Introduction
A total of ~10% of acute myeloid leukemia (AML)
develops following exposure to chemotherapy and/or radiation for a
different primary malignancy and is categorized as therapy-related
AML (t-AML) in the current World Health Organization (WHO)
classification (1). The most common
type of t-AML, which occurs subsequent to exposure to alkylating
agents and/or radiation with a latency period of 5–10 years, is
frequently preceded by therapy-related myelodysplastic syndrome
(t-MDS) and is accompanied with an unbalanced loss of genetic
material, often involving chromosome 5 and/or 7 (1). The less common type of t-AML, occurring
following treatment with agents targeting topoisomerase II,
exhibits a shorter latency period of 1–5 years without the
preceding myelodysplastic phase and is often associated with
balanced recurrent chromosomal translocations, frequently involving
the mixed-lineage leukemia 1 (MLL) gene located at 11q23 or
the runt related transcription factor 1 (RUNX1, or
alternatively designated as AML1) gene at 21q22 (1). Although antimetabolites, including
fludarabine, are also implicated in therapy-related hematological
malignancies, it remains unknown if methotrexate (MTX),
particularly when used at a low dose for the treatment of
rheumatoid arthritis, serves a role in development of MDS/AML.
The t(3;21)(q26.2;q22) translocation is a rare
chromosomal abnormality exhibited in <1% of AML/MDS, primarily
t-MDS/AML or in the blastic crisis phase of chronic myelogenous
leukemia (2–5). Chromosome 3q26.2 abnormalities typically
activate the ecotropic virus integration site 1 (EVI1) gene,
which has been implicated in the pathogenesis of AML and is
associated with a poor prognosis (6–8). EVI1 is a
nuclear transcription factor implicated in the regulation of
proliferation and maintenance of hematopoietic stem cells. EVI1
also exists as the fusion protein MDS1/EVI1 (ME), which is
generated by the alternative splicing of the third exon of
MDS1 to the second exon of EVI1 with the two genes
located nearby to form the MDS1-EVI1 complex locus
(MECOM) on 3q26.2 (6,7). The most common types of 3q26.2
abnormalities are inv (3)
(q21q26.2)/t(3;3)(q21;q26.2), which define a category of AML in the
WHO classification and relocate a distal enhancer of the GATA
binding protein 2 (GATA2) gene to a region between
MDS1 and EVI1, which ectopically activates
EVI1 (1,7,9,10). In contrast, t(3;21)(q26.2;q22) results
in the translocation of a part of RUNX1 at 21q22 to
MECOM and generates various fusion transcripts (2,4). Although
these fusion transcripts, including AML1/MDS1/EVI1
(AME), have been analyzed in several cases of myeloid
neoplasms associated with t(3;21)(q26.2;q22), the expression of the
aberrant fusion protein products in primary leukemic cells has not
previously been confirmed (3,11–16).
The present study examined a patient with MDS/AML
with t(3;21)(q26.2;q22) that developed not with exposure to
chemotherapy or radiotherapy for malignancy, but following
treatment with low-dose MTX for rheumatoid arthritis. The fusion
transcripts of RUNX1/MECOM were analyzed, and two
alternatively-spliced AME transcripts with or without
RUNX1 exon 6 sequences were identified. Additionally, the
expression of the protein products of two of these fusion
transcripts was confirmed, and the expression of
CCAAT/enhancer-binding protein α (CEBPα) and other proteins
implicated in AME-mediated leukemogenesis in leukemic cells from
the patient was evaluated.
Case report
Materials and methods
Ethics statement
The present study was approved by the Ethical
Committee of Tokyo Medical and Dental University (Tokyo, Japan).
Written informed consent was obtained from the patient in
compliance with the Declaration of Helsinki.
Nested reverse transcription-polymerase chain
reaction (RT-PCR) and sequencing analyses of the AME fusion
transcripts
The peripheral blood mononuclear cells from the
patient were obtained by Ficoll-Hypaque density gradient
centrifugation (at 500 × g for 15 min at room temperature). RNA
samples were prepared from the peripheral blood mononuclear cells
of the patient or HL60 cells, used as a negative control, using the
RNeasy® Mini kit (Qiagen GmbH, Hilden, Germany),
according to the manufacturer's protocol, and subjected to RT-PCR
using RUNX1 and EVI1 primers or primers for
β-actin as a control. The RT-PCR for AME transcripts
were performed as described previously (11) using the following primers:
RUNX1 external forward, 5′-AAGTCGCCACCTACCACAGA-3′ and
internal forward, 5′-GCCATCAAAATCACAGTGGA-3′; and EVI1
external reverse, 5′-CCGGCGCCATAGTTTCATGC-3′; and internal reverse,
5′-GGATAGTCTTCGCTCTTCAT-3′. The primers used for β-actin
were: Forward, GGAGAAGCTGTGCTACGTCGCCC; and reverse,
TACATGGTGGTGCCGCCAGACAG. The RT-PCR products were subjected to
agarose gel electrophoresis and stained with ethidium bromide. For
direct sequencing analysis of the AME fusion transcripts,
two species of nested RT-PCR products were isolated from the gel
using the MinElute® Gel Extraction kit (Qiagen GmbH),
according to the manufacturer's protocol, and sequenced using the
same internal primers.
