Introduction
Colon cancer is one of the most common malignant
tumors of the digestive system. With economic development and
changes in lifestyle, the incidence of colon cancer is increasing,
endangering human life and health (1). Chemotherapy is an important treatment
strategy for colon cancer (2);
however, it is a long process involving drug combinations and the
administration of large doses of drugs. Tumor drug resistance has
become increasingly frequent and is a problem for the treatment of
colon cancer and other malignant tumors (3). Notably, 90% of cancer mortalities are
associated with tumor drug resistance (4), therefore the underlying molecular
mechanisms regulating the development of drug resistance are a key
focus of malignant tumor research. Mechanisms of drug resistance in
tumors include the modification of drug targets, repair of damaged
cells, and the activation or inhibition of cell death signaling
pathways. These processes can result from gene mutations, deletions
or amplifications, in addition to epigenetic changes that occur via
abnormal DNA methylation or the post-transcriptional regulation of
microRNAs (miRNAs) (5–7).
Vincristine (VCR), the most frequently used
chemotherapy drug in the clinical treatment of colon cancer, is a
cell cycle-specific drug that binds to tubulin, thereby inhibiting
the assembly of microtubule structures and arresting mitosis in
metaphase (8). The tumor suppressor
breast cancer susceptibility gene 1 (BRCA1) confers increased
susceptibility to breast and ovarian cancers, and mutations in the
BRCA1 gene are present in ≤50% of inherited breast cancers
(9,10). In women <50 years old, the risk of
colorectal cancer is increased in carriers of BRCA1 mutations
(11).
In the current study, the expression of BRCA1 was
verified by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis and western blotting, to investigate
the role of BRCA1 in modulating VCR resistance. The findings
reported here demonstrate potential candidate targets for gene
therapy in VCR-resistant colon cancer.
Materials and methods
Cell culture
Human colon cancer HCT-8 cells were purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Cells were maintained in Dulbecco's modified Eagle's medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 10%
fetal calf serum, 100 µg/ml penicillin, and 100 µg/ml streptomycin
at 37°C with 5% CO2 in a humidified incubator. The cells
were subcultured every 2–3 days through treatment with 0.02% EDTA
and 0.1% trypsin.
Establishment of a VCR-resistant colon
cancer cell line
HCT-8/V cells lines were established through culture
with gradually increasing VCR concentrations. The VCR-sensitive
HCT-8 cell line was cultured in medium containing 5 ng/ml VCR,
following which the VCR concentration was gradually increased to
10, 100, 1,000, and finally 2,000 ng/ml. At each VCR concentration,
some drug resistance was acquired by the cells and those cells that
grew well were cloned by limiting dilution for the next round of
selection at a higher drug concentration. Finally, HCT-8 cells were
cultured in 2,000 ng/ml VCR >20 generations and VCR
supplementation was withdrawn 1 week prior to when the cells were
subjected to further experiments.
MTT assay
Cells were maintained at 37°C with or without VCR
for 24, 48 and 72 h. MTT reagent (5 mg/ml) was added to the medium
and cells were further incubated for 4 h, following which the
culture medium was removed and dimethylsulphoxide (DMSO) was added
to dissolve the crystalline product that had formed. The absorbance
(optical density; OD) in each well was measured using a UV visible
spectrophotometric plate reader (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 490 nm. Cell growth inhibition rates were
calculated as [OD (control)-OD (experimental)/OD (control) ×100%],
allowing the half-maximal inhibitory concentration
(IC50) values to be calculated from triplicate
experiments.
RNA extraction and RT-qPCR
analysis
Cells were collected during the exponential growth
phase and total RNA was isolated using the RNeasy kit (Qiagen,
Inc., Valencia, CA, USA). First strand complementary DNA was
synthesized from total RNA as previously described (12). The PCR reactions were performed on an
Mx3000P qPCR system (Stratagene; Agilent Technologies, Inc., Santa
Clara, CA, USA) and GAPDH was used as an inter-sample control.
