessel common to
the IPDA and FJA emerged between the left and dorsal sides of the
SMA (148±40.0°). In types B and C, the IPDA and PIPDA emerged from
the dorsal side of the SMA, at 187±47.0° and 182±37.4°,
respectively (Fig. 2; Table II). The routes of the IPDA, IPDA/FJA
common vessel and PIPDA exhibited bending.
 | Table I.Location of pancreatic cancers by age
and sex. |
Table I.
Location of pancreatic cancers by age
and sex.
| Location of the
pancreatic cancer | Pancreatic head | Pancreatic body and
tail |
|---|
| Sex, male/female | 51/31 | 25/16 |
| Age, years, mean
(range) | 63.5 (34–81) | 66.5 (45–84) |
 | Table II.IPDA branching types of pancreatic
cancers. |
Table II.
IPDA branching types of pancreatic
cancers.
| IPDA branching
type | Number of patients, n
(%) | Angle formed by the
IPDA or PIPDA |
|---|
| Type A | 79 (64.2) | 148±40.0° |
| Type B | 35 (28.4) | 187±47.0° |
| Type C | 9 (7.3) | 182±37.4° |
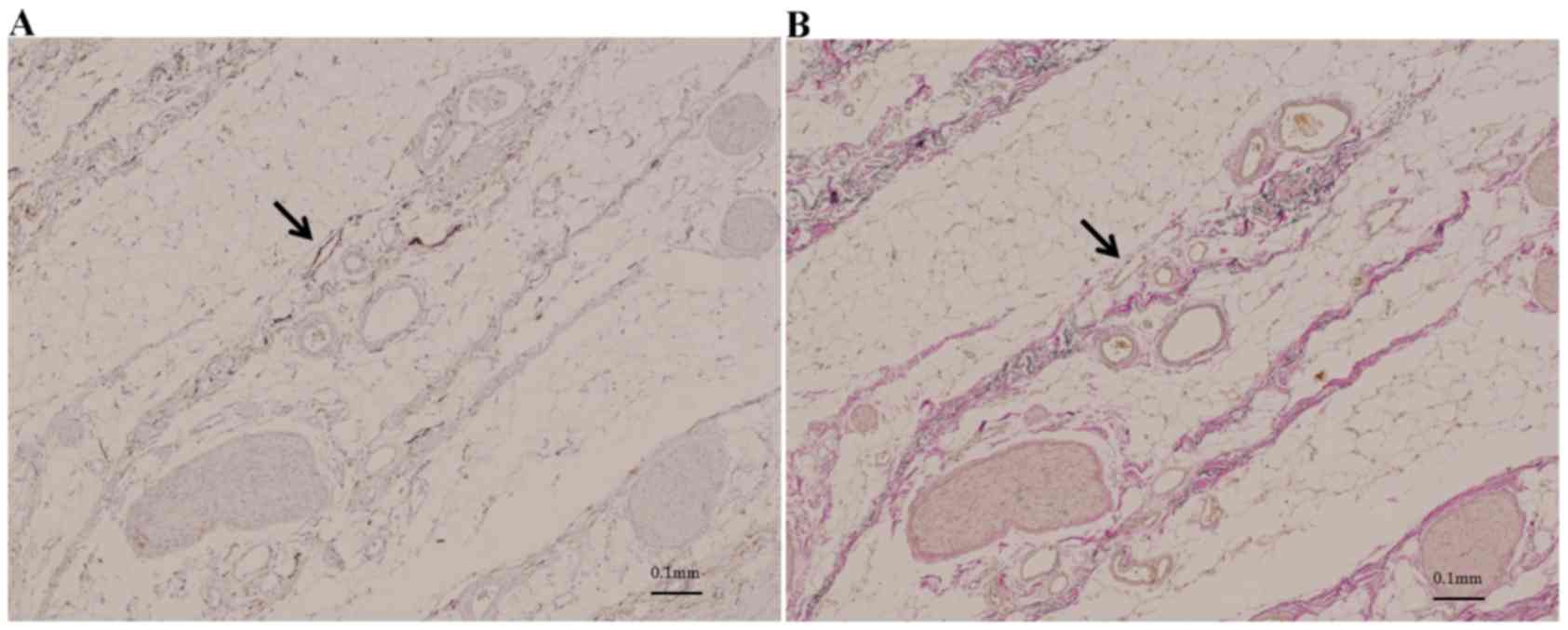
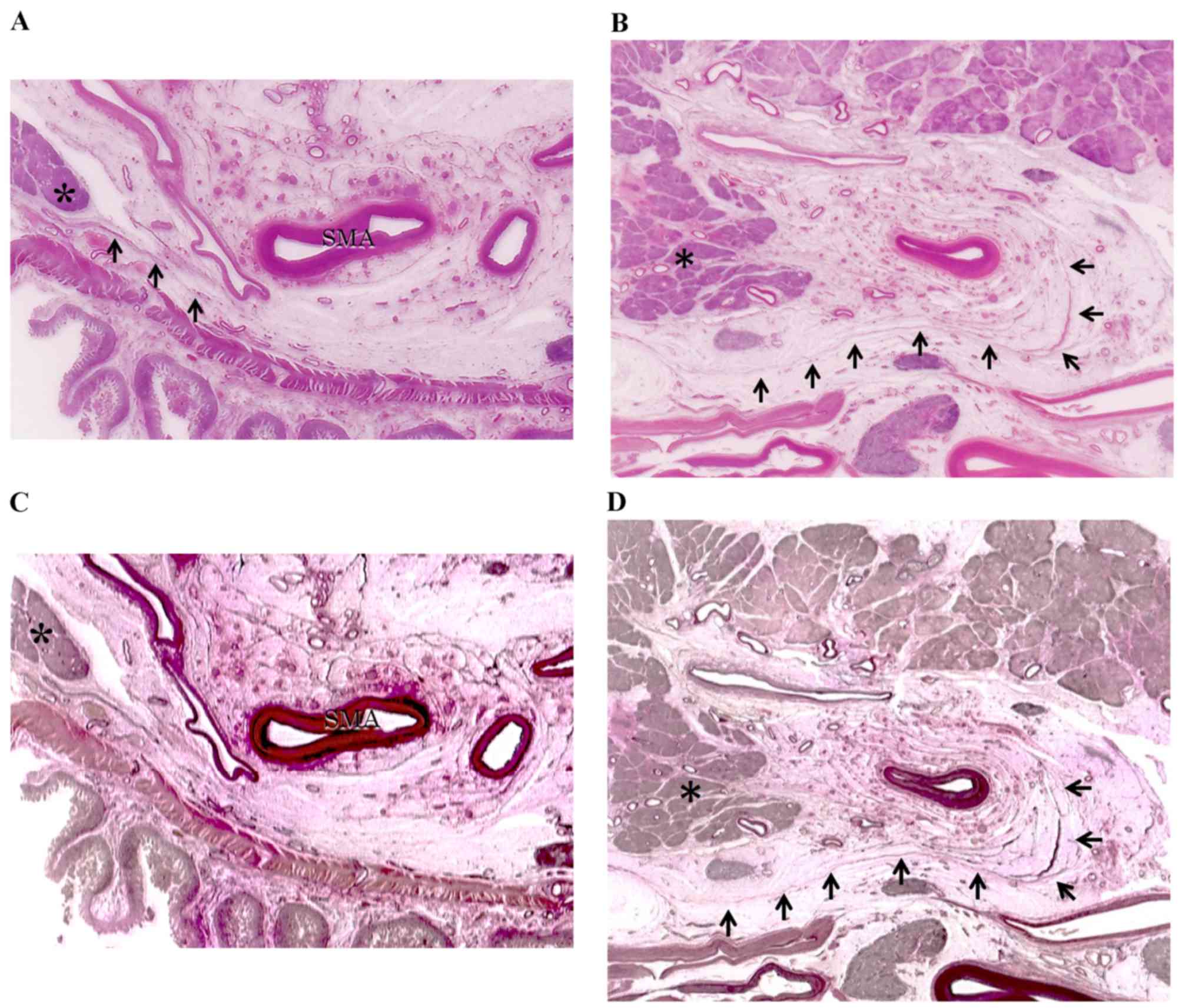
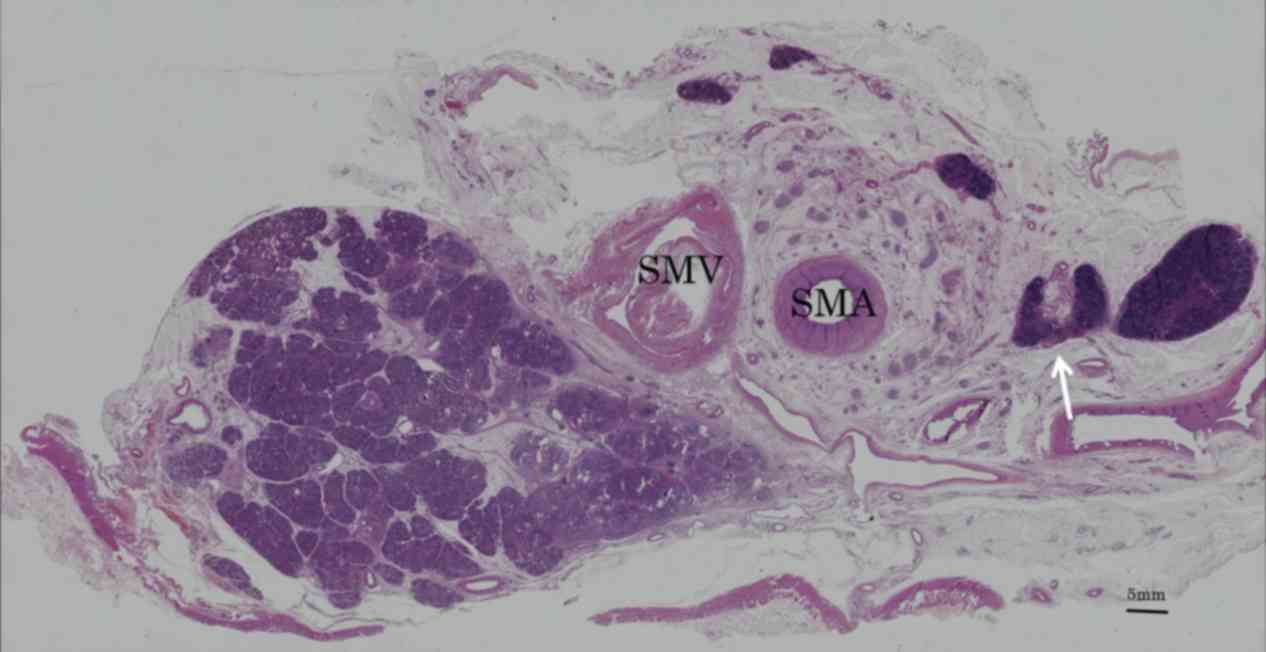
The distribution of the meso-pd was determined by
evaluating the histological continuity of tissue in surgical
specimens and cadavers. Immunohistochemistry with antibodies to
D2-40 revealed lymphatic vessels beside the collagen fibers in this
area (Fig. 3). Collagen fibers,
forming two fibrous bunches, were observed regularly in the soft
tissue. Around the SMA, which was covered grossly by fibrous
tissue, collagen fibers formed concentric circles ~3 mm thick, a
structure called the superior mesenteric arterial plexus (PLsma)
(12). By contrast, collagen fibers
near the uncinated process ran vertically from the uncinated
process of the pancreas to the PLsma, with continuity through the
posterior and left side of the SMA (Fig.
4). These collagen fibers, which were also bent, gradually
fused and joined with the PLsma, making the PLsma thicker as it
proceeded towards the origin of the SMA (Fig. 5).
