Introduction
Human colorectal cancer has multiple steps of
progression, and neoplastic conversion of the colorectal crypt
epithelium is associated with cumulative alterations of numerous
genes, proteins and molecular processes (1). Molecular changes associated with the
earlier steps may be demonstrated in precancerous lesions,
including adenoma and dysplasia, which occasionally coexist with
overt cancer (1–3). Increasing data indicates that a number
of these molecular alterations also occur in morphologically normal
transitional mucosa adjacent to colorectal cancer, under the basic
concept of either field cancerization (areas of preneoplastic
status) (4–7) or molecular crosstalk between cancer and
surrounding non-neoplastic tissues (8).
Cytokeratin (CK)7 is one of the intermediate
filament proteins that constitute the cytoskeleton of numerous
types of epithelial cells. Colorectal crypt epithelia and tumors
derived from it are representative of tissue that is negative for
CK7, and the coordinate expression pattern of CK7 negativity with
CK20-positivity (CK7-/CK20+) is used for confirmation of the
colorectal origin of metastatic tumors (9). A number of studies have noted aberrant
expression of CK7 in a subset of colorectal cancer (10,11).
The type III receptor tyrosine kinase, KIT
proto-oncogene receptor tyrosine kinase (CD117), which is encoded
by the c-kit proto-oncogene, is normally expressed in a variety of
human tissues, including hematopoietic stem cells, melanocytes,
mast cells, germ cells and interstitial cells of Cajal (12–24). CD117
is also expressed in human tumors, including myeloid leukemia,
melanoma, glioblastoma, germ cell tumors, breast cancer, small cell
lung cancer and gastrointestinal stromal tumors (GISTs) (24). CD117 is not expressed in the normal
crypt epithelium in the colon and the rectum, but aberrant
expression of CD117 has been reported in a subset of cancers
(24).
CK7 and CD117 expression has been demonstrated not
only in developed colorectal cancer, but also in adenoma and
dysplasia (2,3,24). To the
best of our knowledge, however, the expression of CK7 and CD117 in
morphologically normal transitional mucosa adjacent to colorectal
cancer has not been investigated to date. The lack of this
information prompted the authors of the present study to assess the
expression of CK7 and CD117 in morphologically normal transitional
mucosa adjacent to colorectal cancer.
Materials and methods
Patients and tissue specimens
The tissue specimens analyzed in the present study
consisted of tissue from 76 lesions of low-grade adenoma (from 65
patients), 18 lesions of high-grade adenoma (from 17 patients), 12
lesions of mucosal adenocarcinoma (from 12 patients), 67 lesions of
small-sized invasive adenocarcinoma (≤2 cm in maximum diameter
extending to the submucosa or deeper; from 66 patients) and 20
lesions of large-sized invasive adenocarcinoma (>2 cm in maximum
diameter extending to the submucosa or deeper; from 20 patients).
The main clinical and pathological characteristics of the patients
and the colorectal tumors are listed in Table I. The archival samples were retrieved
from the database of Kyorin University Hospital (Tokyo, Japan). The
cases were diagnosed and treated by surgical resection (colectomy)
between January 2007 and December 2011. To simplify the
interpretation of obtained results, patients with either preceding
inflammatory bowel disease or hereditary backgrounds, including
familial adenomatous polyposis, or who had received preoperative
chemo- or radiotherapy, were excluded from the present study.
Serrated adenomas and adenocarcinomas developed through serrated
pathways were also excluded. The present study was approved by the
Ethics Committee of Kyorin University, School of Medicine. The need
for written, informed consent was waived due to the retrospective,
non-interventional nature of the present study.
 | Table I.Characteristics of patients with
colorectal adenoma or adenocarcinoma. |
Table I.
Characteristics of patients with
colorectal adenoma or adenocarcinoma.
| Diagnosis | Total number of
neoplasms, n | Age, years (mean,
range) | Male: Female | Left: Right | Size, mm (mean,
range) |
|---|
| Low-grade
adenoma | 76 | 72 (47–91) | 42:23 | 41:35 | 8.2 (3–36) |
| High-grade
adenoma | 18 | 68 (47–91) | 12:5 | 9:9 | 16.2 (8–25) |
| Mucosal
adenocarcinoma | 12 | 70 (59–89) | 11:1 | 8:4 | 19.6 (6–60) |
| Small-sized
invasive adenocarcinoma | 67 | 70 (37–88) | 40:26 | 41:26 | 16.3 (7–20) |
| Large-sized
invasive adenocarcinoma | 20 | 70 (44–86) | 12:8 | 8:12 | 49.9 (24–110) |
Tissue preparation and
immunohistochemistry
Formalin-fixed and paraffin-embedded tissues from
the aforementioned samples were used for immunohistochemical
analysis. The samples were originally fixed in 4%
phosphate-buffered formaldehyde at room temperature for 24 to 72 h
and embedded in paraffin, according to routine procedures. From
each sample, 4 µm sections were cut, dried, dewaxed in xylene and
rehydrated in descending alcohol series (100, 90 and 70%, twice in
each concentration). The subsequent immunohistochemical staining
procedure was conducted using a semiautomatic staining machine
(Ventana ES; Ventana Medical Systems, Inc., Oro Valley, AZ, USA)
according to the manufacturer's protocol. The following primary
antibodies were used for immunohistochemistry: Anti-human CK7 mouse
monoclonal antibody (clone OV-TL 12/30; cat. no. M7018; dilution,
1:200; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) and
anti-human CD117 rabbit polyclonal antibody (cat. no. A4502;
dilution, 1:400; Dako; Agilent Technologies, Inc). Samples were
incubated with these antibodies at 37°C for 16 min. For reference,
hematoxylin and eosin staining was performed on the same samples
(Carrazzi's hematoxylin solution and 1% eosin Y).
Evaluation of CK7 and CD117 expression
in colorectal tumors, transitional mucosa adjacent to the tumor and
distant normal mucosa
For each patient, the expression of CK7 and CD117 in
tumor lesions, in transitional mucosa adjacent to the tumor and in
distant normal mucosa was immunohistochemically evaluated under
light microscope. For each area, 4 fields of view were assessed at
magnification, ×100.
