Introduction
Lung cancer is the most common cancer worldwide,
with estimates revealing that almost half of all new lung cancer
cases occur in Asia, the majority of them in China. Due to the high
prevalence of smoking in China, the rate of lung cancer is higher
than that of the majority of European and American countries
(1). In addition, due to the high
prevalence of smoking, ~30% of lung cancer diagnoses are classified
as the squamous histopathological subtype (2). In total, ~80% of patients with lung
cancer in China exhibit metastases either at the time of
presentation or later in the course of the disease, leading to a
high mortality rate (3).
Myeloid-derived suppressor cells (MDSCs), a type of
immunosuppressive cell, have previously been demonstrated to serve
a role in carcinoma (4). Human MDSCs
are a heterogeneous population composed of cells at several
differentiation stages of the myeloid lineage (5). Different types of tumors harbor distinct
subsets of MDSCs, which can be further divided into granulocytic
cluster of differentiation antigen 15-positive HLA class II
histocompatibility antigen DR-negative/low
(CD15+HLA-DR−/low) and monocytic
CD14+HLA-DR−/low monocytic MDSC subsets
(6). A recent study identified the
existence of a monocytic subset of MDSCs with the
CD14+HLA-DR−/low phenotype that suppresses
the proliferation of T cells (7).
The purpose of the present study was to investigate
the proportion of peripheral CD14+HLA-DR−/low
MDSCs in patients with different stages of lung squamous cell
carcinoma, and to investigate the association between different
tumor stages and MDSC function.
Materials and methods
Patients and healthy donors
A total of 78 patients (67 male and 11 female)
diagnosed from January 2014 to October 2015 with lung squamous cell
carcinoma at NanFang Hospital of Southern Medical University
(Guangzhou, China) were enrolled. The patients were aged between 48
and 72 years old (mean, 58.4 years old). The diagnosis and stage
classification of these patients were performed according to the
American College of Chest Physicians guidelines released in 2013
(8,9).
None of the patients had received chemotherapy or surgery prior to
the blood sample being taken. Patients with autoimmune diseases,
infectious diseases, multi-primary cancers and other serious
diseases were excluded from the current study. All patients were
divided into four stages according to the tumor-node-metastasis
(TNM) diagnostic criteria (10).
Among them, there were 0 patients with stage I, 15 patients with
stage II, 37 patients with stage III and 26 patients with stage IV
lung squamous cell carcinoma. As the healthy control, 30 healthy
volunteers were enrolled in the current study. Blood samples were
collected from the aforementioned patients and healthy controls.
The current study was approved by the Ethics Committee of NanFang
Hospital of Southern Medical University (Guangzhou, China). Written
informed consent was obtained from each patient and healthy
donor.
Cell isolation and sorting
Peripheral blood mononuclear cells (PBMCs) were
isolated from heparinized blood samples using Ficoll-Hypaque
density gradient centrifugation at 2,500 × g for 20 min at 22°C.
MDSCs were isolated from the PBMCs using Miltenyi Macs kit for
CD14+ and HLA-DR− (cat. no. 130-091-632;
Miltenyi Biotech, Inc., Cambridge, MA, USA), according to the
manufacturer's protocol, followed by analysis using a BD FACSAria™
cell sorter (BD Biosciences, Franklin Lakes, NJ, USA). The purity
of the MDSCs was >90%, which was derived using flow cytometry
software FlowJo 7.6.1 (FlowJo LLC, Ashland, OR, USA). The
CD3+ T cells were separated from the PBMCs via
CD3+ selection using a MidiMACS™ separator unit
(Miltenyi Biotech, Inc.), according to the manufacturer's protocol.
The purity of the CD3+ T cells was >95%.
Flow cytometryto determine the
frequency of CD14+HLA-DR−/low cells in PBMCs
from patients
Multicolor fluorescence-activated cell sorting
(FACS) analysis was performed using the following antibodies:
Anti-CD14 (560634, 20 µg/ml), anti-HLA-DR-PerCp (552764, 10 µg/ml),
anti-CD3-APC (565119, 5 µg/ml), anti-CD4-PE (562281, 20 µg/ml) and
anti-CD8-FITC (555366, 20 µg/ml), all supplied by BD Pharmingen,
San Diego, CA, USA. Flow cytometry was performed using a FACS
Calibur™ flow cytometer (BD Biosciences), according to a previously
descibed method (11). Analysis of
the FACS data was performed using FlowJo software (version X.0.7;
TreeStar, Inc., Ashland, OR, USA). Isotype-matched antibodies were
used with all the samples as controls.