Western blot analysis
The peripheral blood mononuclear cells from the
patient and human leukemic HEL and MOLM-1 cells, obtained from the
Fujisaki Cell Center (Okayama, Japan) and cultured in RPMI-1640
medium (Wako Pure Chemicals Industries, Ltd., Osaka, Japan)
containing 10% fetal calf serum (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C in a 5% CO2 incubator, were
lysed and subjected to western blot analysis as described
previously (17). Antibodies directed
against RUNX1 (catalog no., CS4336), EVI1 (catalog no., CS2593) and
calreticulin (catalog no., CS12238) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Antibodies directed
against CEBPα (catalog no., SC61) and heat shock protein 90 (HSP90;
catalog no., SC13119) were from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). These first antibodies were used at 0.2
µg/ml at 4°C in overnight incubation, while the relevant secondary
antibodies (HRP-linked anti-rabbit or -mouse IgG; NA934 or NA9310,
respectively, from GE Healthcare Japan, Tokyo, Japan) were used at
0.2 µg/ml at room temperature for 1 h.
Case results
An 84-year-old Japanese woman was referred to the
Department of Hematology of the Graduate School of Medical and
Dental Sciences at Tokyo Medical and Dental University (Tokyo,
Japan) in September 2013 due to anemia and the appearance of blasts
in the peripheral blood. The patient had been diagnosed with
rheumatoid arthritis in 1997 and subsequently treated with
bucillamine from 1997 until unidentified time, actarit between
August 2001 and February 2002, salazosulfapyridine between February
2002 and September 2002 and penicillamine between September 2002
and June 2005, although clinical information on the dosages of
these drugs was not available. In 2004, treatment with low-dose MTX
(weekly doses of 6–10 mg, adjusted by clinical and laboratory
findings, divided into 2 or 3 doses orally every 12 h) and
prednisolone was started. In 2006, the patient was diagnosed with
lung cancer and underwent radical surgery without any chemotherapy
or radiotherapy. As the patient developed anemia and neutropenia in
August 2010, MTX was discontinued and substituted for tacrolimus
(daily doses of 1–2 mg, adjusted by clinical and laboratory
findings, orally once a day). The duration of the MTX therapy was
