Introduction
To the best of our knowledge, bladder cancer is
currently the fourth most common cancer worldwide (1). In China, the incidence of bladder cancer
had increased from 1991 to 2009, with >20,000 mortalities in
2009 (2,3). In the United States, there were ~76,000
new cases of bladder cancer and 16,000 bladder cancer-associated
mortalities in 2016 (4,5). Currently, although surgical therapies
associated with adjuvant chemotherapy following surgery have made
marked advances in the treatment of bladder cancer, the rate of
recurrence remains high (6).
Furthermore, chemotherapy has a high incidence of side effects.
Consequently, research into and development of highly efficient and
minimally toxic novel drugs is urgently required (7).
Cyclin-dependent kinases (CDKs) are a family of
serine/threonine kinases that are required for cell cycle
progression, particularly CDK2. CDK2 serves an important role in
G1/S phase transition and S phase progression (8,9). In
previous studies, it was also demonstrated that cyclin A-CDK2
activity was markedly upregulated in the early stages of apoptosis
and that this upregulation was required for the progression of
apoptosis induced by treatment with ginsenoside Rh2 (10). Nevertheless, the mechanism by which
CDK2 functions in the progression of apoptosis in T24 cells induced
by isoliquiritigenin (ISL) remains unknown.
Recently, natural products have become one of the
important sources in modern drug discovery for its active
components with anticancer potential and fewer side effects,
particularly for anticancer drugs (6). Licorice is frequently used for culinary
purposes in western countries (11–13), and
is one of the most commonly used herbs in China for therapeutic
effects on different diseases ranging from duodenal ulcers to
cancers (14–16). ISL is a natural flavonoid isolated
from the root of licorice (Glycyrrhiza uralensis). Previous
studies have indicated that ISL possesses distinct biological
properties, including anti-inflammatory, antioxidant, anti-platelet
aggregation, vasorelaxant and estrogenic effects (17–19). A
number of studies have demonstrated marked antitumor activities of
ISL, including apoptosis induction, cell cycle arrest, migration
inhibition and initiation of oxidative stress (20–22).
Previous studies have also demonstrated that ISL possesses
chemopreventive activities (23–26).
In view of previous studies suggesting an
association between CDK2 and apoptosis, the aim of the present
study was to determine the effect of MK-8776, an inhibitor of CDK2,
on the ISL-induced apoptotic rate in T24 cells. In the present
study, the potential underlying molecular mechanism of CDK2 in
apoptosis induced by ISL in T24 cells was investigated.
Materials and methods
Reagents
ISL (purity, ≥98%) was obtained from Jiangxi Herb
Tiangong Technology Co., Ltd. (Jiangxi, China). Culture medium
(RPMI-1640), glutamine and dimethylsulfoxide (DMSO) were purchased
from Sigma; Merck KGaA (Darmstadt, Germany). Fetal bovine serum
(FBS) was purchased from Tianjin Hao Yang Biological Manufacture
Co., Ltd. (Tianjin, China). Penicillin and streptomycin were
obtained from Shandong Sunrise Pharmaceutical Co., Ltd. (Shandong,
China). MK-8776 was obtained from Selleck Chemicals (Shanghai,
China). The CDK2/Cyclin A Kinase Assay kit was obtained from
Shanghai Genmed Gene Pharmaceutical Technology Co., Ltd. (Shanghai,
China). Primary antibodies against β-actin (catalog no. sc-130300),
B cell lymphoma 2 (Bcl-2) (catalog no. sc-130308), caspase-3
(catalog no. sc-56052) and Bcl-2 associated X factor (Bax; catalog
no. sc-80658) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Unless indicated otherwise, other reagents were
purchased from Sigma; Merck KGaA. All other chemicals were of
analytical grade.
Cell culture and treatment
T24 cells were purchased from the Cell Bank of the
Committee on Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). The cells were maintained in RPMI-1640
medium supplemented with 10% (v/v) FBS, 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. Cells were allowed to attach for 24 h before
treatment. ISL was dissolved in DMSO and diluted with fresh medium
to achieve the desired concentration. The final concentration of
DMSO was <0.2% in the fresh medium, and DMSO at this
concentration exhibited no significant effect on cell
viability.
Cell viability assay
The T24 cells were trypsinized and seeded into
96-well plates at 8×104 cells/ml. Subsequently, the
cells were exposed to ISL (0, 15, 30, 45, 60 and 75 µg/ml) at 37°C
for 24 h followed by further incubation in fresh medium at 37°C for
another 24 h. The effect of ISL-induced cytotoxicity was evaluated
using a sulforhodamine B (SRB) assay as described previously
(27). The optical density (OD) was
determined at a wavelength of 490 nm. The inhibition ratio was
calculated as follows.
Inhibitionratio(%)(1–ODofcellsculturedwithISLODofcontrol)X100
Trypan blue exclusion assay
The lethality of ISL on T24 cells was determined
using a trypan blue exclusion assay. After 24 h of incubation with
0, 15, 30, 45, 60 and 75 µg/ml ISL at 37°C, T24 cells were removed
from the culture medium and cells that excluded trypan blue were
counted in a Neubauer chamber using a light microscope. The lethal
ratio was calculated as follows.
Lethalratio(%)=DeadcellcountTotalcellcountx100
Hoechst 33258 staining
The T24 cells were seeded in 6-well culture dishes
(2×105 cells/well). Following treatment with ISL at 0,
30, 50 and 70 µg/ml at 37°C for 24 h, the cells were fixed with
Carnoy's fixative consisting of methanol and glacial acetic acid
(3:1, v/v), washed twice with PBS, stained with Hoechst 33258 (5
mg/ml) for 5 min in the dark, as described previously (28), and washed extensively three times with
PBS. Nuclear staining was examined using a fluorescence microscope
(Nikon LH-M100CB; Jirui Co., Ltd., Suzhou, China) and images were
captured using Image-Pro Plus Premier (version 9.1.4; Media
Cybernetics, Inc., Rockville, MD, USA). Cells that exhibited
decreased nuclear size, chromatin condensation, intense
fluorescence and nuclear fragmentation were considered
apoptotic.
Analysis of apoptosis by Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI)
The cells (2×105 cells/well) were
pretreated with 0.16 µM MK-8776 at 37°C for 1 h, incubated with ISL
at 0, 30, 50 and 70 µg/ml at 37°C for 24 h and harvested. Specific
binding of Annexin V-FITC was carried out by incubating the cells
at room temperature for 15 min in a binding buffer containing a
saturating concentration of Annexin V-FITC and PI, as described
previously (29). Following
incubation, the cells were quantified using a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) and analyzed using CellQuest
Pro acquisition software (version 4.0; FACS Calibur; BD
Biosciences, San Jose, CA, USA).
Detection of mitochondrial membrane
potential (ΔΨm) using
5,5,6,6-tetrachloro-1,1,3,3-tetraethyl benzimidazole carbocyanine
iodide (JC-1)
Following reaching optimal confluence, cells were
pretreated with 0.16 µM MK-8776 for 1 h, and incubated with 50
µg/ml ISL at 37°C for 24 h. In addition, the cells not pretreated
with MK-8776 at 0.16 µM were incubated with ISL at 0, 30, 50 and 70
µg/ml at 37°C for 4, 8, 16 and 24 h washed with ice-cold PBS,
exposed to 500 µl JC-1 dye and incubated in the dark at 37°C for 20
min. Following washing three times with incubation buffer (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China), the cells were diluted
in 500 µl incubation buffer and the fluorescence intensity of the
cells was analyzed using a FACScan flow cytometer (BD Biosciences).