The expression levels of the proteins were scored
between 0 and 3+ according to the extent of the immunoreactive
glands. The scores 1+ to 3+ were defined as follows: 1+, 1–2
consecutive glands; 2+, 3–5 glands; and 3+, ≥6 glands. In solid or
diffusely infiltrating tumors without gland formation, each 100
µm-size area was counted as one gland. Scores of 1+ to 3+ were
considered as positive. Subjects without or with a few scattered
immunoreactive cells were scored as 0 (negative). In cases of
heterogeneous staining, areas of the highest reactivity were
selected for scoring. All immunohistochemical staining was
independently evaluated by two pathologists, and then consensus
scores were adopted.
Statistical analysis
Staining data were analyzed using Statcel v.3
software (OMS, Tokorozawa, Japan; http://www.oms-publ.co.jp). The Mann-Whitney U-test
was applied to determine the difference in the positivity rate for
expression of CK7 and CD117. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of CK7 in colorectal
tumors, in transitional mucosa adjacent to the tumor and in distant
normal mucosa
The immunohistochemical analysis of the expression
of CK7 in the tumor, in transitional mucosa adjacent to the tumor
and in distant normal mucosa is summarized in Table II. Regarding CK7 expression in the
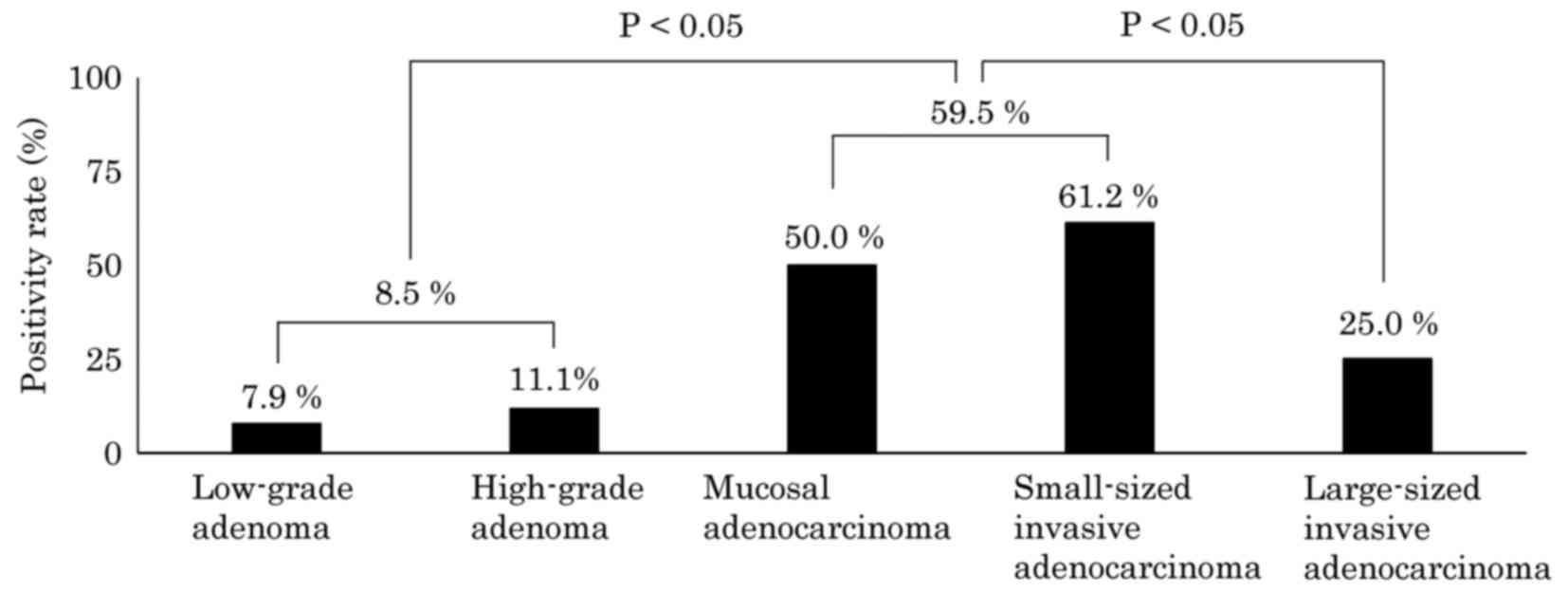
transitional mucosa adjacent to the tumor, a positive result (score
of 1+ to 3+) was obtained in 7.9% (6/76) of low-grade adenomas,
11.1% (2/18) of high-grade adenomas, 50.0% (6/12) of mucosal
adenocarcinomas, 61.2% (41/67) of small-sized invasive
adenocarcinomas and 25.0% (5/20) of large-sized invasive
adenocarcinomas, irrespective of CK7 expression in the
corresponding tumors. The CK7 positivity rate in the transitional
mucosa adjacent to the tumor exhibited a stepwise increase
according to the grade of the corresponding tumor, increasing from
a low-grade adenoma through to a small-sized invasive
adenocarcinoma (Fig. 1). However, in