Apoptosis assay
The CD4+ and CD8+ T cells were
co-cultured with MDSCs in the upper compartment of a Transwell
plate (EMD Millipore, Billerica, MA, USA) at different ratios
(10,000:0, 10,000:1,000, 10,000:5,000, 10,000:10,000 cells) and
treated with monoclonal antibodies anti-CD3 (catalog no. 555337; BD
Biosciences; 10 µg/ml) for 48 h at 37.0°C and anti-CD8 (catalog no.
557084; BD Biosciences; 20 µg/ml) for 48 h at 37°C. The same
proportions of NCI-H226 tumor cells (10,000 cells/well) were
cultured in the lower compartment of the Transwell plate at 37°C
(Shanghai Shun Biotechnology, Shanghai, China). Following
incubation for 48 h, the cells were collected and stained with
annexin-V-fluorescein isothicyanate and 7-amino-actinomycin D
(eBioscience, Inc., San Diego, CA, USA), respectively.
CD3+ cells were stained with anti-CD8-PerCp (catalog no.
560662; 0.5 mg/ml; BD Biosciences) for 20 min at 20°C to analyze
the apoptosis of CD8+ cells. IFN-γ in the supernatant
was tested using an ELISA kit (RapidBio Laboratory, Calabasas, CA,
USA), according to the manufacturer's protocol.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 6; SPSS, Inc., Chicago, IL, USA). Comparisons
between different groups were analyzed using a Mann-Whitney U test.
All data are presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient clinicopathological
characteristics