Fluorescence at excitation/emission wavelengths of 485/580 nm (red)
and 485/530 nm (green) was read using a fluorescence plate reader
(Varioskan Flash 3001; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) and quantitative PCR (qPCR)
RT-PCR and qPCR (30,31) were
performed to examine the mRNA expression of B-cell lymphoma-2
(Bcl-2), Bcl-2-associated X protein (Bax), Bcl-2-interacting
mediator of cell death (Bim), apoptotic protease-activating
factor-1 (Apaf-1), caspase-9 and caspase-3. The primer sequences
are presented in Table I. The cells
were pretreated with 0.16 µM MK-8776 at 37°C for 1 h and incubated
with 50 µg/ml ISL at 37°C for 24 h. The cells were further
incubated with 0, 30, 50 and 70 µg/ml ISL at 37°C for 24 h and
harvested. Total RNA was extracted from the T24 cells using TRIzol
reagent (Shanghai Sangong Biotech Co., Ltd. (Shanghai, China) and
quality was determined according to the
A260/A280 ratio. cDNA synthesis was performed
using a RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Subsequently, the synthesized cDNA was amplified. The cycling
conditions were set as indicated by Sangong Biotech Co. Ltd. The
reaction mixture contained 12.5 µl 2X PCR Master mix (Sangong
Biotech Co., Ltd.), 3 µl cDNA template and 0.5 µl each primer. The
cycling conditions used were as follows: 95°C for 5 min, followed
by 37 cycles of 94°C for 30 sec, heating at specified melting
temperature (Tm, Table I)
for 30 sec, 72°C for 30 sec and 72°C for 5 min. cDNA products were
analyzed using 2% agarose gel electrophoresis. Three independent
experiments were performed. Images were captured using Image-Pro
Plus Premier (version 9.1.4; Media Cybernetics, Inc., Rockville,
MD, USA).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Primer | Tm,
°C |
|---|
| Bcl-2 | Forward:
5′-GGAAATATGGCGCACGCT-3′ | 59 |
|
| Reverse:
5′-TCACTTGTGGCCCA-3′ |
|
| Bax | Forward:
5′-ACGAACTGGACAGTAACATGGAG-3′ 59 |
|
|
| Reverse:
5′-CAGTTTGCTGGCAAAGTAGAAAAG-3′ |
|
| Bim | Forward:
5′-CACATGAGCACATTTCCCTCT-3′ 57 |
|
|
| Reverse:
5′-AAGGCACAAAACCTGCAGTAA-3′ |
|
| Apaf-1 | Forward:
5′-TGGAATGGCAGGCTGTGGGA-3′ | 62 |
|
| Reverse:
5′-TGCACTCCCCCTGGGAAACA-3′ |
|
| Caspase-9 | Forward:
5′-ACGCGTTACTGGCATTGAGG-3′ | 62 |
|
| Reverse:
5′-CAGTGGGCTCACTCTGAAGACC-3′ |
|
| Caspase-3 | Forward:
5′-CTGGACTGTGGCATTGAGAC-3′ 59 |
|
|
| Reverse:
5′-ACAAAGCGACTGGATGAACC-3′ |
|
| GAPDH | Forward:
5′-CAAGGTCATCCATGACAACTTTG-3′ 57 |
|
|
| Reverse:
5′-GTCCACCACCCTGTTGCTGTAG-3′ |
|
Relative gene expression was quantified using qPCR
with a SYBR Green kit (Rotor-Gene Q; Qiagen, Inc. Valencia, CA,
USA), enabling detection of PCR products, according to the
manufacturer's protocol. The cDNA was amplified using qPCR with the
primer pairs given in Table I
(synthesized by Sangon Biotech Co., Ltd.). The cycling conditions
were as follows: 95°C for 5 min, followed by 40 cycles of 95°C for
30 sec, Tm (see Table I)
for 30 sec, and 72°C for 30 sec. A total of three independent
experiments were performed. Quantitative data was analyzed using
the Sequence Detection system software (version 1.0; Applied
Biosystems; Thermo Fisher Scientific, Inc.). The relative quantity
of target mRNA was determined using the 2−ΔΔCq method
(32). Each sample was analyzed using
GAPDH as an endogenous reference gene for mRNA normalization.
CDK2 activity assay
T24 cells were seeded at a density of
2.5×105 cells/ml in flasks and incubated at 37°C for 24
h. The cells were pretreated with 0.16 µM MK-8776 at 37°C for 1 h
and incubated with 50 µg/ml ISL at 37°C for 24 h. The cells were
further incubated with ISL at 0, 30, 50 and 70 µg/ml at 37°C for 4,
8, 16 and 24 h. Following the addition of 2 ml cleanup solution
(CDK2/cyclin A kinase assay reagent A; Shanghai Genmed Gene
Pharmaceutical Technology Co., Ltd., Shanghai, China) and protein
extraction, the CDK2/cyclin A kinase assay kit (Shanghai Genmed
Gene Pharmaceutical Technology Co., Ltd.) was used to determine
CDK2 activity, according to the manufacturer's protocol.
Experiments were performed in triplicate.
Western blot analysis
The cells were harvested, extracted with cell lysis
buffer (catalog no. R0010; Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) and centrifuged at 12,000 × g
for 5 min at 4°C. Protein concentration was determined using a
bicinchoninic acid assay kit (catalog no. PC0020; Beijing Solarbio
Science & Technology Co., Ltd.). The protein lysates were
diluted to equal concentrations and heated at 100°C for 5 min.
Soluble lysates (15 µl/lane) were subjected to SDS-PAGE (10% gel),
transferred onto nitrocellulose membranes (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) and blocked with 5% non-fat milk
in Tris-buffered saline with Tween-20 (TBST) for 2 h at room
temperature. The membranes were incubated with the anti-β-actin
(mouse anti-human; 1:5,000), anti-Bcl-2 (monoclonal, mouse
anti-human; 1:500), anti-caspase-3 (monoclonal, mouse anti-human;
1:500) and anti-Bax (monoclonal, mouse anti-human; 1:400) at 4°C
overnight and incubated with horseradish peroxidase-conjugated
secondary antibody (goat anti-mouse, 1:5,000; catalog no. HS201;
Beijing TransGen Biotech Co., Ltd., Beijing, China). Western blots
were developed using enhanced chemiluminescence (ECL, Thermo Fisher
Scientific, Inc.) and exposed on radiographic film (Kodak,
Rochester, NY, USA). Densitometric analysis was performed using
Image Quant LAS 4000 (Amersham, Buckinghamshire, UK), and the
autoradiographs were quantified using ImageJ software (version
1.49n; National Institutes of Health, Bethesda, Maryland, USA).
Three independent experiments were performed.
Statistical analysis
All results were obtained by ≥3 independent
experiments. The data are expressed as the mean ± standard
deviation. One-way analysis of variance was performed, and the mean
values were compared using Fisher's multiple comparison test. All
statistical analyses were performed using Minitab for Windows
(version 16; Minitab Inc., PA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
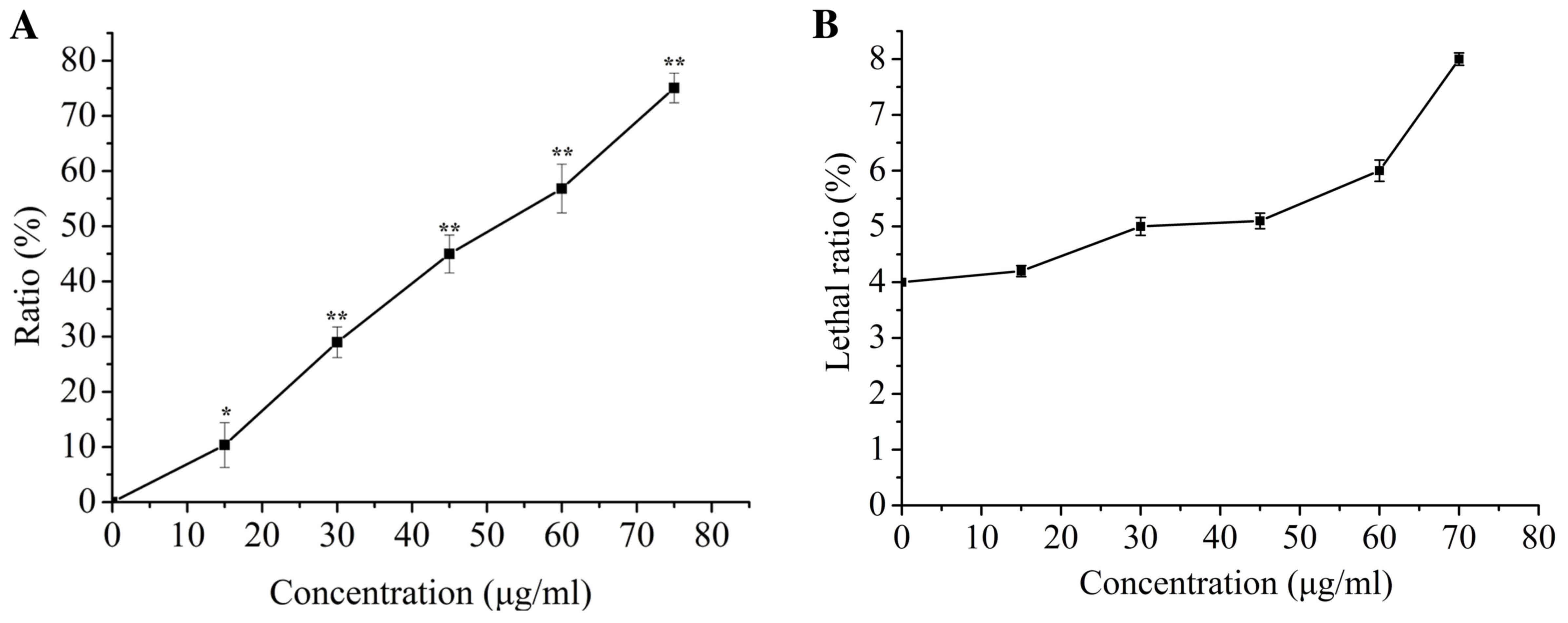
ISL treatment inhibits proliferation
of T24 cells
The effect of ISL-induced cytotoxicity was
determined using an SRB assay. At 24 h following ISL treatment,
cell proliferation decreased with increasing concentrations of ISL,
and the half-maximal inhibitory concentration was ~50 µg/ml
(Fig. 1A). However, the SRB assay did
not reveal whether the decrease in cell proliferation was
associated with cell death or an apparent loss of viability. To
solve this problem, a trypan blue exclusion test was performed on
T24 cells treated with ISL. ISL (<75 µg/ml) did not
significantly affect the lethality ratio of T24 cells (Fig. 1B), which indicated that the inhibitory
effect of ISL on cell proliferation was not due to direct killing
of T24 cells and identified that no significant cytotoxicity was
observed compared with the control.