the large-sized invasive adenocarcinoma cases, the positivity rate
for CK7 in the transitional mucosa adjacent to the tumor was
significantly lower compared with the next lower tumor grades
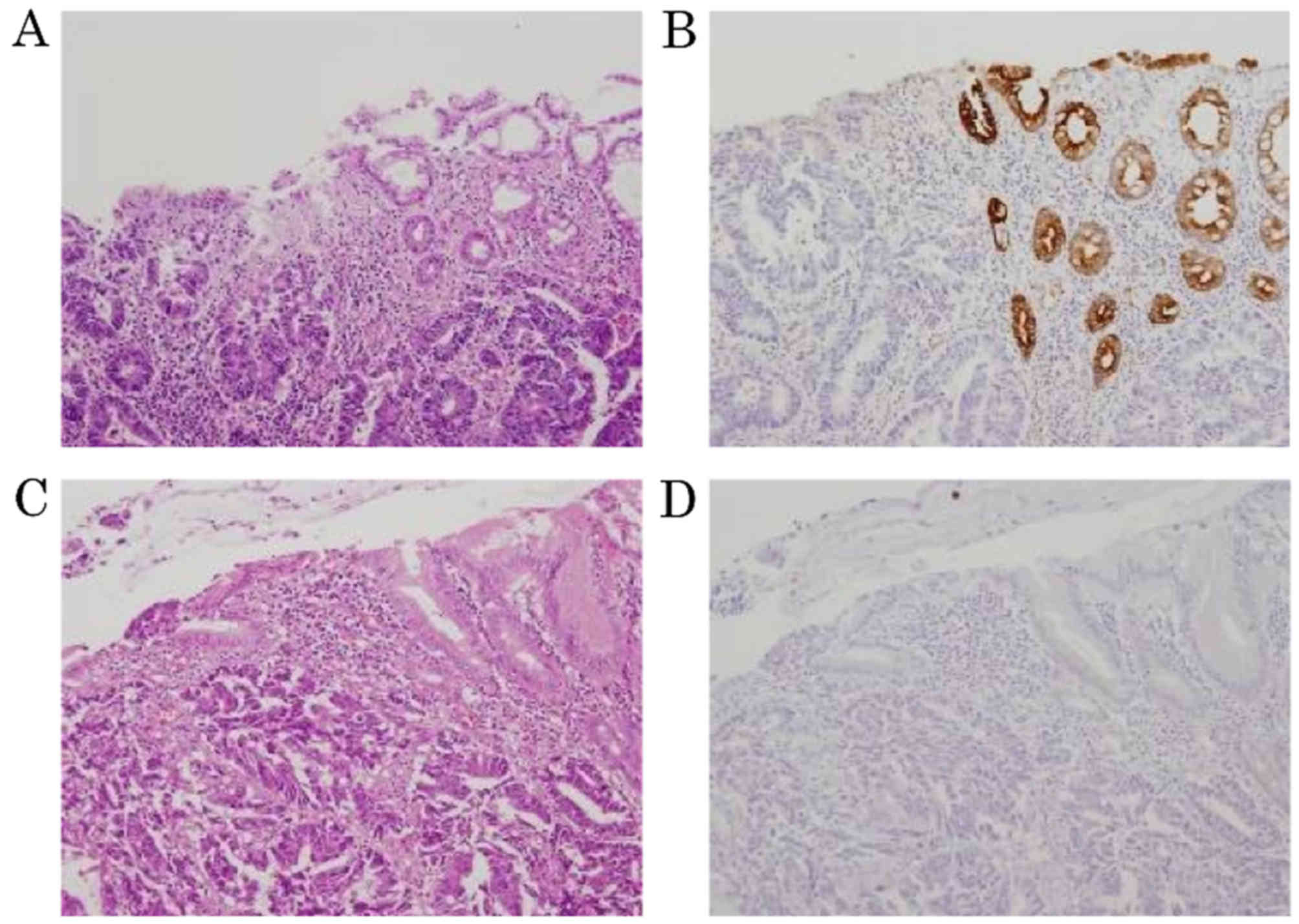
(Fig. 1). Representative images of
the transitional areas revealed that expression of CK7 in the
mucosa adjacent to the small-sized invasive adenocarcinoma (score
3+; positive) differed from that in the mucosa adjacent to the
large-sized invasive adenocarcinoma (score 0; negative; Fig. 2).
 | Table II.Summary of cytokeratin 7 expression
in adjacent mucosa, tumor and distant mucosa. |
Table II.
Summary of cytokeratin 7 expression
in adjacent mucosa, tumor and distant mucosa.
|
|
|
| Score |
|---|
|
|
|
|
|
|---|
| Diagnosis | Region | n | 0 | 1+ | 2+ | 3+ |
|---|
| Low-grade
adenoma | Adjacent
mucosa | 76a | 70 | 4 | 2 | 0 |
|
| Tumor | 76a | 75 | 1 | 0 | 0 |
|
| Distant normal
mucosa | 65 | 65 | 0 | 0 | 0 |
| High-grade
adenoma | Adjacent
mucosa | 18a | 16 | 2 | 0 | 0 |
|
| Tumor | 18a | 17 | 1 | 0 | 0 |
|
| Distant normal
mucosa | 17 | 17 | 0 | 0 | 0 |
| Mucosal
adenocarcinoma | Adjacent
mucosa | 12 | 6 | 3 | 3 | 0 |
|
| Tumor | 12 | 11 | 0 | 1 | 0 |
|
| Distant normal
mucosa | 12 | 12 | 0 | 0 | 0 |
| Small-sized
invasive adenocarcinoma | Adjacent
mucosa | 67a | 26 | 24 | 10 | 7 |
|
| Tumor | 67a | 60 | 1 | 2 | 4 |
|
| Distant normal
mucosa | 66 | 64 | 2 | 0 | 0 |
| Large-sized
invasive adenocarcinoma | Adjacent
mucosa | 20 | 15 | 4 | 1 | 0 |
|
| Tumor | 20 | 15 | 1 | 1 | 3 |
|
| Distant normal
mucosa | 20 | 20 | 0 | 0 | 0 |
As for CK7 expression in the tumor itself, CK7 was
expressed in 1.3% (1/76) of low-grade adenomas, 5.6% (1/18) of
high-grade adenomas, 8.3% (1/12) of mucosal adenocarcinomas, 10.4%
(7/67) of small-sized invasive adenocarcinomas and 25.0% (5/20) of
large-sized invasive adenocarcinomas. Distant normal mucosa was
virtually negative for CK7 except for 3.0% (2/66) of small-sized
invasive adenocarcinoma cases.