75 months and the accumulated dose was 2,204 mg. Despite stopping
MTX treatment, the patient's anemia gradually progressed, with the
hemoglobin level decreasing to 6.6 g/dl (normal range, 12.0–15.0
g/dl) in September 2013, when myeloblasts and hypogranular
neutrophils appeared in the peripheral blood.
On the patient's first visit to the Department of
Hematology (Graduate School of Medical and Dental Sciences, Tokyo
Medical and Dental University) the white blood cell count was
3.7×109/l (normal range, 3.6–9.3×109/l) with
an appearance of 3% blasts, the hemoglobin level was 6.1 g/dl, and
the platelet count was 155×109/l (normal range,
120–410×109/l). Bone marrow examination demonstrated
slightly hypercellular marrow with 6.2% blast (normal range,
0.1–0.7%), pseudo-Pelger-Huet anomaly, hypogranular neutrophils,
megaloblastoid changes, increased ringed sideroblasts (31%), and
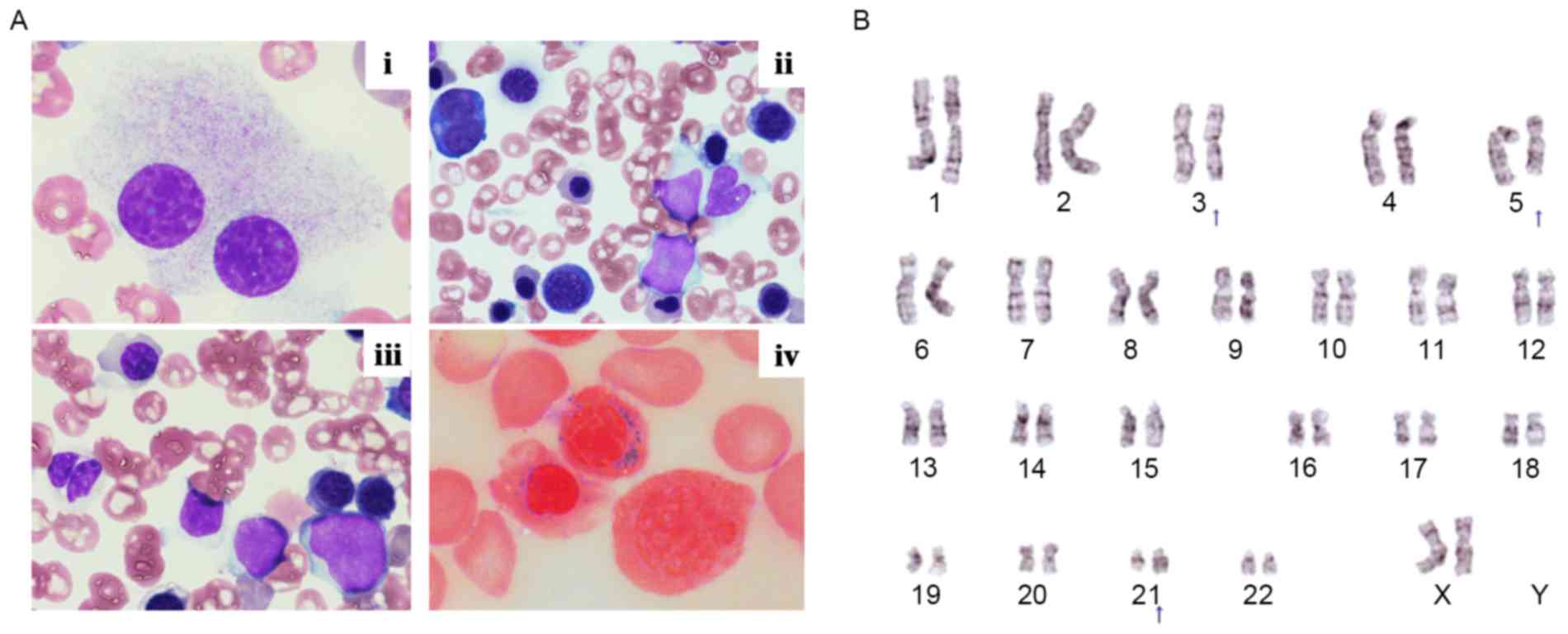
micromegakaryocytes (Fig. 1A). Flow
cytometric analysis demonstrated that the blasts were positive for
cluster of differentiation (CD)13 (82.2%), CD33 (43.2%, dimly
expressed), and CD34 (41.7%), and partially positive for CD7
(18.5%). Cytogenetic analysis illustrated that the karyotype of the
bone marrow cells was as follows: 46, XX, t(3;21)(q26.2;q22)[1]/46,
idem, del(5)(q?) (16) (Fig. 1B).
The position of deletion in 5q [(5)(q?)] could not be determined more
precisely most likely because of the quality of metaphase samples
obtained. Thus, t(3;21)(q26.2;q22) was observed in all the cells in
metaphase (17) that were analyzed.
In addition, the RT-PCR analysis using AML1 and EVI1
primers revealed two AME bands in gel electrophoresis (Fig. 2A). These RT-PCR products were isolated
from the agarose gel and sequenced, which revealed two types of
AML1/MDS1/EVI1 fusion transcripts, in which either exon 5 or
6 of RUNX1 was directly fused with exon 2 of MECOM,
as demonstrated in Fig. 2B.
Consistent with this, two AME fusion proteins with apparent
molecular masses of 210 and 186 kDa were detected in bone marrow
mononuclear cells by western blotting with antibodies directed
against EVI1 and RUNX1 (Fig. 2C).
EVI1 was detectable in HEL and MOLM-1 cells, but not in the
patient's leukemic cells, which also expressed RUNX1, CEBPα and
calreticulin at readily detectable levels.