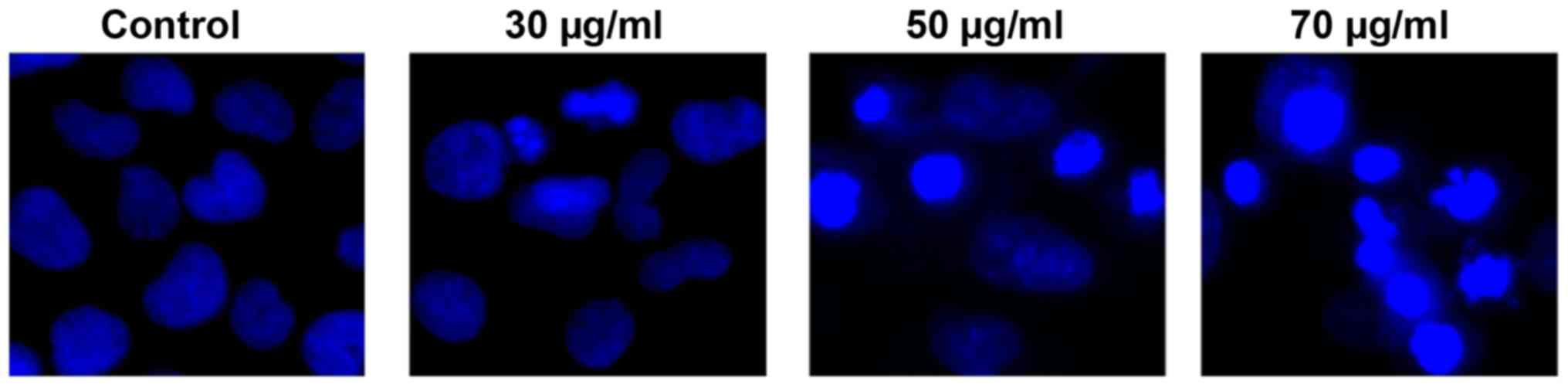
Nuclear morphology
Morphological changes were examined by Hoechst 33258
staining. T24 cells were treated with ISL for 24 h, and evident
apoptotic morphological changes were observed. In the control
group, the nuclei of T24 cells were round and homogeneously stained
(Fig. 2), whereas ISL-treated cells
exhibited evident apoptotic characteristics including cell
shrinkage, loss of membrane integrity or deformation, nuclear
fragmentation and chromatin compaction of late apoptotic
appearance.
Assessment of apoptotic cells
Apoptosis was determined by staining cells with
Annexin V-FITC and PI. A concentration-dependent increase in the
apoptotic ratio of T24 cells was demonstrated by Annexin V and PI
staining using flow cytometry (Fig.
3). After 24 h of incubation, there were limited numbers of
apoptotic cells in the control group (5.57±2.89%), whereas in the
30, 50 and 70 µg/ml ISL treatment groups, the proportion of
apoptotic cells was 19.72±2.03, 35.3±1.93 and 59.77±3.09%,
respectively.
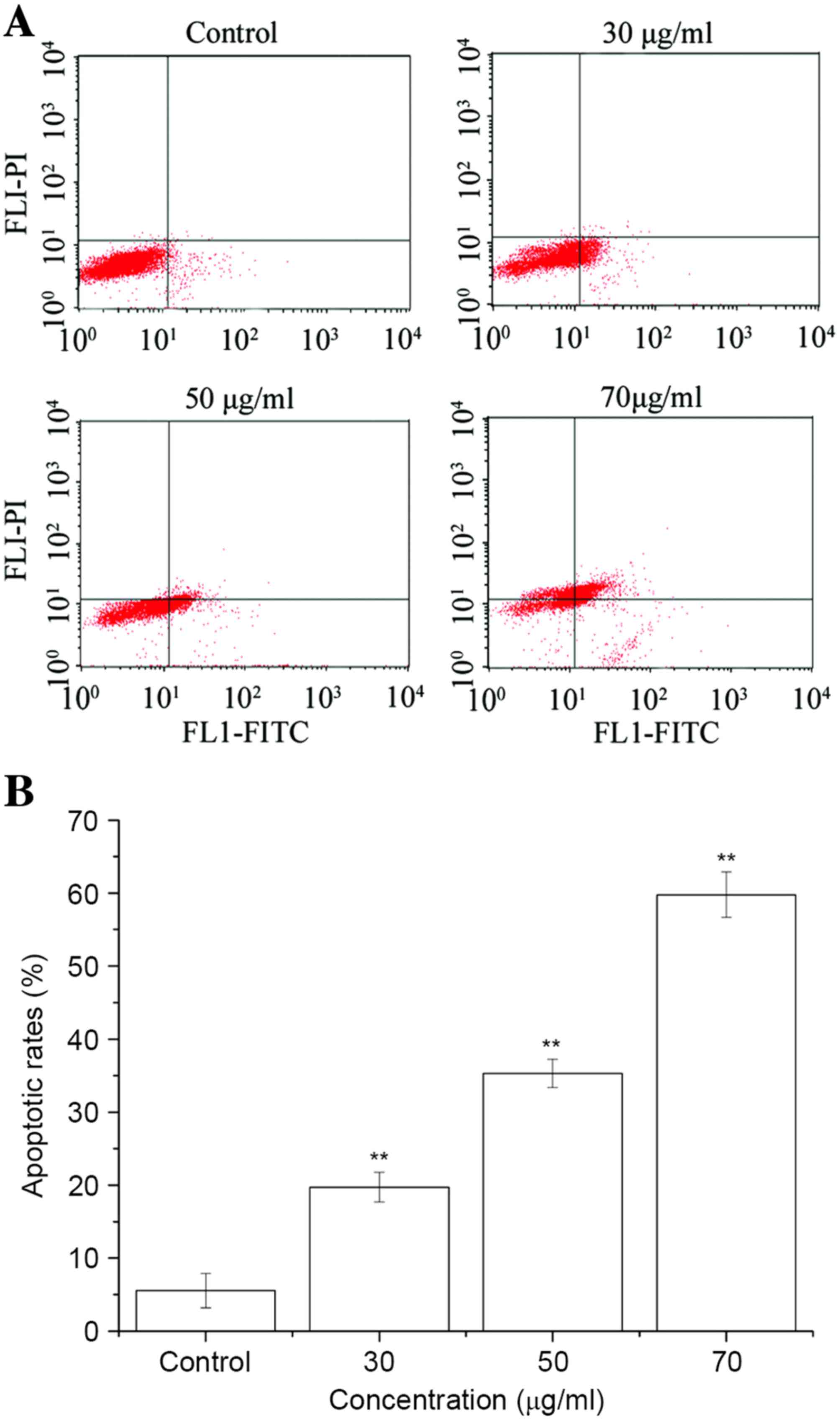 | Figure 3.Effect of ISL on apoptotic rates in
T24 cells. Cells were treated with ISL at 0, 30, 50 and 70 µg/ml
for 24 h, permeabilized, stained with Annexin V, and kept on ice
until analysis using flow cytometry. (A) Cells in the lower left
quadrant were alive, cells in the lower right quadrant were in
early apoptosis, cells in the upper right quadrant were in late
apoptosis and cells in the upper left quadrant were dead. (B)
Quantification of apoptotic rates. Results are presented as the
mean ± standard deviation of three separate experiments.