The clinicopathological characteristics and clinical
stage of the 78 patients and 30 healthy controls are illustrated in
Table I. There was no significant
difference between the age and gender of the study subjects in the
control group and the lung squamous cell carcinoma group. All
patients were diagnosed with lung squamous cell carcinoma by
clinical methods, imaging, bronchoscopy and pathology. The mean
levels of squamous cell carcinoma (SCC) antigen and
carcinoembryonic antigen (CEA) in the patients with lung squamous
cell carcinoma were 23.8±1.5 µg/l and 135.9±34.1 ng/l,
respectively. The normal reference range of SCC is ≤1.5 µg/l and
the normal reference range of CEA is ≤5 ng/ml.
 | Table I.Clinicopathological characteristics
of patients with lung squamous cell carcinoma and healthy
controls. |
Table I.
Clinicopathological characteristics
of patients with lung squamous cell carcinoma and healthy
controls.
| Clinicopathological
characteristics | Healthy
controls | Patients with lung
squamous cell carcinoma |
|---|
| Total, n | 30 | 78 |
| Age, years (mean ±
SD) | 58.4±8.9 | 63.4±9.2 |
| Gender
(male/female) | 26/4 | 68/10 |
|
Tumor-node-metastasis stage |
|
|
| II | ND | 15 |
|
III | ND | 37 |
| IV | ND | 26 |
| SCC antigen, µg/l
(mean ± SD) | ND | 23.8±1.5 |
| CEA, ng/l (mean ±
SD) | ND | 135.9±34.1 |
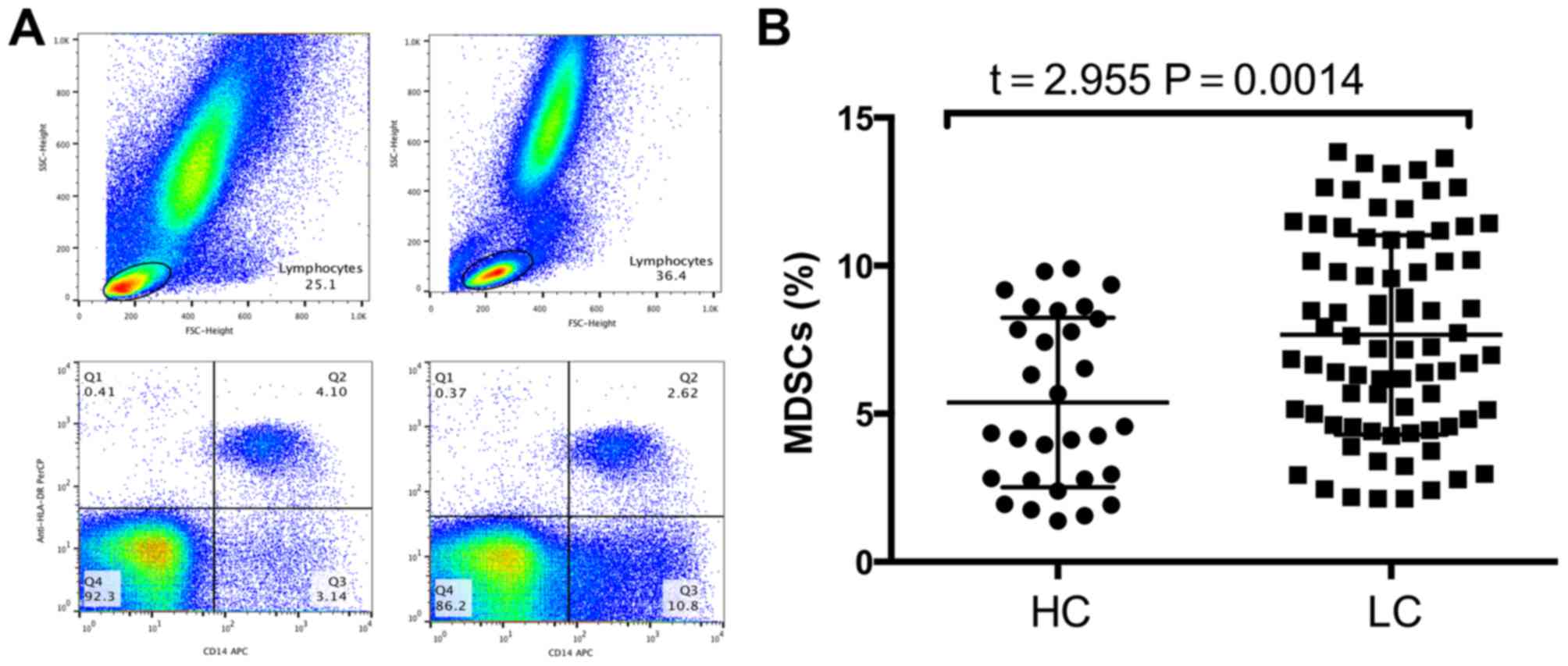