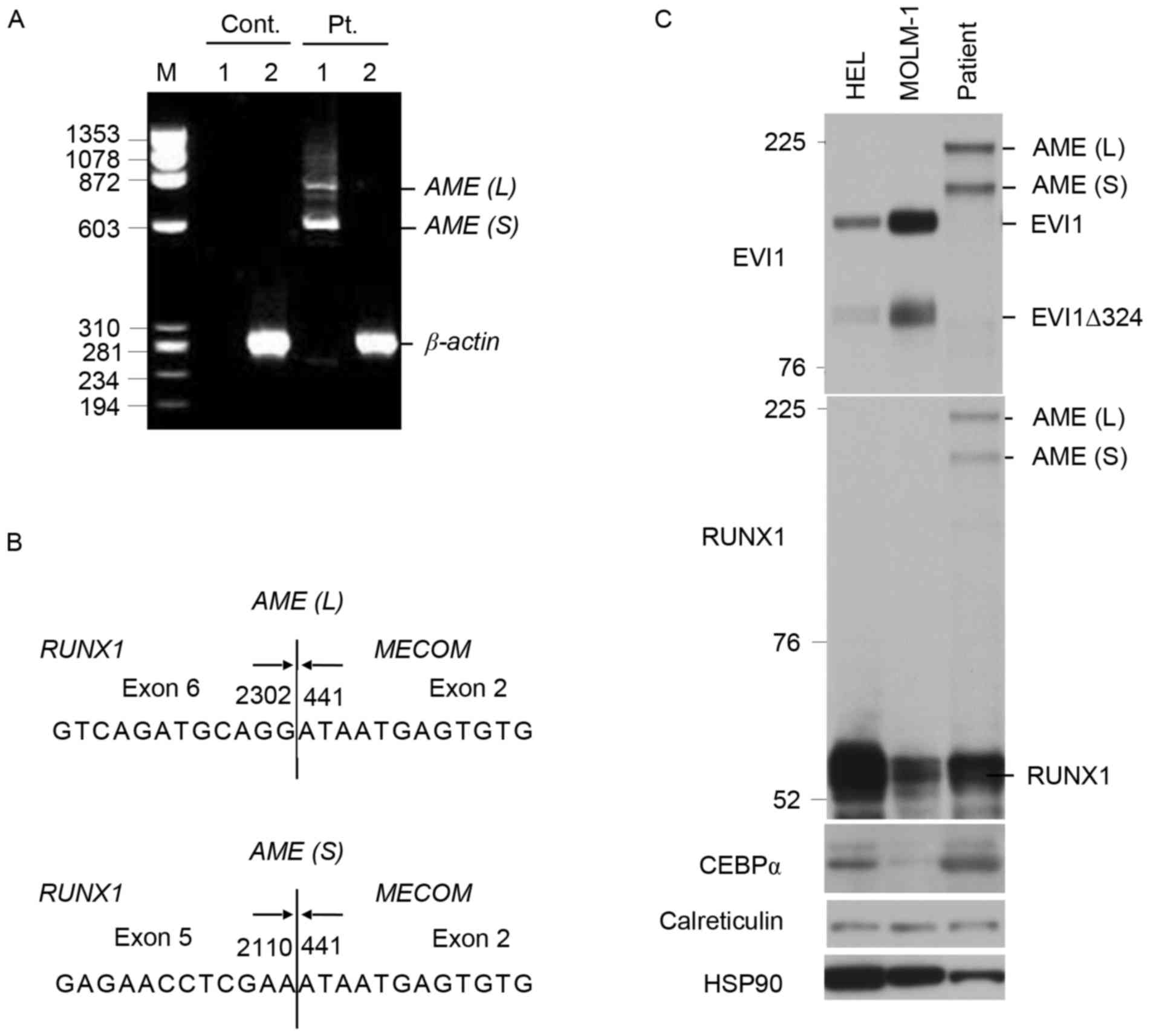 | Figure 2.Analyses of AME fusion transcripts
and proteins in the leukemic cells of the patient. (A) Reverse
transcription (RT)-PCR analyses of the AME fusion transcripts. M,
marker lane; 1, products from RUNX1 and EVI1 primers; 2, β-actin
control. Sizes are given in bp. Pt., patient's bone marrow cells;
Cont., HL60 cells; AME, AML/MDS1/EVI1. The positions of longer and
shorter AME (AML/MDS1/EVI1) fusion transcripts, AME (L) and AME
(S), as well as that of β-actin are also indicated. (B) Sequence
analysis of the AME fusion transcripts obtained from RT-PCR. Arrows
illustrate the boundaries of exons, with the nucleotide number for
RUNX1 (NM_001001890.2) and MECOM (NM_004991.3) indicated. Vertical
lines indicate the junctions of the chimeric transcripts. (C)
Western blot analysis of the AME fusion proteins in patient's
leukemic cells. Total cell lysates from HEL cells, MOLM-1 cells, or
patient's bone marrow cells, as indicated, were subjected to
Western blot analysis using antibodies against indicated proteins.
Sizes are given in KDa. Positions of larger and smaller AME fusion
proteins, AME (L) and AME (S), as well as those for EVI1, the
smaller form of EVI1 lacking 324 amino acids (EVI1∆324), and RUNX1
are also indicated. HSP90 was used for a protein loading
control. |
The patient was diagnosed with MDS: Refractory
anemia with excess blasts [international prognostic scoring system
(IPSS) (18): Int-1; revised IPSS
(19): High; WHO adapted prognostic
scoring system (20): High). Although
treatment with oral metenolone acetate (15 mg/day) was started in
October 2013, the patient's anemia progressed and blasts in the
peripheral blood increased. In December 2013, a bone marrow
examination revealed 25.2% blasts, leading to the diagnosis of AML
with myelodysplasia-related changes. In January 2014, treatment
with azacitidine (75 mg/m2/day for 7 days) was started;
however, the patient's cytopenia progressed (the white blood cell
count, 2.2×109/l; platelet count, 43×109/l)
and the bone marrow blasts increased. The patient was subsequently
treated with low-dose cytarabine chemotherapy (10
mg/m2/day), but succumbed to the disease in July
2014.