Expression of CD117 in colorectal
tumors, transitional mucosa adjacent to the tumor and distant
normal mucosa
The results of immunohistochemical analysis of CD117
expression are summarized in Table
III. Concerning CD117 expression in the transitional mucosa
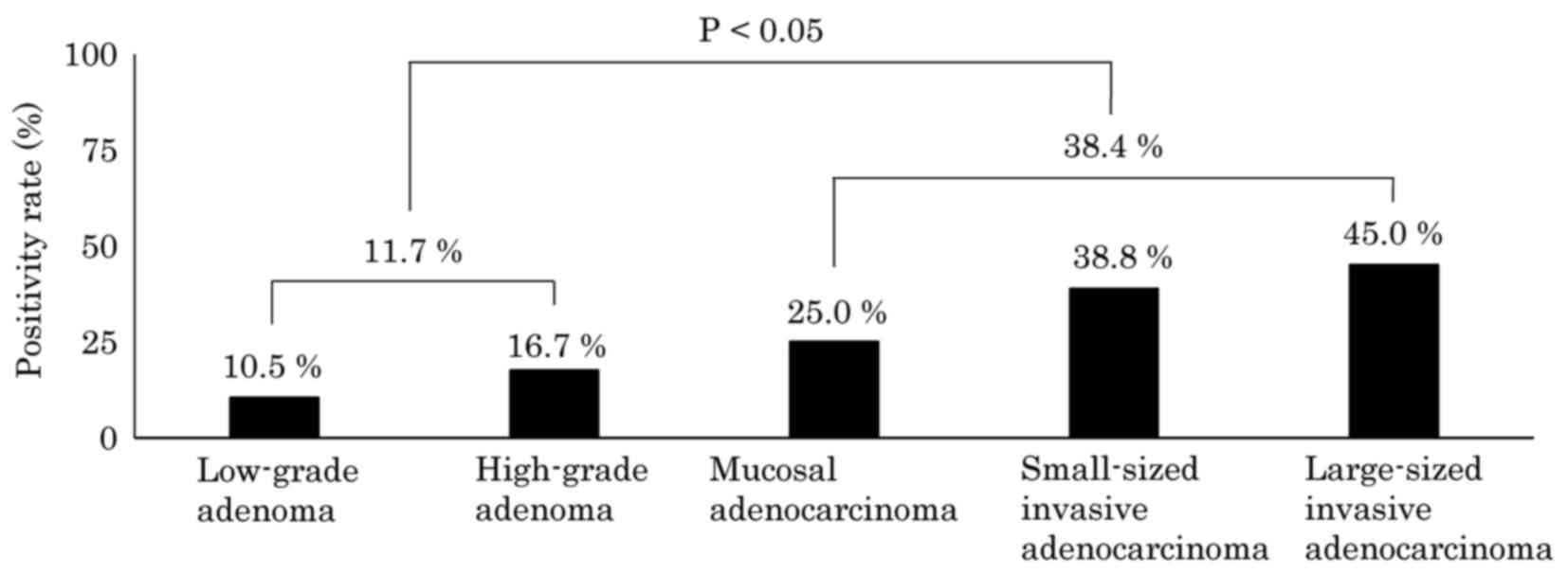
adjacent to the tumor, positive results (a score of 1+ to 3+) were
obtained in 10.5% (8/76) of low-grade adenomas, 16.7% (3/18) of
high-grade adenomas, 25.0% (3/12) of mucosal adenocarcinomas, 38.8%
(26/67) of small-sized invasive adenocarcinomas and 45.0% (9/20) of
large-sized invasive adenocarcinomas, irrespective of CD117
expression in the corresponding tumors. In contrast to CK7, the
positivity rate for CD117 in the transitional mucosa adjacent to
the tumor increased stepwise according to tumor grade, from low
grade adenoma all the way through to large-sized invasive
adenocarcinoma cases, and there was a significant difference in
CD117 expression in this location between the adenoma group and the
adenocarcinoma group (P<0.05; Fig.
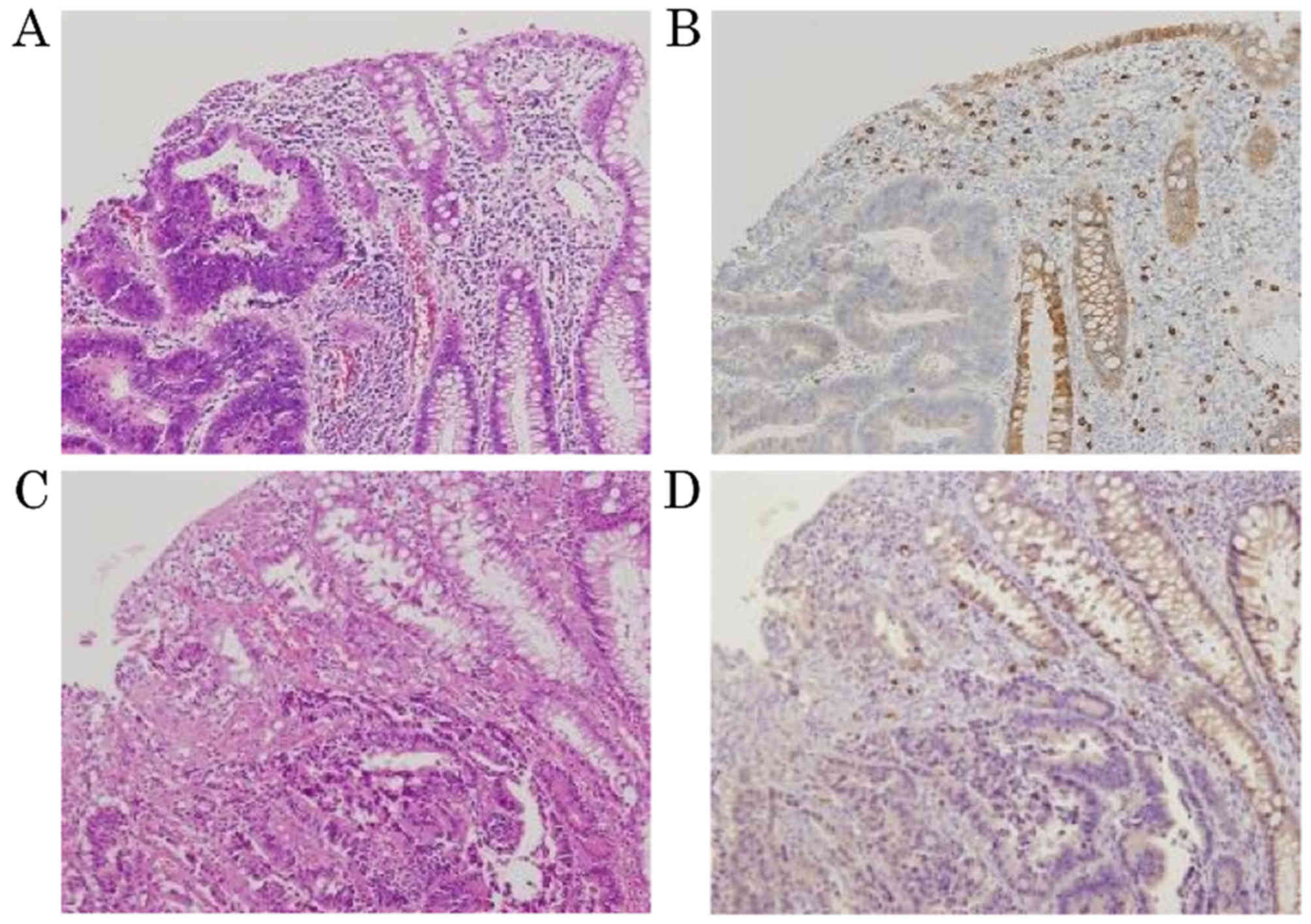
3). Representative images of the transitional areas
demonstrated expression of CD117 in the adjacent mucosa of
small-sized invasive adenocarcinoma (score 3+; positive) and
large-sized invasive adenocarcinoma (score 3+; positive; Fig. 4).
 | Table III.Summary of KIT proto-oncogene
receptor tyrosine kinase expression in adjacent mucosa, tumor and
distant mucosa. |
Table III.