RT-qPCR was performed as previously described (13). Primer sequence information is provided
in Table I.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene name | Primer sequence
(5′-3′) | Product length
(bp) |
|---|
| BRCA1 | F:
AATTAGCCGGTCATGGTG |
|
|
| R:
GCTGGAGTGCCGTGGTAT | 124 |
| GAPDH | F:
ACCCAGAAGACTGTGGATGG |
|
|
| R:
TTCAGCTCAGGGATGACCTT | 125 |
Western blotting
Cellular proteins were extracted with cell lysis
buffer (CST Bio, Inc., Shanghai, China) containing 1 mM
phenylmethylsulfonyl fluoride. Equal amounts (25 µg) of protein per
lane were fractionated on a 7% gel by SDS-PAGE as previously
described (14), transferred to a
polyvinylidene difluoride membrane, and incubated overnight with
primary antibodies against BRCA1 (B1310; 1:1,000 dilution,
Sigma-Aldrich; Merck KGaA), washed and incubated for 1 h with
β-actin (AC-74; 1:2,000 dilution, Sigma-Aldrich; Merck KGaA) at
room temperature. Blot signal Antibody labeling was detected by an
enhanced chemiluminescence system and subsequently exposed to
radiographic film. Blot signal intensities were quantified using
ImageJ 1.37 software (National Institutes of Health, Bethesda, MD,
USA) following normalization to the corresponding loading
controls.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 13.0; SPSS, Inc., Chicago, IL, USA) and are
expressed as the mean ± standard deviation. A Student's t-test with