Discussion
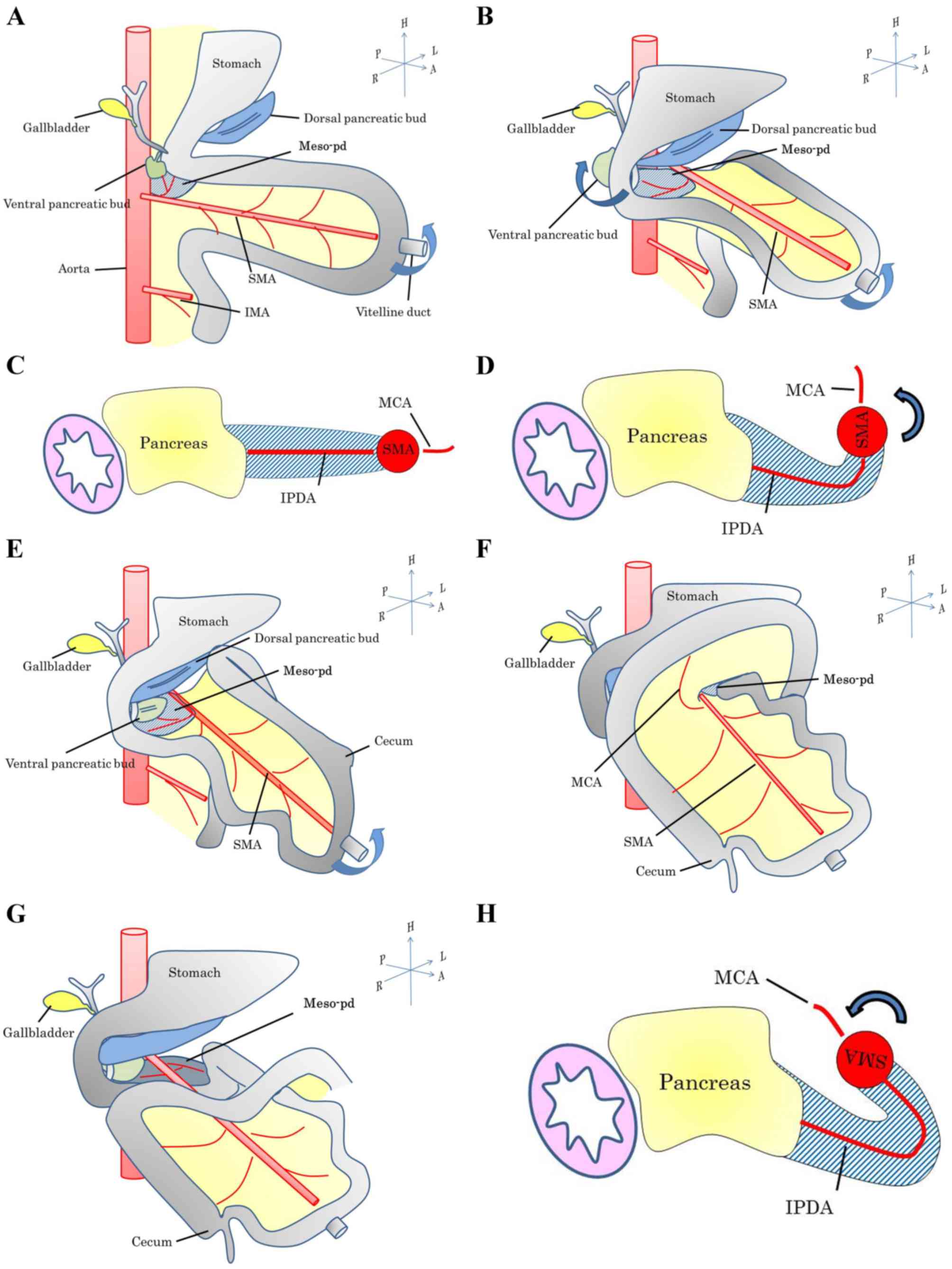
We previously determined that the manner of
lymphatic extension and nerve plexus infiltration of the PHC were
dependent on whether the tumor originated from the embryonic dorsal
or ventral pancreatic bud (12,13).
Tumors confined to the ventral pancreas extend toward the SMA,
whereas tumors confined to the dorsal pancreas extend towards the
common HA or hepatoduodenal ligament. If the tumor infiltrates
deeply into the two areas, the cancer is likely to extend in the
two directions. Therefore, the SMA margin is important for the
carcinoma of the ventral pancreas.