Summary of KIT proto-oncogene
receptor tyrosine kinase expression in adjacent mucosa, tumor and
distant mucosa.
|
|
|
| Score |
|---|
|
|
|
|
|
|---|
| Diagnosis | Region | n | 0 | 1+ | 2+ | 3+ |
|---|
| Low-grade
adenoma | Adjacent
mucosa | 76a | 68 | 2 | 2 | 4 |
|
| Tumor | 76a | 57 | 5 | 7 | 7 |
|
| Distant normal
mucosa | 65 | 61 | 0 | 0 | 4 |
| High-grade
adenoma | Adjacent
mucosa | 18a | 15 | 0 | 0 | 3 |
|
| Tumor | 18a | 12 | 0 | 2 | 4 |
|
| Distant normal
mucosa | 17 | 17 | 0 | 0 | 0 |
| Mucosal
adenocarcinoma | Adjacent
mucosa | 12 | 9 | 0 | 0 | 3 |
|
| Tumor | 12 | 11 | 0 | 1 | 0 |
|
| Distant normal
mucosa | 12 | 12 | 0 | 0 | 0 |
| Small-sized
invasive adenocarcinoma | Adjacent
mucosa | 67a | 41 | 6 | 4 | 16 |
|
| Tumor | 67a | 57 | 0 | 3 | 7 |
|
| Distant normal
mucosa | 66 | 61 | 0 | 0 | 5 |
| Large-sized
invasive adenocarcinoma | Adjacent
mucosa | 20 | 11 | 3 | 2 | 4 |
|
| Tumor | 20 | 19 | 0 | 0 | 1 |
|
| Distant normal
mucosa | 20 | 20 | 0 | 0 | 0 |
As for CD117 expression in the tumor itself, CD117
was expressed in 25.0% (19/76) of low-grade adenoma, 33.3% (6/18)
of high-grade adenoma, 8.3% (1/12) of mucosal adenocarcinoma, 14.9%
(10/67) of small-sized invasive adenocarcinoma and 5.0% (1/20) of
large-sized invasive adenocarcinoma. Notably, the tumor positivity
rate for CD117 was significantly increased in the adenoma group
compared with the adenocarcinoma group (P<0.05). In distant
normal mucosa, CD117 expression was detected only in a small
percentage of low-grade adenoma cases [6.2% (4/65)] and small-sized
invasive adenocarcinoma cases [7.6% (5/66)].
Discussion
In the present study, CK7 and CD117 were
demonstrated to be expressed in the transitional mucosa adjacent to
colorectal tumors. In adenoma, mucosal adenocarcinoma and
small-sized invasive adenocarcinoma cases, the positivity ratio of
CK7 and CD117 increased in a stepwise manner according to tumor
grade. Regarding CK7 and CD117 expression in large-sized invasive
adenocarcinoma cases, there was a difference between whether
expression was increased (CD117) or decreased (CK7) compared with
the expression in small-sized invasive carcinoma cases. The results
of the present study indicated that the expression mechanisms of
CK7 and CD117 were different in the transitional mucosa adjacent to
colorectal cancer.
The positivity ratio for CK7 in the transitional
mucosa adjacent to the tumor was lower in large-sized invasive
adenocarcinoma cases compared with in mucosal and small-sized
invasive adenocarcinoma cases. This lower CK7 positivity ratio in
the large-sized invasive adenocarcinoma cases may be partially
explained by the encroachment of cancer cells into morphologically
normal, CK7-positive mucosa, which predisposes the tissue to
cancerous development.
Cytokeratins are a family of cytoplasmic structural
proteins that have been described in human epithelium, which
demonstrate variable expression depending on the type of tissue and
its differentiation status (25,26). CK7
is expressed in several simple ductal and glandular epithelia,
including the lung, breast, bile and pancreatic ducts, urinary
tracts, ovary and endometrium (27).
In contrast, CK7 expression is generally lacking in normal mucosa
of the colon, rectum and, correspondingly, in colorectal adenoma
and adenocarcinoma (11,28). The biological significance of CK7
expression remains largely unknown. Kirchner et al (29) reported that CK7 is aberrantly
expressed in boundary mucosa adjacent to gastric cancer, and
proposed that this expression may reflect transient
dedifferentiation, which is associated with metaplastic and
dysplastic change in the gastritis-cancer sequence. The same
transient dedifferentiation step may occur in the transitional
mucosa adjacent to colorectal cancer.
Within the concept of field cancerization, also
termed the field effect or the field defect, morphologically normal
mucosa surrounding the cancer is considered to be biologically
different compared with the normal mucosa of subjects with no
malignant or premalignant lesions. This concept was first
introduced by Slaughter et al (30) to explain the presence of multifocal
oral squamous cell carcinomas that developed out of a field of
precancerous change, and the concept has now been applied to a
variety of organs, including the lung, breast, esophagus, stomach
and colon (31). In the colon and
rectum, field cancerization has been described in cancer associated
with chronic inflammatory bowel disease, including ulcerative
colitis and Crohn's disease (32–35), and
also in sporadic cases (5,6,31). Field
cancerization has not been well defined in molecular terms;
however, previous studies have proposed mutated genes, including
tumor protein p53 and DNA methylation genes, as candidate mediators
of the field cancerization in colorectal cancer (4,6,31). Therefore, aberrant expression of CK7
is considered to be another candidate marker for field
cancerization in colorectal cancer.
The areas of CK7-positive crypts observed in the
present study were localized close to the tumor, and the positive
areas were smaller than the fields demonstrated by other studies
that used different markers (5,7,36). Facista et al (37) hypothesized that the molecular
alterations associated with field cancerization progress outwards,
and that the most extensive regions reflect the earliest event in
carcinogenesis. If this is the case, then aberrant expression of
CK7 would be the latest event for tumorigenesis in the colon and
rectum.
With respect to CK7 expression in the tumors,
colorectal cancers commonly lack CK7 expression, and positive
immunostaining for CK7 in metastatic tumors supports the exclusion
of a colorectal origin for these tumors (10,11,26,28).
However, not all colorectal cancers lack CK7 expression, and CK7
positivity in colorectal cancers has been reported to range from
0–16% (reviewed by Harbaum) (38).