Frequency of MDSCs is significantly
increased in patients with lung squamous cell carcinoma compared
with that of healthy controls
MDSC frequency in the peripheral blood was analyzed
using flow cytometry following density gradient centrifugation. To
exclude debris and dead cells, the lymphocytes were selected. Next,
CD14+ cells were selected, followed by gating of the
HLA-DR−/low population (Fig.
1A). The frequency of MDSCs in the peripheral blood of patients
with lung squamous cell carcinoma was significantly higher compared
with that of healthy controls (5.38±0.52 vs. 7.664±0.38%; P=0.0014;
Fig. 1B).
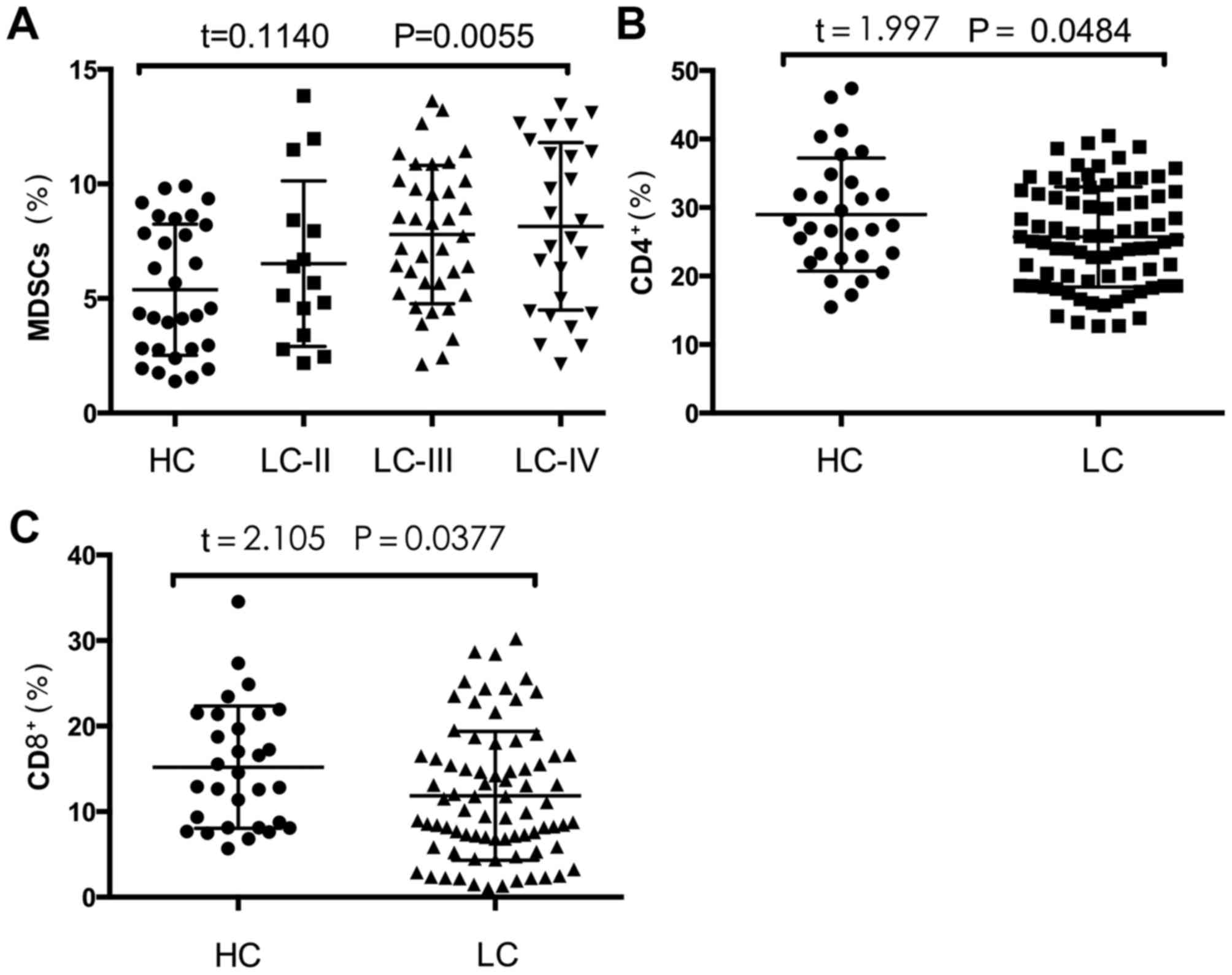
Frequency of MDSCs positively
correlates with disease stage in patients with lung squamous cell
carcinoma
In order to further reveal the role of MDSCs, the
TNM staging method, which is based on tumor size, lymph node
metastasis, tumor-localized metastasis and tumor-distant
metastasis, was used to stage each patient. The frequency of MDSCs
was positively correlated with TNM stage (Fig. 2A). The frequencies of MDSCs in
patients with stage II, III and IV lung squamous cell carcinoma
were 6.51±3.61, 6.51±2.97 and 6.82±3.45%, respectively (P=0.0055;
Fig. 2A).
Frequencies of CD4+ T cells
and CD8+ T cells in PBMCs from patients with lung
squamous cell carcinoma are significantly decreased compared with
those of healthy controls