Discussion
The present study described a case of AML with
t(3;21)(q26.2;q22) that developed from MDS following treatment with
low-dose MTX for rheumatoid arthritis. MDS/AML with
t(3;21)(q26.2;q22) has been identified almost exclusively as
t-MDS/AML. For instance, a large case series examined by the
University of Texas MD Anderson Cancer Center (Houston, TX, USA)
revealed that 16/17 cases (94.1%) of t(3;21)(q26.2;q22) -positive
MDS/AML had previously received chemotherapy or radiotherapy
(5). It is well established that
other balanced recurrent chromosomal translocations in t-AML,
including those involving RUNX1, are typically observed
following treatment with topoisomerase II inhibitors without the
antecedent MDS phase or myelodysplasia-related morphological
changes, and are associated with a better prognosis compared with
other types of t-AML (1). Notably, a
previous study by the same group at the University of Texas MD
Anderson Cancer Center implicated antimetabolites in the
pathogenesis of t-MDS/AML with t(3;21)(q26.2;q22), as prior
treatment with the pyrimidine analog 5-fluorouracil or the purine
analog fludarabine was identified in 6/8 patients with this type of
t-MDS/AML (3). However, the
subsequent study revealed that a number of the 16 patients with
t(3;21)(q26.2;q22)-positive t-MDS/AML also received alkylating
agents and DNA topoisomerase II inhibitors for the treatment of
their primary tumors (5). Thus, in
contrast to t-AML associated with other balanced recurrent
translocations, t-MDS/1-AML with t(3;21)(q26.2;q22) was observed
subsequent to treatment with wide-ranging chemotherapeutics and
demonstrated a very short overall survival rate (median, 4.7
months) (5). In the present study,
the patient exhibited typical clinical and morphological features
of MDS/AML with t(3;21)(q26.2;q22), and survived only 10 months
following the diagnosis of MDS. However, to the best of our
knowledge, t(3;21)(q26.2;q22)-positive MDS/AML following treatment
with MTX has never been reported, and cannot be identified in the
Mitelman Database of Chromosome Aberrations and Gene Fusion in
Cancer (21).
It is well established that treatment with low-dose
MTX for rheumatoid arthritis is associated with the development of
lymphoproliferative disorders (LPDs), which are frequently
associated with Epstein Barr virus infection and often regress
spontaneously following the discontinuation of MTX (1,22). Thus,
immunodeficiency caused by the MTX therapy is strongly implicated
in the pathogenesis of LPDs. Conversely, it remains unknown whether
MTX therapy for rheumatoid arthritis may serve a role in
pathogenesis of MDS/AML. To the best of our knowledge, the cases of
13 patients with AML developing following low-dose MTX treatment
for rheumatoid arthritis have previously been reported (Table I) (23–29).
However, the clinical features, including latency periods,
cumulative dosages of MTX and the presence or absence of antecedent
MDS and myelodysplasia-related morphological changes have been
heterogeneous among these patients. Cytogenetic changes observed in
these patients have also been diverse; while complex karyotypic
abnormalities were exhibited in one patient, t(8;21) was observed
in two other cases (24,25,29).
Conversely, the patient in the present study exhibited typical
clinical and morphological features of MDS/AML associated with
t(3;21)(q26.2;q22), which develops almost exclusively as t-MDS/AML
(3,5).
This indicates that low-dose MTX therapy is causally associated
with the development of MDS/AML in the case reported in the present
study, although it remains unknown whether the mechanism underlying
this association involves DNA mutagenesis induced by MTX as an
antimetabolite or the immunosuppressive effect of MTX. In this
regard, it should be noted that rheumatoid arthritis itself has
been associated with an elevated risk of AML [odds ratio (OR) 1.28,
95% confidence interval (CI) 1.11–1.47] and MDS (OR 1.52, 95% CI
1.27–1.81) (30). Additionally,
rheumatoid arthritis is frequently treated with immunosuppressants
in addition to MTX, as is the case for the patient in the present
study who had received tacrolimus after stopping MTX. This suggests
that patients with rheumatoid arthritis who are treated with MTX
need to be carefully monitored for the development of MDS/AML.
Further reports of patients with t-MDS/AML associated with MTX are
required to clarify the etiological and clinical significance
between MTX and t-MDS/AML.
 | Table I.Identified cases of MDS/AML
developing following low-dose MTX therapy for RA. |
Table I.