CK7 expression in colorectal cancer was reported to be associated
with high-grade, right-sided tumors (39). In the present study, CK7 expression
was observed in 2.1% of colorectal adenomas and 13.1% of
carcinomas, and the frequency of expression increased according to
the tumor grade. These results are in line with those of other
previous studies (9,38,39).
However, no association was observed between CK-7-positivity and
the location of the tumor (data not shown).
In contrast to CK7, the positivity rate for CD117 in
transitional mucosa adjacent to the large-sized invasive
adenocarcinoma cases was increased compared with its expression in
the mucosa adjacent to preinvasive and small-sized invasive tumors.
Based on this result, expression of CD117 in transitional mucosa
adjacent to colorectal cancer may be secondarily induced by the
corresponding cancer rather than predisposing the tissue to
cancerous development.
The proto-oncogene c-kit encodes a transmembrane
tyrosine kinase receptor CD117, which is associated with the
platelet-derived growth factor PDGF/colony stimulating factor 1
(c-fms) receptor subfamily (40,41). The
ligand for CD117 is stem cell factor (SCF), which activates the
tyrosine kinase activity of CD117 via homodimerisation and
autophosphorylation of the receptor at specific tyrosine residues
in the intracellular domain of the receptor (42,43). CD117
is normally expressed in a variety of human tissues, including
hematopoietic stem cells, melanocytes, mast cells, germ cells and
interstitial cells of Cajal (44).
Immunohistochemical analyses indicated that the localization of
CD117 protein in the normal colon was restricted to interstitial
cells of Cajal scattered in the stroma, whereas the non-neoplastic
epithelium was always negative (45).
Abnormal levels of CD117 expression have been
observed in a variety of human tumors, including GISTs, myeloid
leukemia, melanoma, glioblastoma, germ cell cancer, breast cancer,
prostatic cancer and small cell lung cancer (24,44,45). Two
general mechanisms of CD117 activation in these tumors have been
described: Acquisition of activating mutations that result in
constitutive ligand-independent CD117 phosphorylation, and
autocrine or paracrine stimulation of the receptor by its ligand
SCF (24).
Data concerning the expression of CD117 in
colorectal cancer are controversial (24,44–46).
Overall, as determined by immunohistochemistry, the expression of
CD117 is rare, and CD117 expression has been detected in only
1.6–25% of colorectal carcinomas examined (24,44–48).
Colorectal cancer with overexpression of CD117 did not demonstrate
c-kit gene mutations in hot spot regions (44). The majority of CD117-positive
carcinomas co-expressed SCF, and the existence of
autocrine/paracrine mechanisms has been proposed (24,49,50).
There is increasing data to support a central
function for the tumor microenvironment in the mechanisms for the
progression of colorectal cancer (8,51,52). The tumor microenvironment is composed
not only of a heterogeneous population of stromal cells, including
fibroblasts and immune cells, extracellular matrix and secreted
factors within the tumor bulk, but also of the adjacent non-tumor
mucosa, which interacts and communicates with the cancer. This
molecular crosstalk is mediated thorough cytokines and other
factors secreted by the tumor, which activate receptors in the
adjacent colonic tissue; and vice versa, providing novel insights
into the micro-ecology of colorectal tumorigenesis.
In the present study, CD117 expression in
transitional mucosa adjacent to colorectal cancer was considered to
be the result of paracrine stimulation by SCF secreted by the
cancer. CD117 was not included in genes activated in the mucosa
adjacent to colorectal cancer in an extensive transcriptomic
analysis performed by Sanz-Pamplona et al (8). The results of the present study
indicated that molecular crosstalk through SCF and CD117 may be
rather weak, and be restricted to the mucosa just beside the edge
of the colorectal tumor.
In the colorectal tumors of the present study, the
expression of CD117 in carcinoma was less frequent than in adenoma
(12.1 and 26.6%, respectively). This result concurred with that
reported by Bellone et al (24), who also described lower CD117
expression in carcinoma (10.6%) compared with adenoma (12.5%).
Similarly, in the uterus, CD117 was reported to be expressed less
frequently in endometrial carcinoma than in endometrial hyperplasia
(53). Mutational switches for tumor
growth other than the SCF-CD117 autocrine/paracrine system may be
turned on in the course of progression of these tumors.
Although antibodies for CK7 and CD117 are
conventionally used in daily diagnostic practice, they may provide
novel insights into the development and progression of colorectal
cancer (9–24). A potential weakness of the present
study was that it was conducted retrospectively and dealt with
archival histopathological specimens. Further prospective studies,
including those using cancer-grafted model animals, may provide
more evidence to support the results of the present study.
In conclusion, the present study demonstrated that
CK7 and CD117 were expressed in the transitional mucosa adjacent to
colorectal tumors. However, their expression mechanisms were
considered to be different. From the practical point of view,
staining for CK7, as a marker for field cancerization, may be used
for evaluation of the surgical margin and for prediction of the
risk of local recurrence. An approach for analysis of the molecular
crosstalk between receptors, including CD117 and signaling proteins
secreted by the tumor, may be useful in order to gain insight into
the maintenance of the microenvironment of colorectal cancer.
Disruption of this intricate molecular network of cell-cell
communication may be a novel therapeutic strategy for the treatment
of colorectal cancer.
Acknowledgements
The authors would like to thank Ms. Michiru Umino
(Department of Pathology, Kyorin University School of Medicine,
Mitaka, Japan) or her excellent technical assistance.
References
|
1
|
Cho KR and Vogelstein B: Genetic
alterations in the adenoma-carcinoma sequence. Cancer. 70 6
Suppl:S1727–S1731. 1992. View Article : Google Scholar
|
|
2
|
Tatsumi N, Kushima R, Vieth M, Mukaisho K,
Kakinoki R, Okabe H, Borchard F, Stolte M, Okanoue T and Hattori T:
Cytokeratin 7/20 and mucin core protein expression in ulcerative
colitis-associated colorectal neoplasms. Virchows Arch.
448:756–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stenling R, Lindberg J, Rutegård J and
Palmqvist R: Altered expression of CK7 and CK20 in preneoplastic
and neoplastic lesions in ulcerative colitis. APMIS. 115:1219–1226.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baytner S, Mitmaker B, Gordon PH and Wang
E: Immunohistochemical expression of mutant p53 oncogene in
transitional mucosa adjacent to human colon cancer. Clin Invest
Med. 16:379–385. 1993.PubMed/NCBI
|
|
5
|
Jothy S, Ślesak B, Harłozińska A, Lapińska
J, Adarniak J and Rabczyński J: Field effect of human colon
carcinoma on normal mucosa: Relevance of carcinoembryonic antigen
expression. Tumour Biol. 17:58–64. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen L, Kondo Y, Rosner GL, Xiao L,
Hernandez NS, Vilaythong J, Houlihan PS, Krouse RS, Prasad AR,
Einspahr JG, et al: MGMT promoter methylation and field defect in
sporadic colorectal cancer. J Natl Cancer Inst. 97:1330–1338. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hawthorn L, Lan L and Mojica W: Evidence
for field effect cancerization in colorectal cancer. Genomics.