To further investigate the different functions of
the MDSCs in patients and healthy controls, the percentages of
circulating CD4+ T cells and CD8+ T cells
were measured (Fig. 2B and C). The
percentages of CD4+ T cells and CD8+ T cells
in the PBMCs of patients with lung squamous cell carcinoma were
significantly decreased compared with those of healthy controls
(28.97±1.51 vs. 25.71±0.83%, P=0.0484; and 15.20±1.31 vs.
11.84±0.85%, P=0.0377, respectively; Fig.
2B and C).
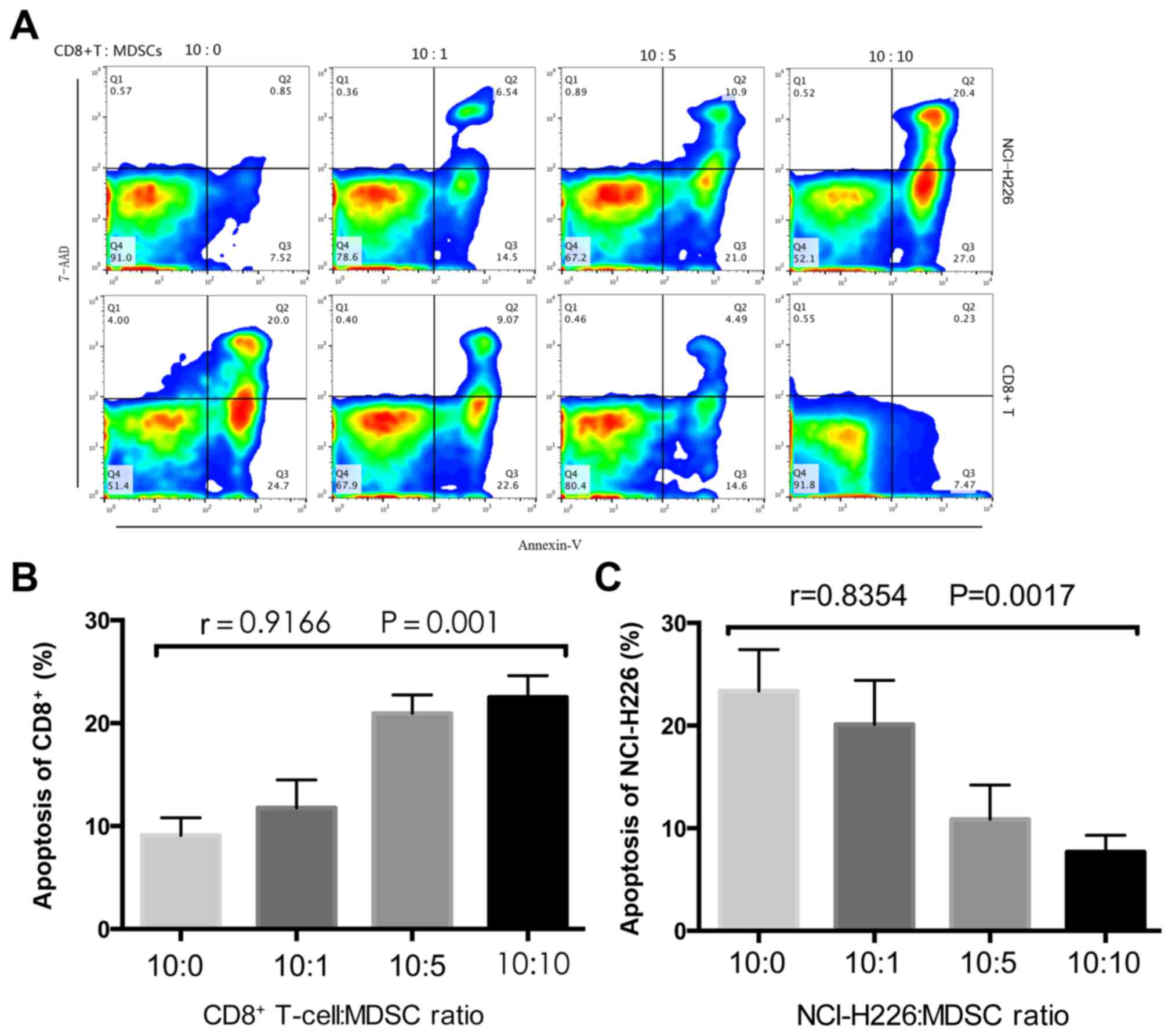
MDSCs inhibit T-cell cytokine
secretion in vitro
To investigate the inhibitory effects that MDSCs
exhibit on CD8+ T cells, MDSCs were sorted and
co-cultured with CD8+ T cells and tumor cells at the
indicated ratios (Fig. 3). Following
48 h, the CD8+ T cells and tumor cells were labeled with
Annexin-V-FITC and 7-AAD respectively, followed by detection using
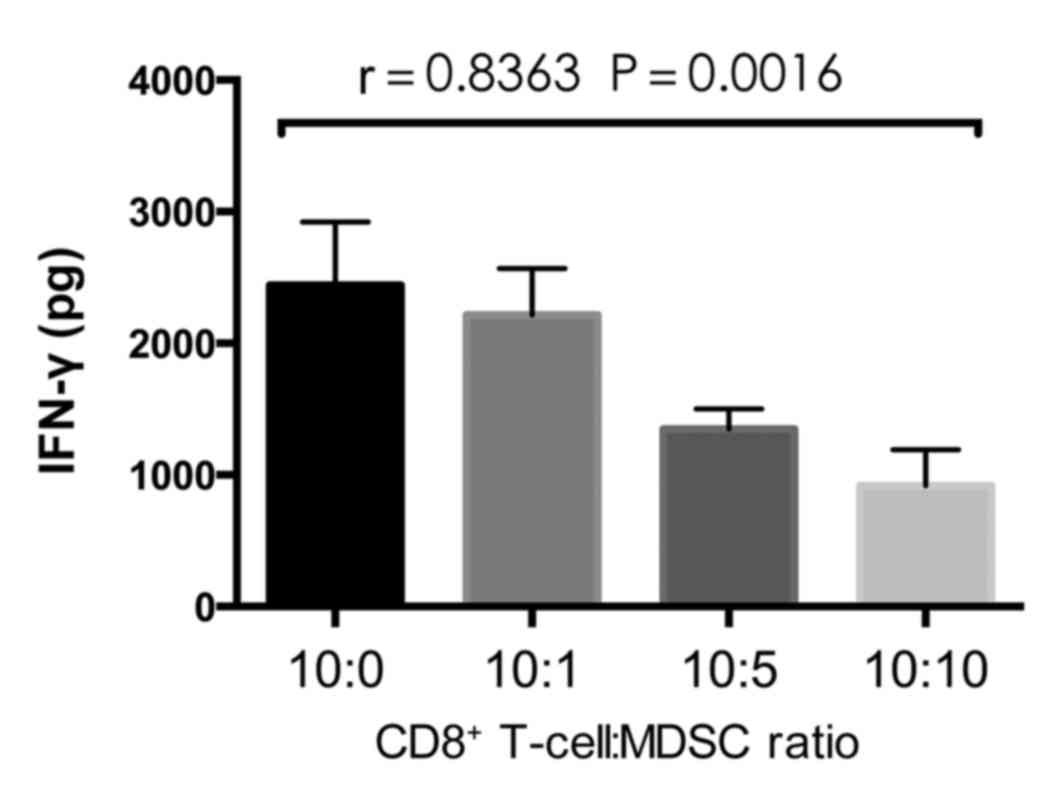
flow cytometry. An ELISA was performed to measure IFN-γ levels in
the co-culture supernatant. The proportion of CD8+
T-cell apoptosis was significantly increased as the proportion of
MDSCs increased (P=0.001; Fig. 3B),
whereas the proportion of tumor cell apoptosis significantly
decreased (P=0.0017; Fig. 3C). The
concentration of IFN-γ significantly decreased with the increase in
MDSCs (P=0.0016; Fig. 4), which
implies that the MDSCs inhibit T cell cytokine secretion.