Identified cases of MDS/AML
developing following low-dose MTX therapy for RA.
| Age/sex | Duration of RA
(y) | Total dose of MTX
(mg) | Diagnosis | Cytogenetic
abnormality | (Refs.) |
|---|
| 83/F | 33 | 690 | AML | 46,XX | (23) |
| 60/F | 23 | 80 | AML-M2 | t(8;21) | (24) |
| NA | 0.6 | NA | CMML to AML-M2 | NA | (25) |
| NA | 11 | NA | MDS/AML-M2 | t(8;21) | (25) |
| NA | 10 | NA | AML-M4 | NA | (25) |
| 71/M | 16 | 750 | AML-M1 | 46,XY | (26) |
| 72/F | 5 | 1200 | AML-M4 | NA | (26) |
| 52/M | 2 | 1250 | AML-M0 | 47,XY, +13 | (26) |
| 70/F | 9 | 500 | AML-M5 | 46,XX | (26) |
| 68/F | 11 | 1700 | AML-M6 | 46,XX | (27) |
| 73/F | 15 | 5850 | AML with MRC | 46,XX | (28) |
| 35/F | 3 | 1170 | AML with MRC | 46,XX | (28) |
| 78/F | 10 | NA | Myeloid
sarcoma | Complex | (29) |
| 84/F | 17 | 2204 | MDS/AML |
t(3;21),del(5)(q?) | * |
The t(3;21)(q26.2;q22) translocation primarily
generates the fusion gene transcript AME, although
transcripts of RUNX1 fused directly with EVI1 or
RPL22 near MECOM have also been identified (2,11,12,31). In
the patient discussed in the present study, two AME fusion
transcripts corresponding with RUNX1 fused at its 3′ end of
exon 5 or 6 to the 5′ end of exon 2 of MECOM were detected.
Thus, these fusion transcripts should encode the N-terminus of
RUNX1 containing the DNA-binding Runt domain fused to the majority
of the MDS1/EVI1 protein. These two different transcripts,
presumably generated by alternative splicing, represent the two
predominant forms of AME, which have previously been
identified in the t(3;21)-carrying leukemic cell line SKH1 and in
primary leukemic cells (3,11–16), with
a few patients revealed to express both forms (14,16).
However, the expression of the protein products of these fusion
transcripts in primary leukemic cells has not yet been documented,
to the best of our knowledge. In the present study, it was
confirmed that the fusion products were expressed in present
patient at a high level, comparable to that of EVI1 in human
leukemic MOML-1 cells with inv(3)
(q21.2;q26) (7,9) and HEL cells (32). The Runt domain maintained in AME is
the DNA-binding domain of RUNX1, which is the DNA-binding subunit
of the core-binding transcription factor that regulates the
expression of genes essential for hematopoiesis. By recruiting
histone deacetyltransferase and transcriptional co-repressors
through the C-terminal domain of EVI1, AME represses the
RUNX1-dependent promoters in a dominant-negative manner, thus
serving an essential role in leukemogenesis (2,4). This is
consistent with the observation of the present study that the
leukemic cells of the patient did not express EVI1 despite the fact
that wild-type RUNX1 was expressed at a readily detectable level,
since EVI1 has been suggested to be a target gene of RUNX1
(32).
AME has also been demonstrated to serve a role in
leukemogenesis through the inhibition of CEBPα, a transcription
factor that regulates the expression of genes associated with
myeloid differentiation, including its own gene (2,4,31,33).
Helbling et al (33)
previously suggested that the conditional expression of AME in the
myeloid leukemic cell line U937 suppressed the expression of CEBPα
through the induction and activation of calreticulin, a putative
inhibitor of CEBPα translation. The authors additionally
demonstrated that leukemic cells from none of the 8 patients with
t(3;21)-positive AML examined expressed CEBPα, although the
expression of AME in these cells was not confirmed (33). By contrast, CEBPα was readily
detectable in the leukemic cells from the patient in the present
study. In addition, the expression of calreticulin was not
distinctively enhanced in the present study, although the possible
activation of calreticulin in the leukemic cells of the patient was
not examined. Conversely, Tokita et al (31) revealed that AME inhibited
CEBPα-mediated transcriptional activity in the murine hematopoietic
progenitor cell line LG-3, although the mRNA levels of
CEBPα, a target gene of CEBPα itself, and the expression
level of calreticulin was not altered by the expression of AME in
these cells. These observations suggest that the effects of AME on
various cellular factors and events associated with leukemogenesis
may depend on the cellular context. Thus, a detailed analysis of
these factors, particularly at the protein level, in primary
leukemic cells is warranted to generate more data in regards to the
mechanism of AME-mediated leukemogenesis.