The present study examined the distribution of the
meso-pd, which was considered to be the mesentery of the embryonic
ventral pancreas. The SMA margin is the most frequent site of PHC
recurrence (3), despite the soft
tissue on the right side of the SMA being regularly resected by
pancreaticoduodenectomy. Lymphatic vessel involvement is common in
PHC. Regional lymphatic basin resection is required to avoid local
recurrence, since this area is thought to remain the regional
lymphatic basin of PHC subsequent to soft tissue resection of the
right side of the SMA, resulting in local recurrence. The
mesopancreas (3,6,7), defined
as the structure located on the right side of the SMA, is the
primary site of cancer cell infiltration. However, lymph node
metastasis is often observed on the left side of the SMA in
patients with PHC (8,9). Resection based on the mesopancreas may
therefore be insufficient for curative resection of the regional
lymphatic basin.
The meso-pd, consisting of a cluster of soft
connective tissue situated along the IPDA and the FJA, is regarded
as the site of lymphatic spread, with tMPDe regarded as necessary
for pathological cure (10). Patterns
of arterial branches differ in the pancreatic head. Dissection of
the lymphatic basin in PHC patients requires assessment of these
patterns by multi detector CT, thus clarifying the direction of the
SMA. At emergence, the arteries feeding the pancreatic head can be
classified into three types (11).
All of these branches emerged from the back or left side of the SMA
and ran to the far side of the pancreatic head in an arc.
Therefore, the meso-pd was located in a roll shape around the trunk
arteries, extending to the left side of the SMA.
By investigating the continuity of the meso-pd
histologically in this area of soft tissue, it was revealed by
immunohistochemistry that the lymphatic vessels were alongside the
collagen fibers. The present study therefore examined the
distribution of the meso-pd relative to the continuity of the
collagen fibers. The meso-pd originates from the uncinated process
of the pancreas and connects to the PLsma, defined as the left back
side of the soft tissue around the SMA (14). The distribution of the meso-pd is the
same as the route of the IPDA. Its lower limit was vertically above
the third duodenal portion. This soft tissue is also the mesentery
of the jejunum, which is the route of the lymphatics and the nerves
connecting the jejunum. These findings suggest that the regional
lymphatic basin of the pancreatic head spreads towards the left
side of the SMA, with an appearance resembling a Swiss roll. This
appearance was likely caused by intestinal rotation in the fetus
(Figs. 6 and 7) (15). The
mid-gut rotated 270° anticlockwise around the SMA, which acted as
the axis. This resulted in a bend in the meso-pd, which
subsequently extended to the left of the SMA.
The artery first approached during
pancreatoduodenectomy has been revealed to contribute to the
determination of resectability and reduction of bleeding; several
procedures have been described (16).
Early shutdown of arterial blood feeding the head of the pancreas
is therefore important. The present evaluation of the location of
the branches of the pancreatic head from the SMA supports the
artery first approach. Among the various surgical methods, the
mesenteric approach is considered optimal for en bloc resection
since the meso-pd, which contains the nerve plexus and lymphatics
associated with the head of the pancreas, extends from the right to
the back side of the SMA. Thus, tMPDe has theoretical advantages
for en bloc and curative resection of carcinomas of the ventral
pancreas. By contrast, the mesentery corresponding to the embryonic
dorsal pancreas is currently unclear, although it has been
associated with the HA. Survival rate following HA resection was
poor in the present study and procedure, which focuses on the
meso-pd, and was shown to be insufficient for treatment of
carcinomas of the dorsal pancreas (17).
The present study concluded that the meso-pd, which
spans the dorsal and left sides of the SMA (Fig. 8), is the proper mesentery of the
pancreas and duodenum. Therefore, in patients with PHC, dissecting
the right and left sides of the SMA during lymphadenectomy may be
advantageous.