SPSS 17.0 software was used for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Establishment of VCR-resistant colon
cancer cells
The VCR-resistant cell line referred to as HCT-8/V
was established by gradually increasing the concentration of VCR
during cell culture. The HCT-8/V cells grew well in medium
containing 2,000 ng/ml VCR which was 12.7 times the concentration
tolerated by the HCT-8 cells. The HCT-8 and HCT-8/V colon cancer
cells were subsequently treated with different concentrations of
VCR, and the IC50 of VCR in the HCT-8 and HCT-8/V cells
was 13.56 and 165.49 µg/ml, respectively (data not shown). The MTT
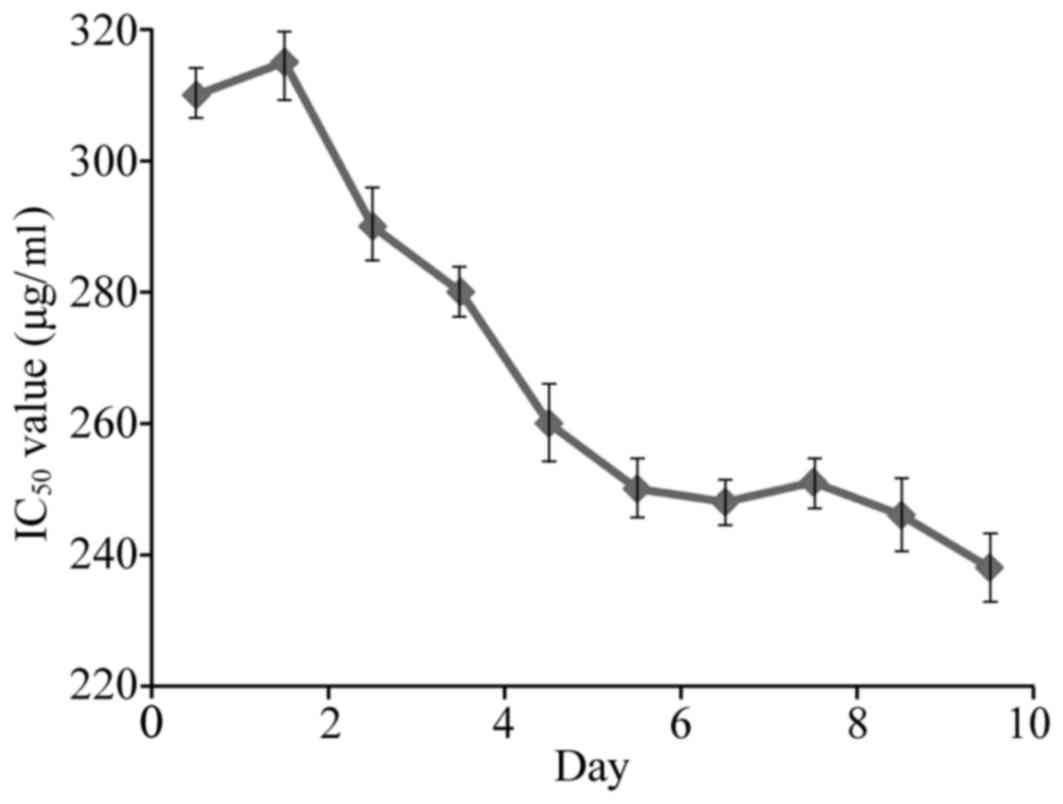
assay results indicate that the resistance phenotype of the cells
was stable 6 days following withdrawal of VCR supplementation in
the culture medium (Fig. 1).
BRCA1 mRNA expression is increased in
VCR-resistant colon cancer cells
Total RNA was isolated from the VCR-resistant and
VCR-sensitive colon cancer cells, and the expression levels of
BRCA1 in these cells was assessed using RT-qPCR analysis with
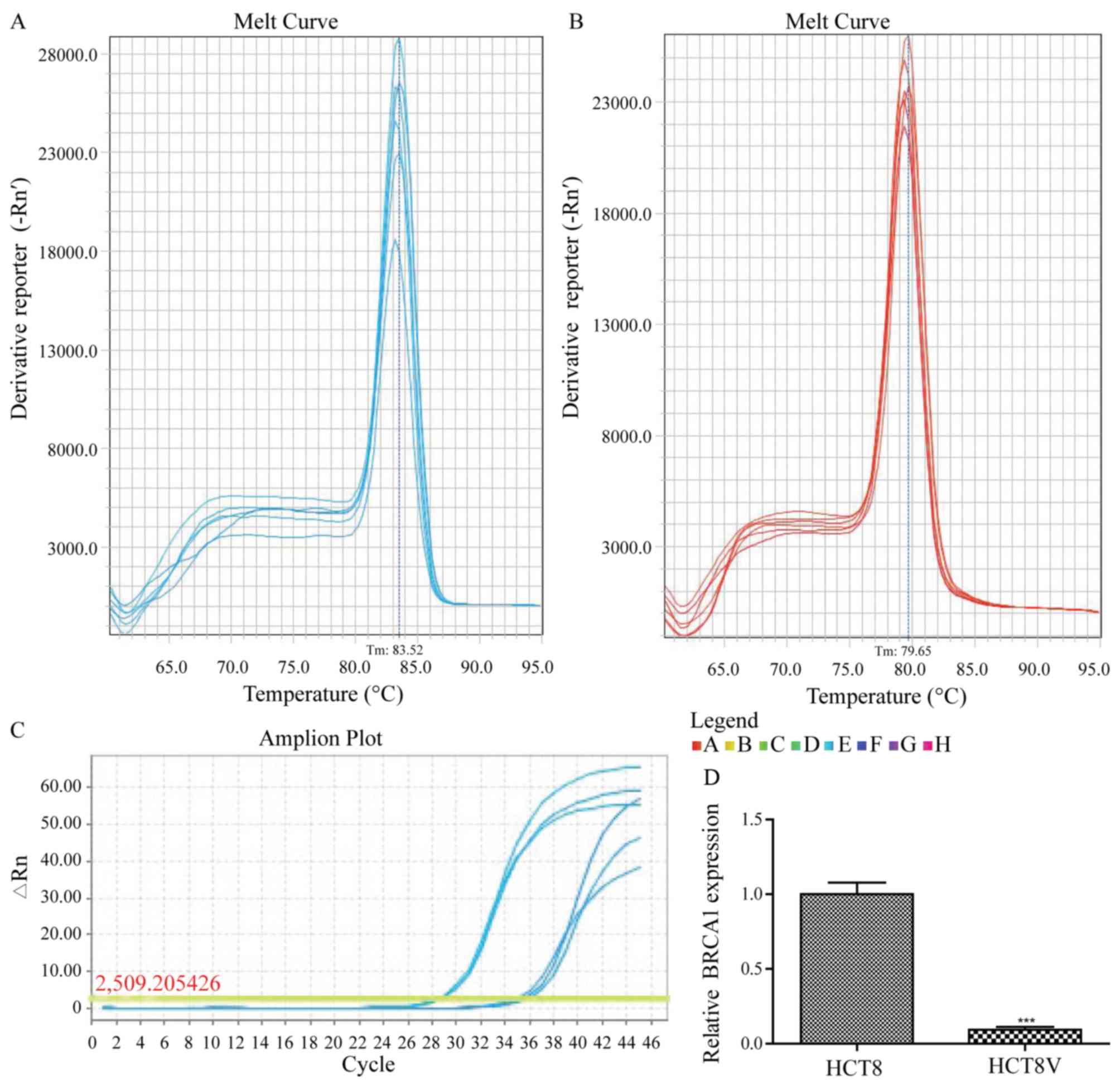
gene-specific primers (Table I). The
melting curves of BRCA1 and GAPDH amplification are illustrated in
Fig. 2A and B, respectively. The
relative expression of BRCA1 was significantly lower (10.72-fold)
in VCR-sensitive HCT-8 cells compared with the VCR-resistant cells