**P<0.01 vs. untreated control group cells. ISL,
isoliquiritigenin; FITC, fluorescein isothiocyanate; PI, propidium
iodide. |
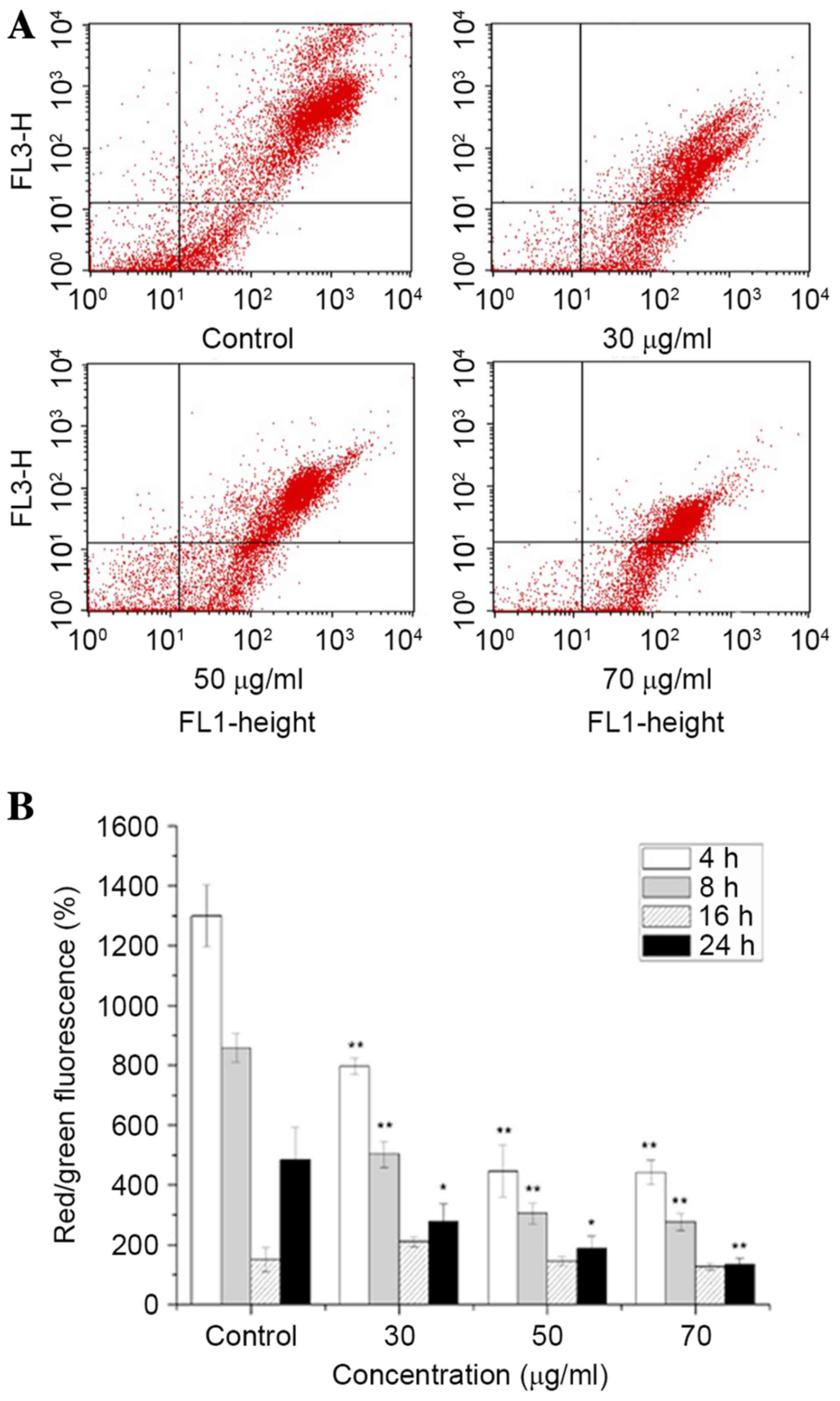
Effects of ISL on the ΔΨm
of the cells
Mitochondria serve an important role in the
regulation of apoptosis, and apoptosis mediated by the
mitochondrial signaling pathway is often associated with a decrease
in ΔΨm. The ΔΨm changes in T24 cells were
determined by staining with JC-1 dye following various treatment
periods, and detection using flow cytometry and fluorescence
microscopic analysis. The decrease in intensity of JC-1 dye
staining reflected a decrease in the ΔΨm. As presented
in Fig. 4, a concentration- and
time-dependent decrease in ΔΨm was observed in
ISL-treated cells.
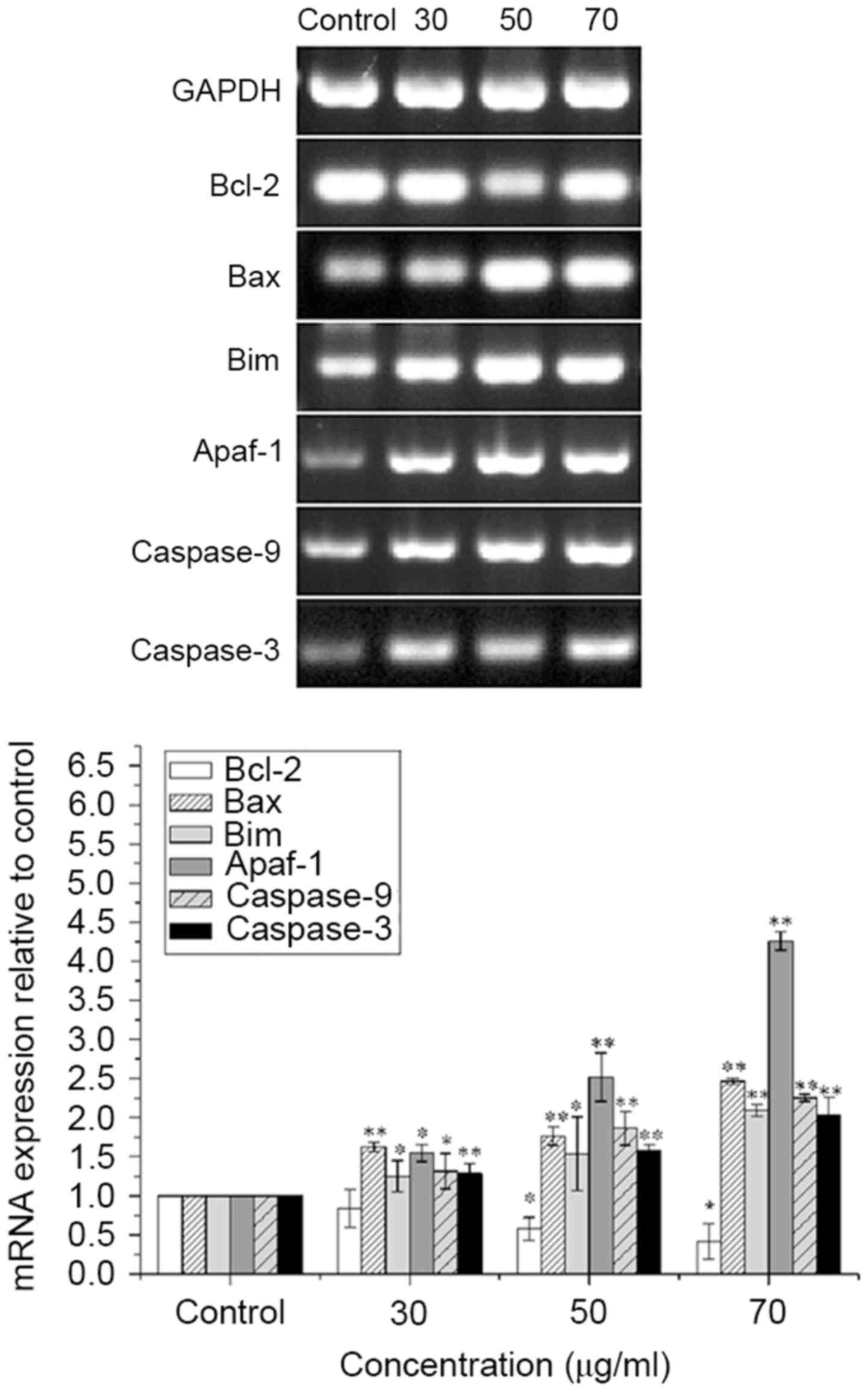
ISL increases the mRNA expression of
Bax, Bim, Apaf-1, caspase-9 and caspase-3, and decreases the mRNA
expression of Bcl-2 in T24 cells
The mRNA expression levels of Bax, Bim, Apaf-1,
caspase-9 and caspase-3 were significantly increased, and that of
Bcl-2 was significantly decreased in ISL-treated T24 cells in a
concentration-dependent manner (P<0.05 or <0.01; Fig. 5).