Discussion
The human immune system has evolved over millions of
years and can protect the body from pathogens, including bacteria,
and parasites (12). Designing an
immunotherapy that can enhance the anticancer effects of the immune
system remains a challenge and immunotherapies have had little
success in clinical trials (13). It
has previously been demonstrated that tumor size is not
significantly affected following the administration of
immunotherapy, which may be due to certain cell types that suppress
the immune response (14,15). An optimistic trend in the treatment of
lung disease, which may change the immune suppressive effect, is
emerging (16). Immunotherapies are
designed to stimulate the immune system in order to restore its
anticancer effects (17,18). The purpose of the current study was to
evaluate whether lung squamous cell carcinoma cells are affected by
MDSCs, as there is currently little information on changes in MDSC
proportion in patients with tumors. A number of recent studies,
performed independently in patients with non-small cell lung cancer
(NSCLC) and other carcinomas, demonstrated that these cells are
immunosuppressive and that their frequency is upregulated in
carcinoma (19–21).
Previous studies have demonstrated the functions of
human MDSCs in hepatocellular, renal carcinoma and prostate cancer
types, among others (22,23). The proliferation and aggregation of
MDSCs in human malignant neoplasms may have an effect on tumor
progression and prognosis. The definition of these
immunosuppressive cells in patients is problematical, since there
is no human homolog of Gr-1 marker, and no correlation between
phenotype and immune suppressive properties has been reported
(11,24). Identifying MDSCs may aid in developing
anticancer treatments that target these cell populations. MDSCs are
a heterogeneous group of cells that include monocytic (M)-MDSCs,
polymorphonuclear MDSCs and immature myeloid cells. These three
subsets can express different combinations of myeloid markers that
are associated with different differentiations of myeloid cells
(CD14, CD15, HLA-DR, CD33, CD11b, CD15 and CD16), and can possess
the immunosuppressive activity of MDSCs (25).
M-MDSCs were the first subtype of human MDSCs to be
identified in the peripheral blood of melanoma patients, and are
defined as CD14+ and HLA-DR−/low cells
(26). M-MDSCs have also been
identified in a number of other cancer types, including renal cell
carcinoma, hepatocellular carcinoma and advanced NSCLC (27). In the present study, following the
exclusion of debris and granulocytes, CD14+ and
HLA-DR−/low cells were selected, as these cells have
been widely studied. The frequency of these cells has been reported
to be elevated in a number of cancer types (28); however, further studies are required
in patients with lung squamous cell carcinoma. Data from the
present study demonstrated that the frequency of MDSCs in the PBMCs
of 78 patients with lung squamous cell carcinoma was significantly
increased compared with that of healthy controls. Additionally, the
frequency of MDSCs was associated with TNM stage, and the levels of
CD4+ and CD8+ T-cells were significantly
decreased in patients with lung squamous cell carcinoma compared
with healthy controls. Previous studies have demonstrated that the
decreased number of lymphocytes described in patients with cancer
is partially due to the immunosuppressive effects of MDSCs
(29,30).
According to previous studies, MDSCs mediate
immunosuppression through a number of molecular mechanisms. MDSCs
deplete essential metabolites for T lymphocytes through the
activation of arginase-1 and nitric oxide synthase 2 (31). High levels of reactive oxygen species
affect T cells by downregulating T-cell surface glycoprotein CD3 ζ
chain expression and reducing cytokine secretion. MDSCs interfere
with T cell migration and viability by expressing the
metalloproteinase disintegrin and metalloproteinase
domain-containing protein 17 that is able to cleave the integrin
CD62L on T cells. MDSCs promote the clonal expansion of
antigen-specific natural regulatory T cells (Tregs) and induce the
conversion of CD4+ T cells into induced Tregs through
the release of transforming growth factor-β (32). In order to further verify the
immunosuppressive effect of MDSCs in lung squamous carcinoma cell
immunity, MDSCs from patients with lung squamous cell carcinoma and
healthy controls were sorted and subsequently cultured with
NCI-H226 cells. As the ratio of MDSCs increased, the proportion of
CD8+ T cell apoptosis significantly increased, whereas
NICI-H226 cell apoptosis signficantly decreased. Additionally, the
concentration of IFN-γ significantly decreased with the increase in
MDSCs, which implies that MDSCs inhibit T cell cytokine secretion.