103:211–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sanz-Pamplona R, Berenguer A, Cordero D,
Molleví DG, Crous-Bou M, Sole X, Paré-Brunet L, Guino E, Salazar R,
Santos C, et al: Aberrant gene expression in mucosa adjacent to
tumor reveals a molecular crosstalk in colon cancer. Mol Cancer.
13:462014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bayrak R, Yenidünya S and Haltas H:
Cytokeratin 7 and cytokeratin 20 expression in colorectal
adenocarcinomas. Pathol Res Pract. 207:156–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moll R, Franke WW, Schiller DL, Geiger B
and Krepler R: The catalog of human cytokeratins: Patterns of
expression in normal epithelia, tumors and cultured cells. Cell.
31:11–24. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramaekers F, van Niekerk C, Poels L,
Schaafsma E, Huijsmans A, Robben H, Schaart G and Vooijs P: Use of
Monoclonal antibodies to keratin 7 in the differential diagnosis of
adenocarcinomas. Am J Pathol. 136:641–655. 1990.PubMed/NCBI
|
|
12
|
Wang C, Curtis JE, Geissler EN, McCulloch
EA and Minden MD: The expression of the proto-oncogene C-kit in the
blast cells of acute myeloblastic leukemia. Leukemia. 3:699–702.
1989.PubMed/NCBI
|
|
13
|
Ikeda H, Kanakura Y, Tamaki T, Kuriu A,
Kitayama H, Ishikawa J, Kanayama Y, Yonezawa T, Tarui S and Griffin
JD: Expression and functional role of the proto-oncogene c-kit in
acute myeloblastic leukemia cells. Blood. 78:2962–2968.
1991.PubMed/NCBI
|
|
14
|
Natali PG, Nicotra MR, Winkler AB,
Cavaliere R, Bigotti A and Ullrich A: Progression of human
cutaneous melanoma is associated with loss of expression of c-kit
proto-oncogene receptor. Int J Cancer. 52:197–201. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berdel WE, De Vos S, Maurer J, Oberberg D,
von Marschall Z, Schroeder JK, Li J, Ludwig WD, Kreuser ED, Thiel
E, et al: Recombinant human stem cell factor stimulates growth of a
human glioblastoma cell line expressing c-kit protooncogene. Cancer
Res. 52:3498–3502. 1992.PubMed/NCBI
|
|
16
|
Natali PG, Nicotra MR, Sures I, Mottolese
M, Botti C and Ullrich A: Breast cancer is associated with loss of
the c-kit oncogene product. Int J Cancer. 52:713–717. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hida T, Ueda R, Sekido Y, Hibi K, Matsuda
R, Ariyoshi Y, Sugiura T and Takahashi T and Takahashi T: Ectopic
expression of c-kit in small-cell lung cancer. Int J Cancer Suppl.
8:108–109. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hines SJ, Organ C, Kornstein MJ and
Krystal GW: Coexpression of the c-kit and stem cell factor genes in
breast carcinomas. Cell Growth Differ. 6:769–779. 1995.PubMed/NCBI
|
|
19
|
Bokemeyer C, Kuczyk MA, Dunn T, Serth J,
Hartmann K, Jonasson J, Pietsch T, Jonas U and Schmoll HJ:
Expression of stem-cell factor and its receptor c-kit protein in
normal testicular tissue and malignant germ-cell tumours. J Cancer
Res Clin Oncol. 122:301–306. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krystal GW, Hines SJ and Organ CP:
Autocrine growth of small cell lung cancer mediated by coexpression
of c-kit and stem cell factor. Cancer Res. 56:370–376.
1996.PubMed/NCBI
|
|
21
|
Montone KT, van Belle P, Elenitsas R and
Elder DE: Protooncogene c-kit expression in malignant melanoma:
Protein loss with tumor progression. Mod Pathol. 10:939–944.
1997.PubMed/NCBI
|
|
22
|
Tian Q, Frierson HF Jr, Krystal GW and
Moskaluk CA: Activating c-kit gene mutations in human germ cell
tumors. Am J Pathol. 154:1643–1647. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng F, Liu XH, Xie Q, Liu WQ, Bai CG and
Ma DL: Expression and mutation of c-kit gene in gastrointestinal
stromal tumors. World J Gastroenterol. 9:2548–2451. 2003.PubMed/NCBI
|
|
24
|
Bellone G, Smirne C, Carbone A, Buffolino
A, Scirelli T, Prati A, Solerio D, Pirisi M, Valente G, Nano M and
Emanuelli G: KIT/stem cell factor expression in premalignant and
malignant lesions of the colon mucosa in relationship to disease
progression and outcomes. Int J Oncol. 29:851–859. 2006.PubMed/NCBI
|
|
25
|
Quinlan RA, Schiller DL, Hatzfeld M,
Achtstätter T, Moll R, Jorcano JL, Magin TM and Franke WW: Patterns
of expression and organization of cytokeratin intermediate
filaments. Ann N Y Acad Sci. 455:282–306. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moll R, Schiller DL and Franke WW:
Identification of protein IT of the intestinal cytoskeleton as a
novel type I cytokeratin with unusual properties and expression
patterns. J Cell Biol. 111:567–580. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamagishi H, Imai Y, Okamura T, Fukuda K,
Ono Y, Ban S, Inoue T and Ueda Y: Aberrant cytokeratin expression
as a possible prognostic predictor in poorly differentiated
colorectal carcinoma. J Gastroenterol Hepatol. 28:1815–1822. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Varadhachary GR, Abbruzzese JL and Lenzi
R: Diagnostic strategies for unknown primary cancer. Cancer.
100:1776–1785. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kirchner T, Müller S, Hattori T, Mukaisyo
K, Papadopoulos T, Brabletz T and Jung A: Metaplasia,
intraepithelial neoplasia and early cancer of the stomach are
related to dedifferentiated epithelial cells defined by
cytokeratin-7 expression in gastritis. Virchows Arch. 439:512–522.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Slaughter DP, Southwick HW and Smejkal W:
Field cancerization in oral stratified squamous epithelium;
clinical implications of multicentric origin. Cancer. 6:963–968.