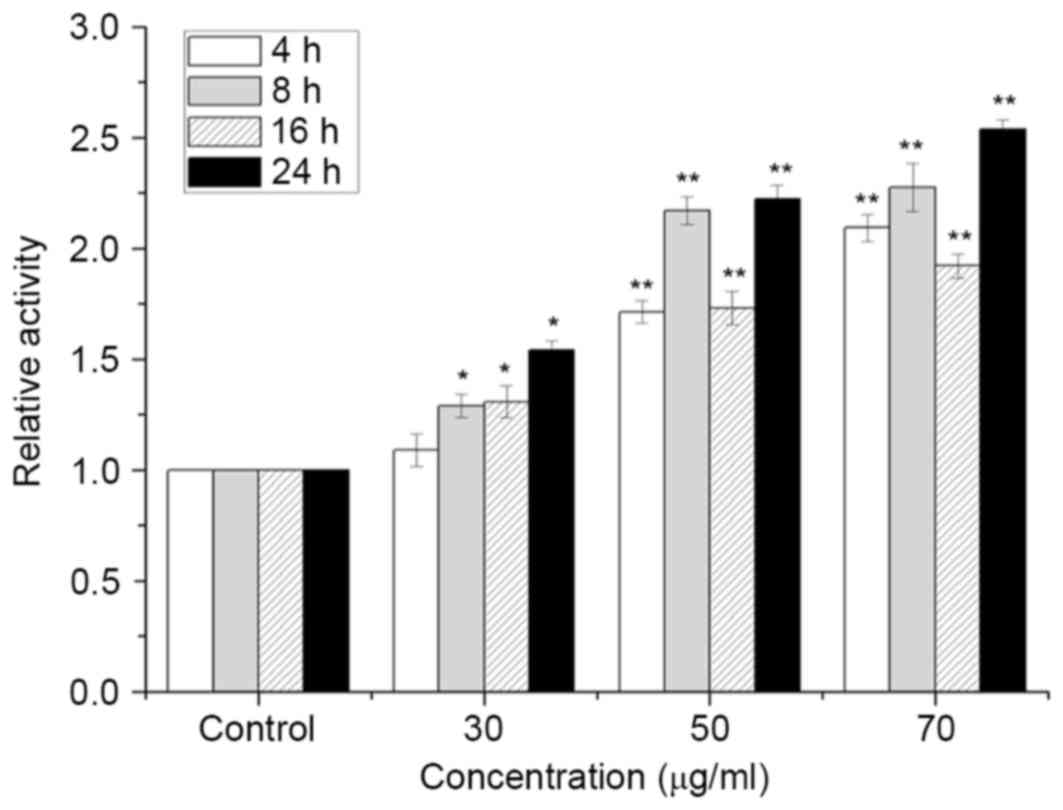
ISL increases the activity of CDK2 in
T24 cells
As presented in Fig.
6, CDK2 activity was increased significantly following
treatment with ISL compared with that of the control (P<0.05).
The maximum enhancement in CDK2 activity was observed following
treatment with 70 µg/ml ISL at all time points.
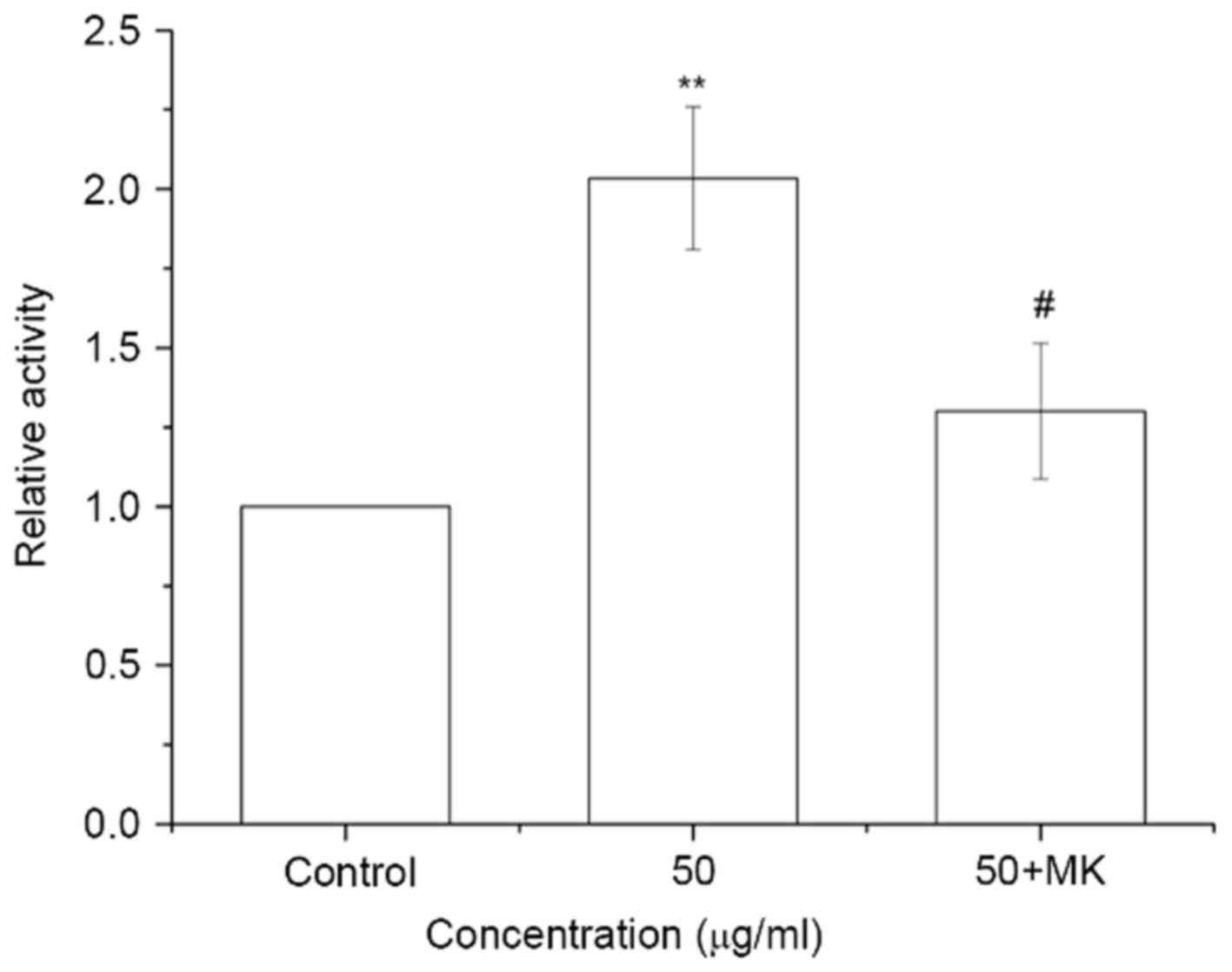
MK-8776 reverses the ISL-induced
increase in CDK2 activity in T24 cells
The cells were pretreated with 0.16 µM MK-8776 at
37°C for 1 h and incubated with ISL at 50 µg/ml at 37°C for 24 h.