(0.09333±0.01856 vs. 1.0000±0.07810; P<0.01; Fig. 2C).
BRCA1 protein expression is increased
in VCR-resistant colon cancer cells
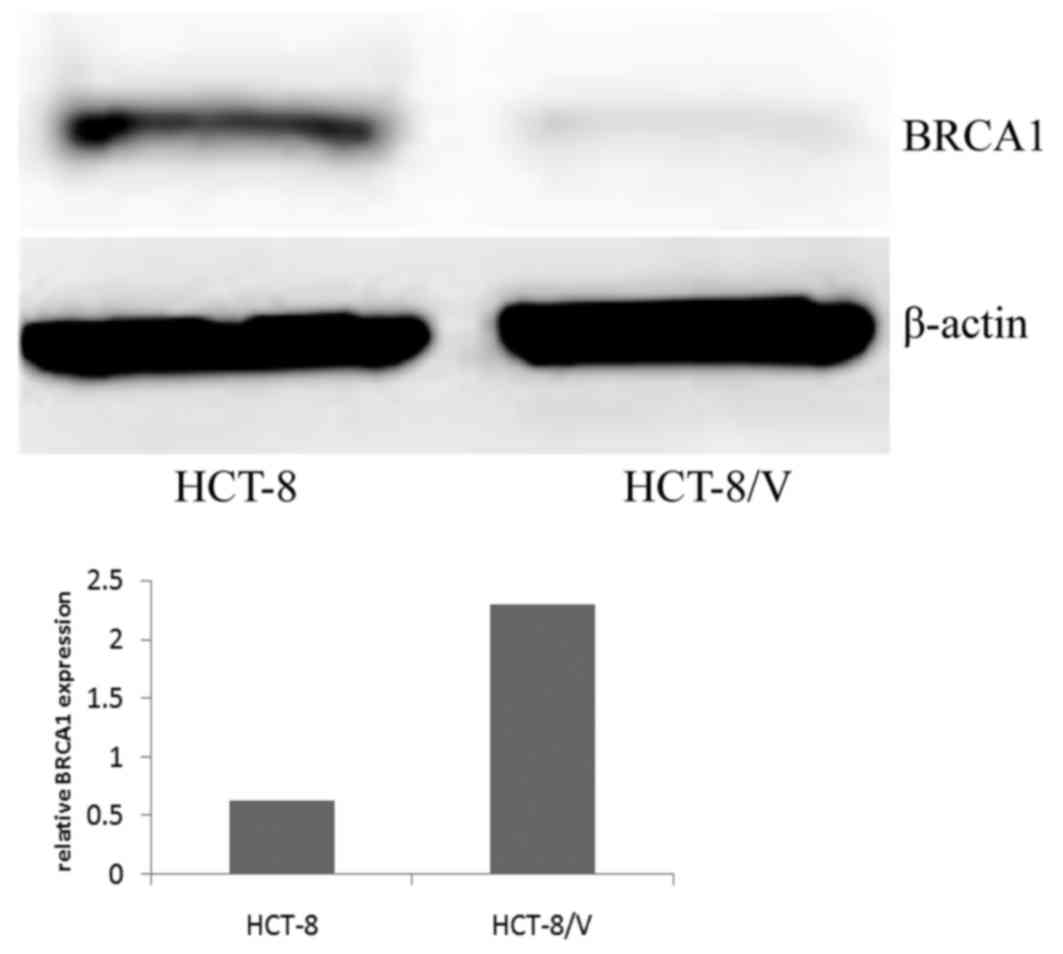
Total protein was extracted from the HCT-8 and
HCT-8/V cells for western blot analysis. BRCA1 expression was
normalized to that of the reference protein (β-actin) to determine
the relative expression levels. The expression of BRCA1 was
significantly lower in the drug-sensitive cells compared with their
VCR-resistant counterparts. BRCA1 protein expression in the HCT-8/V
cells (2.2937±0.5421) was 3.68 times higher compared with that in
the drug-sensitive cells (0.6233±0.3654; P<0.05; Fig. 3).
Discussion
VCR is widely used in the clinical treatment of
leukemia, lung cancer and other malignant tumors; however, during
VCR use, tumors gradually exhibit resistance to the drug (15–19). The
molecular mechanism underlying VCR resistance is complex and
involves a number of genes, including insulin-like growth
factor-binding protein 7 and multidrug resistance protein 1, in
addition to long non-coding RNA (20–22). In
the present study, a VCR-resistant cell line (HCT-8/V) was
established to explore the mechanism of drug resistance in colon
cancer cells to VCR by gradually increasing the drug concentration
during cell culture. The concentration of VCR tolerated by the
HCT-8/V cells was 2,000 ng/ml, which was 12.7 times the
concentration tolerated by the HCT-8 cells.
Germline mutations in the BRCA1 and BRCA2 genes
account for 5% of all breast cancers and ~80% of families with
these mutations suffer from hereditary breast cancer and ovarian
cancer (23). Lohse et al
(24) identified that BRCA1 and BRCA2
mutant xenografts were significantly more sensitive to cisplatin
compared with the control, while the BRCA1 and BRCA2 wild type
models exhibited sensitivity to gemcitabine but not to cisplatin.
However, BRCA1 expression was significantly decreased in the HCT-8
cells compared with in the drug-resistant cells.
Following treatment with vinca alkaloids, including
vincristine, tumor cells may acquire drug resistance in the
following ways: Modifications to the action site of drugs,
including tubulin sequence mutations or changes in the cytoskeletal
protein (25); high expression of
drug efflux pumps resulting in reduced intracellular drug
concentrations (26); activation of
the detoxification system by other non-toxic compounds (27); or inhibition of apoptotic signal
transduction resulting in reduced apoptosis (28). The findings reported in the present
study demonstrate that the drug resistance of colon cancer cells
can increase the expression of BRCA1. Further investigation into
VCR-resistance in these cells is required to improve understanding
of the underlying molecular mechanisms of chemotherapeutic drug
resistance in tumors.