This confirms the immunosuppressive effect of MDSCs in lung
squamous cell carcinoma (33). These
results may aid in developing novel treatments that inhibit
malignant neoplasm progression and metastasis. It has previously
been reported that it is possible to use MDSCs as a therapeutic
target (34).
The present study investigated the proportion of
peripheral CD14+HLA-DR−/low MDSCs in patients
with different stages of lung squamous cell carcinoma, and the
association between different tumor stages and MDSC function. The
frequency of MDSCs is significantly increased in patients with lung
squamous cell carcinoma. The frequencies of CD4+ T cells
and CD8+ T cells in PBMCs from patients with lung
squamous cell carcinoma were significantly decreased compared with
those from the healthy controls. MDSCs inhibit T-cell cytokine
secretion in vitro. In conclusion, MDSCs participate in the
immune escape of lung squamous cell carcinoma, and may provide a
possible therapeutic strategy for the treatment of this
disease.
References
|
1
|
Zhou C: Lung cancer molecular epidemiology
in China: Recent trends. Transl Lung Cancer Res. 3:270–279.
2014.PubMed/NCBI
|
|
2
|
Land SR, Liu Q, Wickerham DL, Costantino
JP and Ganz PA: Cigarette smoking, physical activity, and alcohol
consumption as predictors of cancer incidence among women at high
risk of breast cancer in the NSABP P-1 trial. Cancer Epidemiol
Biomarkers Prev. 23:823–832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou QH, Fan YG, Bu H, Wang Y, Wu N, Huang
YC, Wang G, Wang XY and Qiao YL: China national lung cancer
screening guideline with low-dose computed tomography (2015
version). Thorac Cancer. 6:812–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motallebnezhad M, Jadidi-Niaragh F,
Qamsari ES, Bagheri S, Gharibi T and Yousefi M: The immunobiology
of myeloid-derived suppressor cells in cancer. Tumour Biol.
37:1387–1406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qu P, Wang LZ and Lin PC: Expansion and
functions of myeloid-derived suppressor cells in the tumor
microenvironment. Cancer Lett. 380:253–256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ochando J, Conde P and Bronte V:
Monocyte-derived suppressor cells in transplantation. Curr
Transplant Rep. 2:176–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haile LA, von Wasielewski R,
Gamrekelashvili J, Krüger C, Bachmann O, Westendorf AM, Buer J,
Liblau R, Manns MP, Korangy F and Greten TF: Myeloid-derived
suppressor cells in inflammatory bowel disease: A new
immunoregulatory pathway. Gastroenterology. 135:871–881, 881.e1-e5.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Detterbeck FC, Postmus PE and Tanoue LT:
The stage classification of lung cancer: Diagnosis and management
of lung cancer, III ed: American College of Chest Physicians
evidence-based clinical practice guidelines. Chest. 143 5
Suppl:e191S–e210S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jett JR, Schild SE, Kesler KA and
Kalemkerian GP: Treatment of small cell lung cancer: Diagnosis and
management of lung cancer, 3rd ed: American College of Chest
Physicians evidence-based clinical practice guidelines. Chest. 143
5 Suppl:e400S–e419S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Travis WD, Brambilla E and Riely GJ: New
pathologic classification of lung cancer: Relevance for clinical
practice and clinical trials. J Clin Oncol. 31:992–1001. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang G, Huang H, Zhu Y, Yu G, Gao X, Xu
Y, Liu C, Hou J and Zhang X: A novel subset of
B7-H3+CD14+HLA-DR-/low myeloid-derived suppressor cells are
associated with progression of human NSCLC. Oncoimmunology.
4:e9771642015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simon AK, Hollander GA and McMichael A:
Evolution of the immune system in humans from infancy to old age.
Proc Biol Sci. 282:pp. 201430852015; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alizadeh D and Larmonier N:
Chemotherapeutic targeting of cancer-induced immunosuppressive
cells. Cancer Res. 74:2663–2668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keskinov AA and Shurin MR: Myeloid
regulatory cells in tumor spreading and metastasis. Immunobiology.
220:236–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laborde RR, Lin Y, Gustafson MP, Bulur PA
and Dietz AB: Cancer vaccines in the world of immune suppressive
monocytes (CD14(+)HLA-DR (lo/neg) cells): The gateway to improved
responses. Front Immunol. 5:1472014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bruchard M and Ghiringhelli F: Impact of
chemotherapies on immunosuppression and discovery of new
therapeutic targets. Bull Cancer. 101:605–607. 2014.(In French).