CDK2 activity was significantly increased in ISL-treated cells
(P<0.01), whereas CDK2 activity in ISL-treated cells was
significantly decreased by pretreatment with MK-8776 for 1 h
compared with ISL-treated cells without MK-8776 pre-treatment
(P<0.05; Fig. 7).
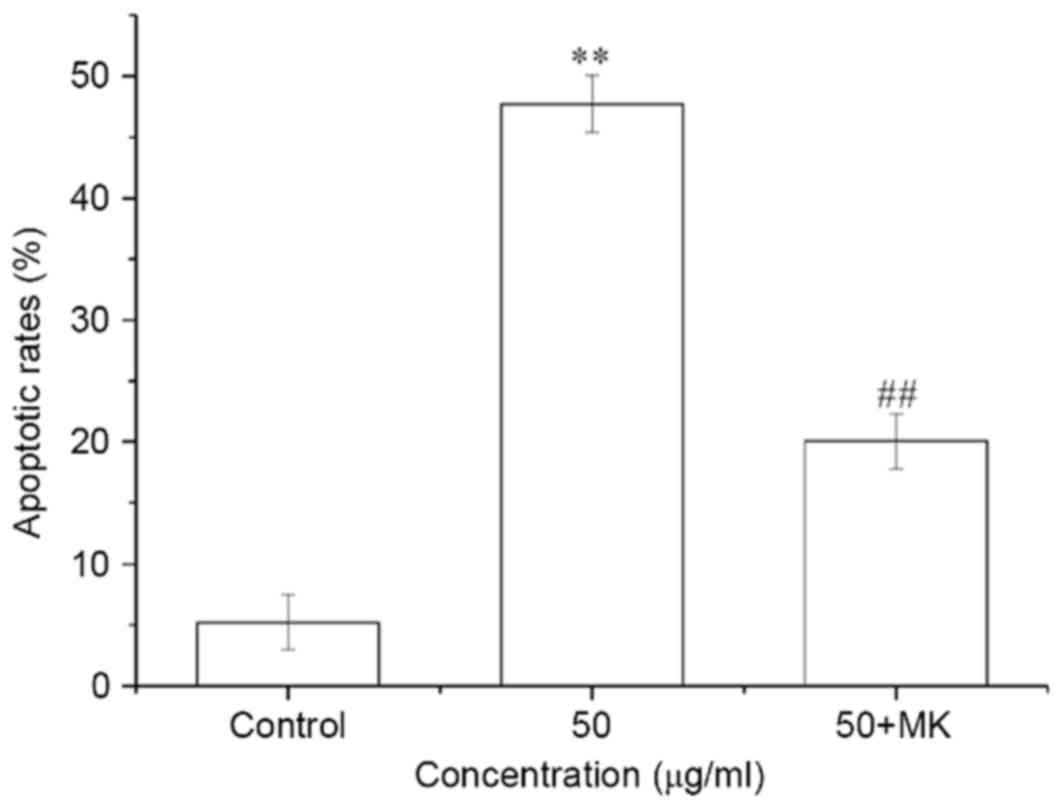
MK-8776 decreases the apoptotic ratio
induced by ISL in T24 cells
The cells were pretreated with 0.16 µM MK-8776 at
for 1 h and incubated with 50 µg/ml ISL for 24 h. The apoptotic
ratio of T24 cells was significantly increased by ISL treatment at
50 µg/ml for 24 h, whereas the apoptotic ratio of ISL-treated cells
was significantly decreased by pretreatment with MK-8776 for 1 h
compared with ISL-treated cells not pretreated with MK-8776
(P<0.01; Fig. 8).
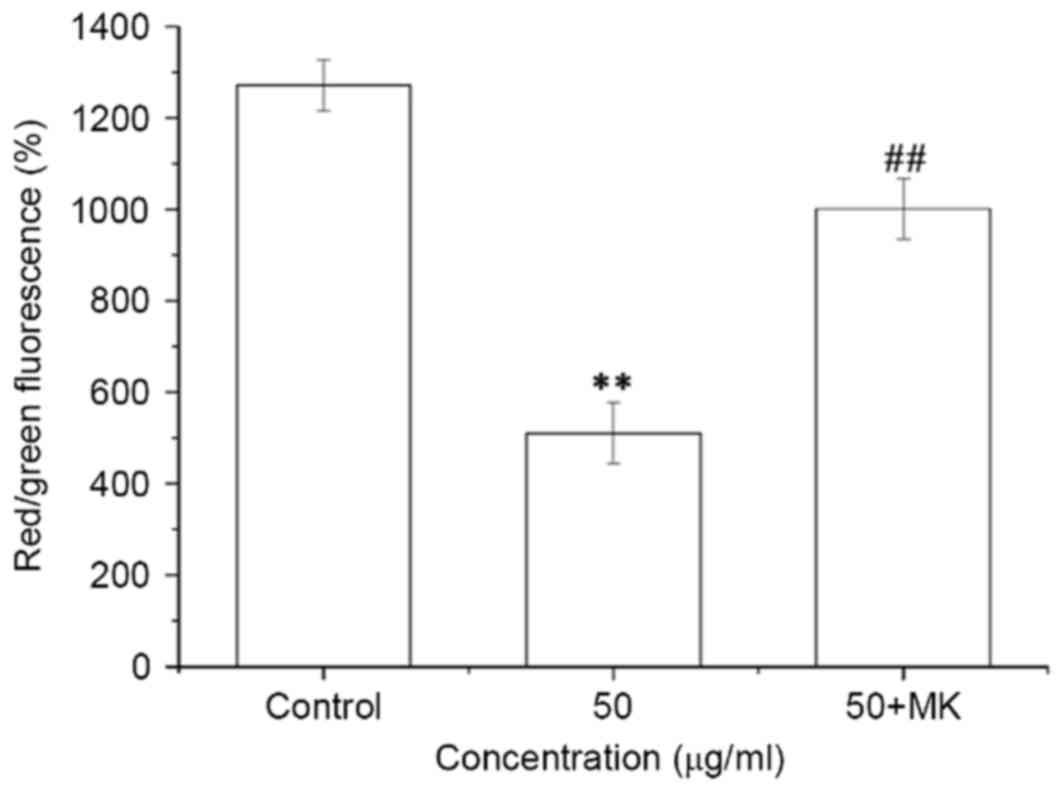
MK-8776 reverses the ISL-induced
decrease in the ΔΨm in T24 cells
ISL treatment at 50 µg/ml for 24 h led to a
significant decrease in ΔΨm in T24 cells, whereas
pretreatment with MK-8776 for 1 h significantly reversed the
decrease in ΔΨm induced by ISL in T24 cells (P<0.01;
Fig. 9).
MK-8776 reverses the increases in Bax,
Bim, Apaf-1, caspase-9 and caspase-3 mRNA expression, and the
decrease in Bcl-2 mRNA expression in T24 cells induced by ISL
treatment
Pretreatment with MK-8776 for 1 h led to a reversal
of the decrease in the mRNA expression level of antiapoptotic
Bcl-2, and of the increase in the mRNA expression of proapoptotic
Bax, Bim, Apaf-1, caspase-9 and caspase-3 induced by treatment with
ISL at 50 µg/ml (P<0.05 or <0.01; Fig. 10).