References
|
1
|
Das V, Kalita J and Pal M: Predictive and
prognostic biomarkers in colorectal cancer: A systematic review of
recent advances and challenges. Biomed Pharmacother. 87:8–19. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pabla B, Bissonnette M and Konda VJ: Colon
cancer and the epidermal growth factor receptor: Current treatment
paradigms, the importance of diet and the role of chemoprevention.
World J Clin Oncol. 6:133–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shekhar MP: Drug resistance: Challenges to
effective therapy. Curr Cancer Drug Targets. 11:613–623. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kimura M, Endo H, Inoue T, Nishino K,
Uchida J, Kumagai T, Kukita Y, Kato K, Imamura F and Inoue M:
Analysis of ERBB ligand-induced resistance mechanism to crizotinib
by primary culture of lung adenocarcinoma with EML4-ALK fusion
gene. J Thorac Oncol. 10:527–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Ngo JA, Wetzel MD and Marchetti
D: Heparanase mediates a novel mechanism in lapatinib-resistant
brain metastatic breast cancer. Neoplasia. 17:101–113. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borges S, Döppler HR and Storz P: A
combination treatment with DNA methyltransferase inhibitors and
suramin decreases invasiveness of breast cancer cells. Breast
Cancer Res Treat. 144:79–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma J, Fang B, Zeng F, Ma C, Pang H, Cheng
L, Shi Y, Wang H, Yin B, Xia J, et al: Down-regulation of miR-223
reverses epithelial-mesenchymal transition in gemcitabine-resistant
pancreatic cancer cells. Oncotarget. 6:1740–1749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Camplejohn RS: A critical review of the
use of vincristine (VCR) as a tumour cell synchronizing agent in
cancer therapy. Cell Tissue Kinet. 13:327–335. 1980.PubMed/NCBI
|
|
9
|
Antoniou A, Pharoah PD, Narod S, Risch HA,
Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, et
al: Average risks of breast and ovarian cancer associated with
BRCA1 or BRCA2 mutations detected in case Series unselected for
family history: A combined analysis of 22 studies. Am J Hum Genet.
72:1117–1130. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh KK, Shukla PC, Quan A, Al-Omran M,
Lovren F, Pan Y, Brezden-Masley C, Ingram AJ, Stanford WL, Teoh H
and Verma S: BRCA1 is a novel target to improve endothelial
dysfunction and retard atherosclerosis. J Thorac Cardiovasc Surg.
146:949–960. e4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Phelan CM, Iqbal J, Lynch HT, Lubinski J,
Gronwald J, Moller P, Ghadirian P, Foulkes WD, Armel S, Eisen A, et
al: Incidence of colorectal cancer in BRCA1 and BRCA2 mutation
carriers: Results from a follow-up study. Br J Cancer. 110:530–534.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang TY, Zhang QQ, Zhang X, Sun QL, Zhao
CP and Wang XY: The effect of recombinant lentiviral vector
encoding miR-145 on human esophageal cancer cells. Tumour Biol.
36:9733–9738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang JH, Du AL, Wang L, Wang XY, Gao JH
and Wang TY: Episomal lentiviral vector-mediated miR-145
overexpression inhibits proliferation and induces apoptosis of
human esophageal carcinomas cells. Recent Pat Anticancer Drug
Discov. 11:453–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong WH, Wang TY, Wang F and Zhang JH:
Simple, time-saving dye staining of proteins for sodium dodecyl
sulfate-polyacrylamide gel electrophoresis using Coomassie blue.
PLoS One. 6:e223942011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chao MW, Lai MJ, Liou JP, Chang YL, Wang
JC, Pan SL and Teng CM: The synergic effect of vincristine and
vorinostat in leukemia in vitro and in vivo. J Hematol Oncol.