Acknowledgements
The present study was supported by the Ministry of
Education, Culture, Sports, Science and Technology of Japan (grant
nos. 15K09467 and 24591384).
References
|
1
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO classification of
tumours of haematopoietic and lymphoid tissues. 2. 4th. IARC;
2008
|
|
2
|
Nucifora G, Laricchia-Robbio L and Senyuk
V: EVI1 and hematopoietic disorders: History and perspectives.
Gene. 368:1–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin CC, Cortes J, Barkoh B, Hayes K,
Kantarjian H and Jones D: t(3;21)(q26;q22) in myeloid leukemia: An
aggressive syndrome of blast transformation associated with
hydroxyurea or antimetabolite therapy. Cancer. 106:1730–1738. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maki K, Yamagata T and Mitani K: Role of
the RUNX1-EVI1 fusion gene in leukemogenesis. Cancer Sci.
99:1878–1883. 2008.PubMed/NCBI
|
|
5
|
Li S, Yin CC, Medeiros LJ, Bueso-Ramos C,
Lu G and Lin P: Myelodysplastic syndrome/acute myeloid leukemia
with t(3;21)(q26.2;q22) is commonly a therapy-related disease
associated with poor outcome. Am J Clin Pathol. 138:146–152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glass C, Wilson M, Gonzalez R, Zhang Y and
Perkins AS: The role of EVI1 in myeloid malignancies. Blood Cell
Mol Dis. 53:67–76. 2014. View Article : Google Scholar
|
|
7
|
Hinai AA and Valk PJ: Review: Aberrant
EVI1 expression in acute myeloid leukaemia. Br J Haematol.
172:870–888. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morishita K, Parker DS, Mucenski ML,
Jenkins NA, Copeland NG and Ihle JN: Retroviral activation of a
novel gene encoding a zinc finger protein in IL-3-dependent myeloid
leukemia cell lines. Cell. 54:831–840. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gröschel S, Sanders MA, Hoogenboezem R, De
Wit E, Bouwman BA, Erpelinck C, van der Velden VH, Havermans M,
Avellino R, van Lom K, et al: A single oncogenic enhancer
rearrangement causes concomitant EVI1 and GATA2 deregulation in
leukemia. Cell. 157:369–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamazaki H, Suzuki M, Otsuki A, Shimizu R,
Bresnick EH, Engel JD and Yamamoto M: A remote GATA2 hematopoietic
enhancer drives leukemogenesis in inv(3)(q21;q26) by activating
EVI1 expression. Cancer Cell. 25:415–427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitani K, Ogawa S, Tanaka T, Miyoshi H,
Kurokawa M, Mano H, Yazaki Y, Ohki M and Hirai H: Generation of the
AML1-EVI-1 fusion gene in the t(3;21)(q26;q22) causes blastic
crisis in chronic myelocytic leukemia. EMBO J. 13:504–510.
1994.PubMed/NCBI
|
|
12
|
Nucifora G, Begy CR, Kobayashi H, Roulston
D, Claxton D, Pedersen-Bjergaard J, Parganas E, Ihle JN and Rowley
JD: Consistent intergenic splicing and production of multiple
transcripts between AML1 at 21q22 and unrelated genes at 3q26 in
(3;21)(q26;q22) translocations. Proc Natl Acad Sci USA. 91:pp.
4004–4008. 1994; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Motomura S, Fujisawa S, Tsunooka S,
Fujimaki K, Harano H, Mohri H, Okubo T, Fujita H, Maruta A and
Kodama F: Translocation (3;21)(q26;q22) in de novo acute
myelogenous leukemia. Leukemia. 11:172–173. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park TS, Choi JR, Yoon SH, Song J, Kim J,
Kim SJ, Kwon O and Min YH: Acute promyelocytic leukemia relapsing
as secondary acute myelogenous leukemia with translocation
t(3;21)(q26;q22) and RUNX1-MDS1-EVI1 fusion transcript. Cancer
Genet Cytogenet. 187:61–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang JJ, Cho SY, Suh JT, Lee HJ, Lee WI,
Yoon HJ, Baek SK and Park TS: Detection of RUNX1-MECOM fusion gene
and t(3;21) in a very elderly patient having acute myeloid leukemia
with myelodysplasia-related changes. Ann Lab Med. 32:362–365. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forghieri F, Bigliardi S, Morselli M,
Potenza L, Fantuzzi V, Faglioni L, Nasillo V, Messerotti A, Paolini
A and Luppi M: An unusual case of splenomegaly and increased
lactate dehydrogenase heralding acute myeloid leukemia with
eosinophilia and RUNX1-MECOM fusion transcripts. Leuk Res Rep.