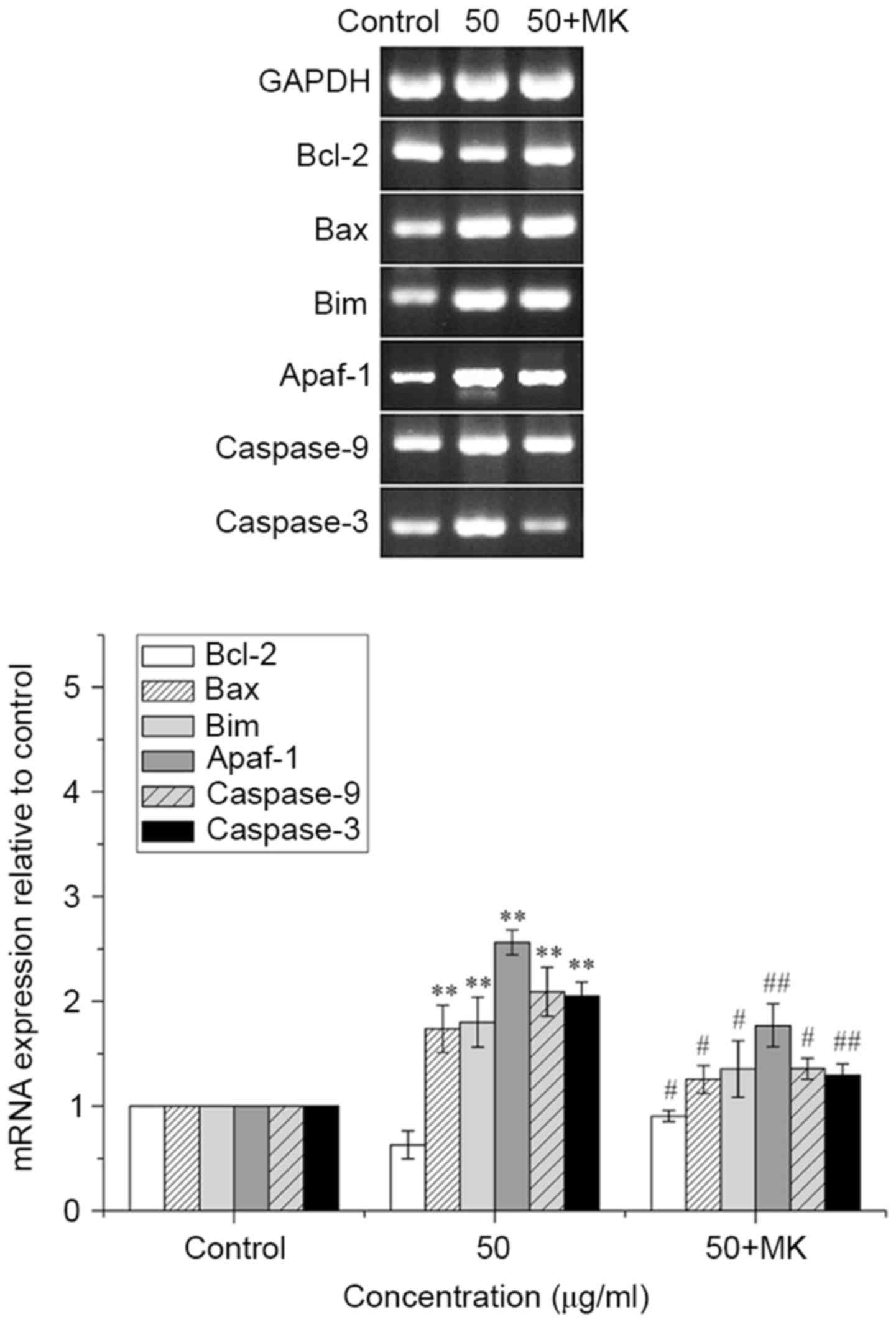 | Figure 10.MK-8776 antagonizes the increases in
Bax, Bim, Apaf-1, caspase-9 and caspase-3 mRNA expression, and the
decrease in Bcl-2 mRNA expression in T24 cells. T24 cells
pretreated or not with MK-8776 were treated with ISL at 50 µg/ml
and mRNA expression was determined using the reverse
transcription-polymerase chain reaction and agarose gel
electrophoresis. Quantification results are presented as the mean ±
standard deviation of three independent experiments. **P<0.01
vs. untreated control group cells; #P<0.05,
##P<0.01 vs. 50 µg/ml ISL-treated group cells. Bax,
Bcl-2-associated X protein; Bim, Bcl-2-interacting mediator of cell
death; Apaf-1, apoptotic protease-activating factor-1; Bcl-2,
B-cell lymphoma 2; ISL, isoliquiritigenin. |
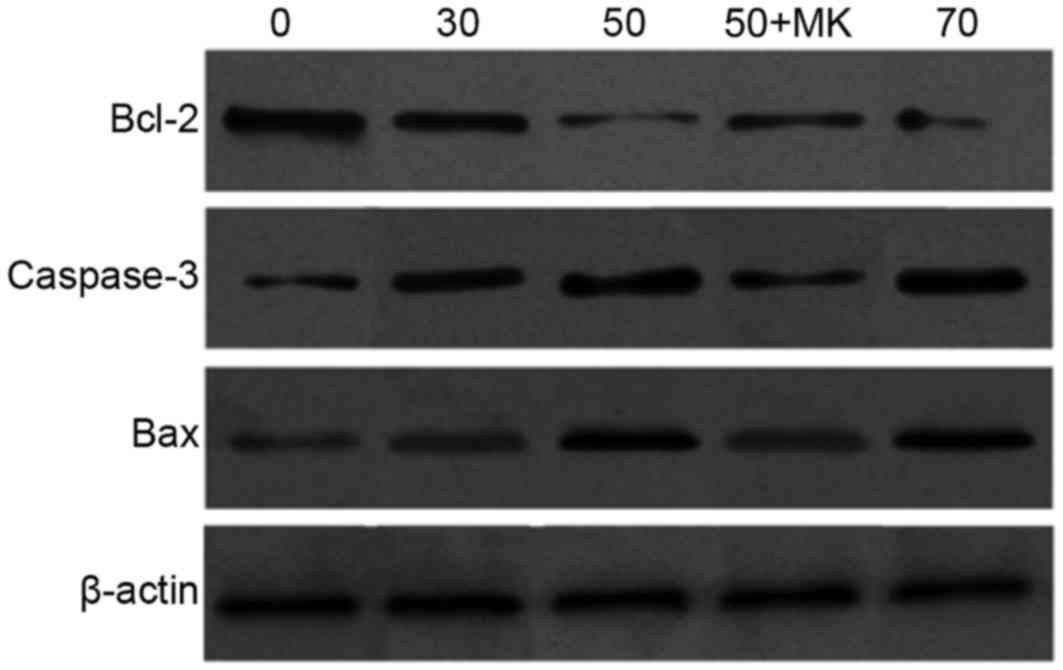
ISL increases the protein expression
of Bax and caspase-3, and decreases the expression of Bcl-2 in T24
cells
To further investigate the potential underlying
molecular mechanism of ISL-induced apoptosis, the protein levels of
Bcl-2, Bax and caspase-3 were determined by western blot analysis.
It was observed that the Bcl-2 expression level was decreased, and
the Bax and caspase-9 expression levels were increased, in a ISL
concentration-dependent manner. Compared with the ISL-treated cells
alone, pretreatment with MK-8776 led to an increase in the level of
antiapoptotic Bcl-2 mRNA, and a decrease in the level of the
proapoptotic Bax and caspase-3 (active form with minor molecular
mass) (Fig. 11).
Discussion
Although ISL has been implicated in the induction of
apoptosis in a variety of cell types (33–37), the
underlying molecular mechanism by which ISL induces apoptosis
remains unclear. In particular, the signaling pathway by which ISL
induces apoptosis in T24 cells remains unknown. The alteration in
mitochondrial membrane depolarization is intimately associated with
apoptosis, which indicates that the ISL-mediated arrest of
proliferation has an association with T24 cell apoptosis.
Prosurvival Bcl-2 family members localized in the outer membrane of
the mitochondria are important regulatory factors in the process of
cell apoptosis. Bcl-2 family members are divided into two groups:
Antiapoptotic proteins, including Bcl-2, B-cell lymphoma extra
large and B-cell lymphoma W, and proapoptotic proteins, including
Bcl-2 antagonist/killer, Bax, Bcl-2-associated death promoter and
Bim. The balance of these two groups determines the status of cells
as alive or dead (38,39). First, proapoptotic proteins open the
permeability transition pore on the mitochondrial membrane and
promote the release of cytochrome c which combines with
Apaf-1. Secondly, procaspase-9 is activated, and caspase-3,
downstream of the activator caspases, is activated through the
mitochondrial signaling pathway. Finally, activation of these
signaling pathways leads to cell apoptosis. In the present study,
following treatment of T24 cells with various concentrations of
ISL, a decrease in the ΔΨm was induced in a
time-dependent manner. In addition, ISL significantly upregulated
the activities of the related apoptotic signaling molecules Bax,
Bim, Apaf-1, caspase-9 and caspase-3, and downregulated the
activation of Bcl-2. Furthermore, the results of the present study
identified that ISL treatment upregulated the pro-apoptotic
proteins Bax and caspase-3, and downregulated the anti-apoptotic
protein Bcl-2. These results suggested that ISL induced apoptosis
of T24 cells by a molecular mechanism responsible for
depolarization of ΔΨm, and that ISL led to the apoptosis
of T24 cells via mitochondrial signaling pathways.