8:822015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung SO, Kim SY, Kim JO, Jung SS, Park HS,
Moon JY, Kim SM and Lee JE: Promising effects of 3rd line
cyclophosphamide, adriamycin and vincristine (CAV) and 4th line
ifosfamide and carboplatin chemotherapy in refractory small cell
lung cancer. Thorac Cancer. 6:659–663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Y and Qiu L: Nonspecifically enhanced
therapeutic effects of vincristine on multidrug-resistant cancers
when coencapsulated with quinine in liposomes. Int J Nanomedicine.
10:4225–4237. 2015.PubMed/NCBI
|
|
18
|
Qiu JG, Zhang YJ, Li Y, Zhao JM, Zhang WJ,
Jiang QW, Mei XL, Xue YQ, Qin WM, Yang Y, et al: Trametinib
modulates cancer multidrug resistance by targeting ABCB1
transporter. Oncotarget. 6:15494–15509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsubaki M, Takeda T, Ogawa N, Sakamoto K,
Shimaoka H, Fujita A, Itoh T, Imano M, Ishizaka T, Satou T and
Nishida S: Overexpression of survivin via activation of ERK1/2,
Akt, and NF-κB plays a central role invincristine resistance in
multiple myeloma cells. Leuk Res. 39:445–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bartram I, Erben U, Ortiz-Tanchez J,
Blunert K, Schlee C, Neumann M, Heesch S and Baldus CD: Inhibition
of IGF1-R overcomes IGFBP7-induced chemotherapy resistance in
T-ALL. BMC Cancer. 15:6632015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tivnan A, Zakaria Z, O'Leary C, Kögel D,
Pokorny JL, Sarkaria JN and Prehn JH: Inhibition of multidrug
resistance protein 1 (MRP1) improves chemotherapy drug response in
primary and recurrent glioblastoma multiforme. Front Neurosci.
9:2182015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun QL, Zhao CP, Wang TY, Hao XB, Wang XY,
Zhang X and Li YC: Expression profile analysis of long non-coding
RNA associated with vincristine resistance in colon cancer cells by
next-generation sequencing. Gene. 572:79–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elia AE and Elledge SJ: BRCA1 as tumor
suppressor: Lord without its RING? Breast Cancer Res. 14:3062012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lohse I, Borgida A, Cao P, Cheung M,
Pintilie M, Bianco T, Holter S, Ibrahimov E, Kumareswaran R,
Bristow RG, et al: BRCA1 and BRCA2 mutations sensitize to
chemotherapy in patient-derived pancreatic cancer xenografts. Br J
Cancer. 113:425–432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dumontet C, Jaffrezou JP, Tsuchiya E,
Duran GE, Chen G, Derry WB, Wilson L, Jordan MA and Sikic BI:
Resistance to microtubule-targeted cytotoxins in a K562 leukemia
cell variant associated with altered tubulin expression and
polymerization. Bull Cancer. 91:E81–E112. 2004.PubMed/NCBI
|
|
26
|
Cousein E, Barthélémy C, Poullain S, Simon
N, Lestavel S, Williame V, Joiris E, Danel C, Clavey V, Brossard D,
et al: P-glycoprotein and cytochrome P450 3A4 involvement in
risperidone transport using an in vitro Caco-2/TC7 model and an in
vivo model. Prog Neuropsychopharmacol Biol Psychiatry. 31:878–886.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu CL, Lim YP and Hu ML: Fucoxanthin
attenuates rifampin-induced cytochrome P450 3A4 (CYP3A4) and
multiple drug resistance 1 (MDR1) gene expression through pregnane
X receptor (PXR)-mediated pathways in human hepatoma HepG2 and
colon adenocarcinoma LS174T cells. Mar Drugs. 10:242–257. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu CJ, Ou JH, Wang ML, Jialielihan N and
Liu YH: Elevated survivin mediated multidrug resistance and reduced
apoptosis in breast cancer stemcells. J BUON. 20:1287–1294.
2015.PubMed/NCBI
|