3:83–85. 2014.PubMed/NCBI
|
|
17
|
Nogami A, Oshikawa G, Okada K, Fukutake S,
Umezawa Y, Nagao T, Kurosu T and Miura O: FLT3-ITD confers
resistance to the PI3K/Akt pathway inhibitors by protecting the
mTOR/4EBP1/Mcl-1 pathway through STAT5 activation in acute myeloid
leukemia. Oncotarget. 6:9189–9205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greenberg P, Cox C, LeBeau MM, Fenaux P,
Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, et al:
International scoring system for evaluating prognosis in
myelodysplastic syndromes. Blood. 89:2079–2088. 1997.PubMed/NCBI
|
|
19
|
Greenberg PL, Tuechler H, Schanz J, Sanz
G, Garcia-Manero G, Solé F, Bennett JM, Bowen D, Fenaux P, Dreyfus
F, et al: Revised international prognostic scoring system for
myelodysplastic syndromes. Blood. 120:2454–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malcovati L, Porta MG, Pascutto C,
Invernizzi R, Boni M, Travaglino E, Passamonti F, Arcaini L,
Maffioli M, Bernasconi P, et al: Prognostic factors and life
expectancy in myelodysplastic syndromes classified according to WHO
criteria: A basis for clinical decision making. J Clin Oncol.
23:7594–7603. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitelman F, Johansson B and Mertens FE:
Mitelman Database of Chromosome Aberrations and Gene Fusions in
Cancer. http://cgap
ncinihgov/Chromosomes/MitelmanFebruary 16–2016
|
|
22
|
Ichikawa A, Arakawa F, Kiyasu J, Sato K,
Miyoshi H, Niino D, Kimura Y, Takeuchi M, Yoshida M, Ishibashi Y,
et al: Methotrexate/iatrogenic lymphoproliferative disorders in
rheumatoid arthritis: Histology, Epstein-Barr virus, and clonality
are important predictors of disease progression and regression. Eur
J Haematol. 91:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pointud P, Prudat M and Peron JM: Acute
leukemia after low dose methotrexate therapy in a patient with
rheumatoid arthritis. J Rheumatol. 20:1215–1216. 1993.PubMed/NCBI
|
|
24
|
Kerr L Dubin, Troy K and Isola L: Temporal
association between the use of methotrexate and development of
leukemia in 2 patients with rheumatoid arthritis. J Rheumatol.
22:2356–2358. 1995.PubMed/NCBI
|
|
25
|
Rosenthal NS and Farhi DC: Myelodysplastic
syndromes and acute myeloid leukemia in connective tissue disease
after single-agent chemotherapy. Am J Clin Pathol. 106:676–679.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kolte B, Baer AN, Sait SN, O'Loughlin KL,
Stewart CC, Barcos M, Wetzler M and Baer MR: Acute myeloid leukemia
in the setting of low dose weekly methotrexate therapy for
rheumatoid arthritis. Leuk Lymphoma. 42:371–378. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi BR, Ahn MJ, Lee WS, Kim TH, Bae SC
and Jun JB: Acute erythroleukemia in a rheumatoid arthritis patient
during low-dose methotrexate therapy. Rheumatol Int. 25:311–313.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al-Anazi KA, Eltayeb KI, Bakr M and
Al-Mohareb FI: Methotrexate-induced acute leukemia: Report of three
cases and review of the literature. Clin Med Case Rep. 2:43–49.
2009.PubMed/NCBI
|
|
29
|
Sakai T, Tamura S, Miyoshi T, Nesumi N,
Nagai K and Oshima K: Development of myeloid sarcoma after
long-term methotrexate use for rheumatoid arthritis. Int J Hematol.
99:493–498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anderson LA, Pfeiffer RM, Landgren O,
Gadalla S, Berndt SI and Engels EA: Risks of myeloid malignancies
in patients with autoimmune conditions. Br J Cancer. 100:822–828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tokita K, Maki K and Mitani K: RUNX1/EVI1,
which blocks myeloid differentiation, inhibits CCAAT-enhancer
binding protein alpha function. Cancer Sci. 98:1752–1757. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maicas M, Vázquez I, Vicente C,
García-Sánchez MA, Marcotegui N, Urquiza L, Calasanz MJ and Odero
MD: Functional characterization of the promoter region of the human
EVI1 gene in acute myeloid leukemia: RUNX1 and ELK1 directly
regulate its transcription. Oncogene. 32:2069–2078. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Helbling D, Mueller BU, Timchenko NA,
Hagemeijer A, Jotterand M, Meyer-Monard S, Lister A, Rowley JD,
Huegli B, Fey MF and Pabst T: The leukemic fusion gene
AML1-MDS1-EVI1 suppresses CEBPA in acute myeloid leukemia by
activation of Calreticulin. Proc Natl Acad Sci USA. 101:pp.
13312–13317. 2004; View Article : Google Scholar : PubMed/NCBI
|