1953. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo Y, Yu M and Grady WM: Field
cancerization in the colon: A role for aberrant DNA methylation?
Gastroenterol Rep (Oxf). 2:16–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lyda MH, Noffsinger A, Belli J and
Fenoglio-Preiser CM: Microsatellite instability and K-ras mutations
in patients with ulcerative colitis. Hum Pathol. 31:665–671. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leedham SJ, Graham TA, Oukrif D, McDonald
SA, Rodriguez-Justo M, Harrison RF, Shepherd NA, Novelli MR,
Jankowski JA and Wright NA: Clonality, founder mutations, and field
cancerization in human ulcerative colitis-associated neoplasia.
Gastroenterology. 136:542–550.e6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koizumi K, Alonso S, Miyaki Y, Okada S,
Ogura H, Shiiya N, Konishi F, Taya T, Perucho M and Suzuki K:
Array-based identification of common DNA methylation alterations in
ulcerative colitis. Int J Oncol. 40:983–994. 2011.PubMed/NCBI
|
|
35
|
Galandiuk S, Rodriguez-Justo M, Jeffery R,
Nicholson AM, Cheng Y, Oukrif D, Elia G, Leedham SJ, McDonald SA,
Wright NA and Graham TA: Field cancerization in the intestinal
epithelium of patients with Crohn's ileocolitis. Gastroenterology.
142:855–864.e8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lochhead P, Chan AT, Nishihara R, Fuchs
CS, Beck AH, Giovannucci E and Ogino S: Etiologic field effect:
Reappraisal of the field effect concept in cancer predisposition
and progression. Mod Pathol. 28:14–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Facista A, Nguyen H, Lewis C, Prasad AR,
Ramsey L, Zaitlin B, Nfonsam V, Krouse RS, Bernstein H, Payne CM,
et al: Deficient expression of DNA repair enzymes in early
progression to sporadic colon cancer. Genome Integr. 3:32012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harbaum L, Pollheimer MJ, Kornprat P,
Lindtner RA, Schlemmer A, Rehak P and Langner C: Keratin 7
expression in colorectal cancer-freak of nature or significant
finding? Histopathology. 59:225–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park SY, Kim HS, Hong EK and Kim WH:
Expression of cytokeratins 7 and 20 in primary carcinomas of the
stomach and colorectum and their value in the differential
diagnosis of metastatic carcinomas to the ovary. Hum Pathol.
33:1078–1085. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Besmer P, Murphy JE, George PC, Qiu FH,
Bergold PJ, Lederman L, Snyder HW Jr, Brodeur D, Zuckerman EE and
Hardy WD: A new acute transforming feline retrovirus and
relationship of its oncogene v-kit with the protein kinase gene
family. Nature. 320:415–421. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yarden Y, Kuang WJ, Yang-Feng T, Coussens
L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U and
Ullrich A: Human proto-oncogene c-kit: A new cell surface receptor
tyrosine kinase for an unidentified ligand. EMBO J. 6:3341–3351.
1986.
|
|
42
|
Ashman LK: The biology of stem cell factor
and its receptor C-kit. Int J Biochem Cell Biol. 31:1037–1051.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rönnstrand L: Signal transduction via the
stem cell factor receptor/c-Kit. Cell Mol Life Sci. 61:2535–2548.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Preto A, Moutinho C, Velho S, Oliveira C,
Rebocho AP, Figueiredo J, Soares P, Lopes JM and Seruca R: A subset
of colorectal carcinomas express c-KIT protein independently of
BRAF and/or KRAS activation. Virchows Arch. 450:619–626. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sammarco I, Capurso G, Coppola L, Bonifazi
AP, Cassetta S, Fave G Delle, Carrara A, Grassi GB, Rossi P, Sette
C and Geremia R: Expression of the protooncogene c-KIT in normal
and tumor tissues from colorectal carcinoma patients. Int J
Colorectal Dis. 19:545–553. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Friederichs J, von Weyhern CW, Rosenberg
R, Doll D, Busch R, Lordick F, Siewert JR and Sarbia M:
Immunohistochemical detection of receptor tyrosine kinases c-kit,
EGF-R, and PDGF-R in colorectal adenocarcinomas. Langenbecks Arch
Surg. 395:373–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Reed J, Ouban A, Schickor FK, Muraca P,
Yeatman T and Coppola D: Immunohistochemical staining for c-Kit
(CD117) is a rare event in human colorectal carcinoma. Clin
Colorectal Cancer. 2:119–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Medinger M, Kleinschmidt M, Mross K,
Wehmeyer B, Unger C, Schaefer HE, Weber R and Azemar M: c-kit
(CD117) expression in human tumors and its prognostic value: An
immunohistochemical analysis. Pathol Oncol Res. 16:295–301. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Toyota M, Hinoda Y, Takaoka A, Makiguchi
Y, Takahashi T, Itoh F, Imai K and Yachi A: Expression of c-kit and
kit ligand in human colon carcinoma cells. Tumour Biol. 14:295–302.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lahm H, Amstad P, Yilmaz A, Borbenyi Z,
Wyniger J, Fischer JR, Suardet L, Givel JC and Odartchenko N:
Interleukin 4 down-regulates expression of c-kit and autocrine stem
cell factor in human colorectal carcinoma cells. Cell Growth
Differ. 6:1111–1118. 1995.PubMed/NCBI
|
|
51
|
Kitamura T, Kometani K, Hashida H,
Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M,
Takabayashi A, et al: SMAD4-deficient interstinal tumors recruit
CCR1+ myeloid cells that promote invasion. Nat Genet. 39:467–475.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kitamura T, Fujishita T, Loetscher P,
Revesz L, Hashida H, Kizaka-Kondoh S, Aoki M and Taketo MM:
Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses
colon cancer liver metastasis by blocking accumulation of immature
myeloid cells in a mouse model. Proc Natl Acad Sci USA. 107:pp.
13063–13068. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yilmaz E, Celik O, Simsek Y, Turkcuoglu I,
Celik E, Gül M, Hascalik S and Aydin NE: c-Kit proto-oncogene
expression in endometrial hyperplasia and endometrial cancer. Arch
Gynecol Obstet. 286:197–200. 2012. View Article : Google Scholar : PubMed/NCBI
|