References
|
1
|
Wagner M, Redaelli C, Lietz M, Seiler CA,
Friess H and Büchler MW: Curative resection is the single most
important factor determining outcome in patients with pancreatic
adenocarcinoma. Br J Surg. 91:586–594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stojadinovic A, Brooks A, Hoos A, Jaques
DP, Conlon KC and Brennan MF: An evidence-based approach to the
surgical management of resectable pancreatic adenocarcinoma. J Am
Coll Surg. 196:954–964. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaedcke J, Gunawan B, Grade M, Szöke R,
Liersch T, Becker H and Ghadimi BM: The mesopancreas is the primary
site for R1 resection in pancreatic head cancer: Relevance for
clinical trials. Langenbecks Arch Surg. 395:451–458. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heye T, Zausig N, Klauss M, Singer R,
Werner J, Richter GM, Kauczor HU and Grenacher L: CT diagnosis of
recurrence after pancreatic cancer: Is there a pattern? World J
Gastroenterol. 17:1126–1134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pedrazzoli S, DiCarlo V, Dionigi R, Mosca
F, Pederzoli P, Pasquali C, Klöppel G, Dhaene K and Michelassi F:
Standard versus extended lymphadenectomy associated with
pancreaticoduodenectomy in the surgical treatment of adenocarcinoma
of the head of the pancreas: A multicenter, prospective, randomized
study. Lymphadenectomy Study Group. Ann Surg. 228:508–517. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gockel I, Domeyer M, Wolloscheck T,
Konerding MA and Junginger T: Resection of the mesopancreas (RMP):
A new surgical classification of a known anatomical space. World J
Surg Oncol. 5:442007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adham M and Singhirunnusorn J: Surgical
technique and results of total mesopancreas excision (TMpE) in
pancreatic tumors. Eur J Surg Oncol. 38:340–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kayahara M, Nagakawa T, Ueno K, Ohta T,
Tsukioka Y and Miyazaki I: Surgical strategy for carcinoma of the
pancreas head area based on clinicopathologic analysis of nodal
involvement and plexus invasion. Surgery. 117:616–623. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noto M, Miwa K, Kitagawa H, Kayahara M,
Takamura H, Shimizu K and Ohta T: Pancreas head carcinoma:
Frequency of invasion to soft tissue adherent to the superior
mesenteric artery. Am J Surg Pathol. 29:1056–1061. 2005.PubMed/NCBI
|
|
10
|
Kawabata Y, Tanaka T, Nishi T, Monma H,
Yano S and Tajima Y: Appraisal of a total meso-pancreatoduodenum
excision with pancreaticoduodenectomy for pancreatic head
carcinoma. Eur J Surg Oncol. 38:574–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Horiguchi A, Ishihara S, Ito M, Asano Y,
Yamamoto T and Miyakawa S: Three-dimensional models of arteries
constructed using multidetector-row CT images to perform
pancreatoduodenectomy safely following dissection of the inferior
pancreaticoduodenal artery. J Hepatobiliary Pancreat Sci.
17:523–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitagawa H, Ohta T, Makino I, Tani T,
Tajima H, Nakagawara H, Ohnishi I, Takamura H, Kayahara M, Watanabe
H, et al: Carcinomas of the ventral and dorsal pancreas exhibit
different patterns of lymphatic spread. Front Biosci. 13:2728–2735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makino I, Kitagawa H, Ohta T, Nakagawara
H, Tajima H, Ohnishi I, Takamura H, Tani T and Kayahara M: Nerve
plexus invasion in pancreatic cancer: Spread patterns on
histopathologic and embryological analyses. Pancreas. 37:358–365.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Japan Pancreas Society, . Classification
of Pancreatic Carcinoma. 3rd. Kanehara & Co., Ltd.; Tokyo:
2011
|
|
15
|
Shinohara H, Kurahashi Y, Kanaya S, Haruta
S, Ueno M, Udagawa H and Sakai Y: Topographic anatomy and
laparoscopic technique for dissection of no. 6 infrapyloric lymph
nodes in gastric cancer surgery. Gastric Cancer. 16:615–620. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inoue Y, Saiura A, Yoshioka R, Ono Y,
Takahashi M, Arita J, Takahashi Y and Koga R: Pancreatoduodenectomy
with systematic mesopancreas dissection using a supracolic anterior
artery-first approach. Ann Surg. 262:1092–1101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kitagawa H, Tajima H, Nakagawara H, Makino
I, Miyashita T, Shoji M, Nakanuma S, Hayashi N, Takamura H, Ohta T
and Ohtake H: En bloc vascular resection for the treatment of
borderline resectable pancreatic head carcinoma. Mol Clin Oncol.
2:369–374. 2014.PubMed/NCBI
|