PubMed/NCBI
|
|
17
|
Kennedy DE and Knight KL: Inhibition of B
lymphopoiesis by adipocytes and IL-1-producing myeloid-derived
suppressor cells. J Immunol. 195:2666–2674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Michaud HA, Eliaou JF, Lafont V, Bonnefoy
N and Gros L: Tumor antigen-targeting monoclonal antibody-based
immunotherapy: Orchestrating combined strategies for the
development of long-term antitumor immunity. Oncoimmunology.
3:e9556842014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albeituni SH, Ding C, Liu M, Hu X, Luo F,
Kloecker G, Bousamra M II, Zhang HG and Yan J: Yeast-derived
particulate beta-glucan treatment subverts the suppression of
myeloid-derived suppressor cells (MDSC) by inducing
polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation
to APC in cancer. J Immunol. 196:2167–2180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koinis F, Vetsika EK, Aggouraki D,
Skalidaki E, Koutoulaki A, Gkioulmpasani M, Georgoulias V and
Kotsakis A: Effect of first-line treatment on myeloid-derived
suppressor cells' subpopulations in the peripheral blood of
patients with non-small cell lung cancer. J Thorac Oncol.
11:1263–1272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang A, Zhang B, Wang B, Zhang F, Fan KX
and Guo YJ: Increased CD14(+)HLA-DR(−/low) myeloid-derived
suppressor cells correlate with extrathoracic metastasis and poor
response to chemotherapy in non-small cell lung cancer patients.
Cancer Immunol Immunother. 62:1439–1451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Umansky V, Sevko A, Gebhardt C and Utikal
J: Myeloid-derived suppressor cells in malignant melanoma. J Dtsch
Dermatol Ges. 12:1021–1027. 2014.(In English, German). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Albeituni SH, Ding C, Liu M, Hu X, Luo F,
Kloecker G, Bousamra M II, Zhang HG and Yan J: Yeast-derived
particulate β-glucan treatment subverts the suppression of
myeloid-derived suppressor cells (MDSC) by inducing
polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation
to APC in cancer. J Immunol. 196:2167–2180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang A, Zhang B, Wang B, Zhang F, Fan KX
and Guo YJ: Increased CD14(+)HLA-DR(−/low) myeloid-derived
suppressor cells correlate with extrathoracic metastasis and poor
response to chemotherapy in non-small cell lung cancer patients.
Cancer Immunol Immunother. 62:1439–1451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang J, Guo W and Liang X: Phenotypes,
accumulation, and functions of myeloid-derived suppressor cells and
associated treatment strategies in cancer patients. Hum Immunol.
75:1128–1137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gutknecht MF and Bouton AH: Functional
significance of mononuclear phagocyte populations generated through
adult hematopoiesis. J Leukoc Biol. 96:969–980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin Y, Huang X, Lynn KD and Thorpe PE:
Phosphatidylserine-targeting antibody induces M1 macrophage
polarization and promotes myeloid-derived suppressor cell
differentiation. Cancer Immunol Res. 1:256–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du Four S, Maenhout SK, Niclou SP,
Thielemans K, Neyns B and Aerts JL: Combined VEGFR and CTLA-4
blockade increases the antigen-presenting function of intratumoral
DCs and reduces the suppressive capacity of intratumoral MDSCs. Am
J Cancer Res. 6:2514–2531. 2016.PubMed/NCBI
|
|
29
|
Liu J, Zhou Y, Huang Q and Qiu L:
CD14+HLA-DRlow/− expression: A novel prognostic factor in chronic
lymphocytic leukemia. Oncol Lett. 9:1167–1172. 2015.PubMed/NCBI
|
|
30
|
Shi G, Wang H and Zhuang X:
Myeloid-derived suppressor cells enhance the expression of
melanoma-associated antigen A4 in a Lewis lung cancer murine model.
Oncol Lett. 11:809–816. 2016.PubMed/NCBI
|
|
31
|
Draghiciu O, Lubbers J, Nijman HW and
Daemen T: Myeloid derived suppressor cells-An overview of combat
strategies to increase immunotherapy efficacy. Oncoimmunology.
4:e9548292015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ostrand-Rosenberg S: Myeloid-derived
suppressor cells: More mechanisms for inhibiting antitumor
immunity. Cancer Immunol Immunother. 59:1593–1600. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Solito S, Marigo I, Pinton L, Damuzzo V,
Mandruzzato S and Bronte V: Myeloid-derived suppressor cell
heterogeneity in human cancers. Ann N Y Acad Sci. 1319:47–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Sanctis F, Solito S, Ugel S, Molon B,
Bronte V and Marigo I: MDSCs in cancer: Conceiving new prognostic
and therapeutic targets. Biochim Biophys Acta. 1865:35–48.
2016.PubMed/NCBI
|