The association of CDK2 and cyclin E/cyclin A allows
progression through G1 phase and entry into DNA
synthesis, which are associated with cell cycle regulation
(39–41). Besides being important proteins of
cell cycle regulation, CDK2 and cyclin A are involved in the
apoptotic process. It has been demonstrated that upregulation of
CDK2 activity is required for etoposide-induced apoptosis in human
cervical carcinoma HeLa cells and mitochondrial translocation of
Bax, as well as the decrease in ΔΨm (38). In order to determine whether CDK2
serves a vital role in ISL-induced apoptosis in T24 cells, T24
cells were treated with various concentrations of ISL, and the
activity of CDK2 increased in a time-dependent manner, whereas the
activity of CDK2 was decreased by pretreatment with MK-8776 in the
ISL-treated cells.
In order to determine whether CDK2 is involved in
ISL-induced apoptosis in T24 cells, the effect of MK-8776 on
ISL-induced apoptosis was investigated. The apoptotic ratio was
decreased markedly in the MK-8776 treatment group. Furthermore,
MK-8776 inhibited the decrease in ΔΨm and downregulated
the mRNA expression of Bax, Bim, Apaf-1, caspase-9 and caspase-3,
and upregulated the expression of Bcl-2. In addition, compared with
the ISL-treated alone, the level of antiapoptotic Bcl-2 mRNA
increased, and the mRNA expression of proapoptotic protein Bax and
caspase-3 was decreased by pretreatment with MK-8776, suggesting
that CDK2 served an important role in ISL-induced apoptosis. The
apoptotic mechanism may be associated with CDK2-mediated
depolarization of ΔΨm (42–44), which
initiates a caspase cascade by facilitating a decrease in
ΔΨm and finally leading to mitochondrial apoptosis.
ISL induced apoptosis in T24 cells. CDK2 activation
was the critical step, which induced irreversible apoptotic cell
death in the cells. However, the regulatory molecular mechanism for
CDK2 activity in association with ISL-induced apoptotic pathway
remains unclear.
Glossary
Abbreviations
Abbreviations:
|
CDK
|
cyclin-dependent kinase
|
|
ISL
|
isoliquiritigenin
|
|
SRB
|
sulforhodamine B
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
ΔΨm
|
mitochondrial membrane potential
|
|
JC-1
|
5,5,6,6-tetrachloro-1,1,3,3-tetraethyl
benzimidazole carbocyanine iodide
|
|
Bcl-2
|
B-cell lymphoma-2
|
|
Bax
|
Bcl-2-associated X protein
|
|
Bim
|
Bcl-2-interacting mediator of cell
death
|
|
Apaf-1
|
apoptotic protease-activating
factor-1
|
References
|
1
|
Huang W, Chen Y, Liu Y, Zhang Q, Yu Z, Mou
L, Wu H, Zhao L, Long T, Qin D and Gui Y: Roles of ERβ and GPR30 in
proliferative response of human bladder cancer cell to estrogen.
Biomed Res Int. 2015:2517802015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yee DS, Ishill NM, Lowrance WT, Herr HW
and Elkin EB: Ethnic differences in bladder cancer survival.
Urology. 78:544–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM: International variation.
Oncogene. 23:6329–6340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Braithwaite D, Demb J, Henderson LM,
Mandelblatt JS and Kerlikowske K: American Cancer Society: Cancer
Facts & Figures. 2016.
|
|
5
|
Douglass L and Schoenberg M: The future of
intravesical drug delivery for non-muscle invasive bladder cancer.
Bladder Cancer. 2:285–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang J, Yuan X, Zhao H, Yan X, Sun X and
Zheng Q: Licochalcone A inhibiting proliferation of bladder cancer
T24 cells by inducing reactive oxygen species production. Biomed
Mater Eng. 24:1019–1025. 2014.PubMed/NCBI
|
|
7
|
Stein JP and Skinner DG: Radical
cystectomy for invasive bladder cancer: Long-term results of a
standard procedure. World J Urol. 24:296–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng C, Zeng W, Su J, Kuang Y, He Y, Zhao
S, Zhang J, Ma W, Bode AM, Dong Z and Chen X: Cyclin-dependent
kinase 2 (CDK2) is a key mediator for EGF-induced cell
transformation mediated through the ELK4/c-Fos signaling pathway.
Oncogene. 35:1170–1179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu JW, Sun P, Zhang DX, Xiong WJ and Mi J:
Hexokinase 2 regulates G1/S checkpoint through CDK2 in
cancer-associated fibroblasts. Cell Signal. 26:2210–2216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin YH, Yim H, Park JH and Lee SK: Cdk2
activity is associated with depolarization of mitochondrial
membrane potential during apoptosis. Biochem Biophys Res Commun.
305:974–980. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kinghorn AD, Pan L, Fletcher JN and Chai
H: The relevance of higher plants in lead compound discovery
programs. J Nat Prod. 74:1539–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fintelmann V: Modern phytotherapy and its
uses in gastrointestinal conditions. Planta Med. 57 Suppl
7:S48–S52. 1991. View Article : Google Scholar
|
|
13
|
Madak-Erdogan Z, Gong P, Zhao YC, Xu L,
Wrobel KU, Hartman JA, Wang M, Cam A, Iwaniec UT, Turner RT, et al:
Dietary licorice root supplementation reduces diet-induced weight
gain, lipid deposition, and hepatic steatosis in ovariectomized
mice without stimulating reproductive tissues and mammary gland.
Mol Nutr Food Res. 60:369–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukai T, Marumo A, Kaitou K, Kanda T,
Terada S and Nomura T: Anti-Helicobacter pylori flavonoids from
licorice extract. Life Sci. 71:1449–1463. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Funakoshi-Tago M, Nakamura K, Tsuruya R,
Hatanaka M, Mashino T, Sonoda Y and Kasahara T: The fixed structure
of Licochalcone A by alpha, beta-unsaturated ketone is necessary
for anti-inflammatory activity through the inhibition of NF-kappaB
activation. Int Immunopharmacol. 10:562–571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni
SJ, Wang LS and Du X: Licochalcone A inhibits growth of gastric
cancer cells by arresting cell cycle progression and inducing
apoptosis. Cancer Lett. 302:69–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YM, Kim TH, Kim YW, Yang YM, Ryu DH,
Hwang SJ, Lee JR, Kim SC and Kim SG: Inhibition of liver X
receptor-α-dependent hepatic steatosis by isoliquiritigenin, a
licorice antioxidant flavonoid, as mediated by JNK1 inhibition.
Free Radic Biol Med. 49:1722–1734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yadav VR, Prasad S, Sung B and Aggarwal
BB: The role of chalcones in suppression of NF-κB-mediated
inflammation and cancer. Int Immunopharmacol. 11:295–309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chin YW and Kinghorn AD: Structural
characterization, biological effects, and synthetic studies on
xanthones from mangosteen (Garcinia mangostana), a popular
botanical dietary supplement. Mini Rev Org Chem. 5:355–364. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Z, Wang N, Liu P, Chen Q, Situ H, Xie
T, Zhang J, Peng C, Lin Y and Chen J: MicroRNA-25 regulates
chemoresistance-associated autophagy in breast cancer cells, a
process modulated by the natural autophagy inducer
isoliquiritigenin. Oncotarget. 5:7013–7026. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao H, Yuan X, Li D, Chen H, Jiang J,
Wang Z, Sun X and Zheng Q: Isoliquiritigen enhances the antitumour
activity and decreases the genotoxic effect of cyclophosphamide.
Molecules. 18:8786–8798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haraguchi H, Ishikawa H, Mizutani K,
Tamura Y and Kinoshita T: Antioxidative and superoxide scavenging
activities of retrochalcones in Glycyrrhiza inflata. Bioorg Med
Chem. 6:339–347. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukai T, Satoh K, Nomura T and Sakagami H:
Preliminary evaluation of antinephritis and radical scavenging
activities of glabridin from Glycyrrhiza glabra. Fitoterapia.
74:624–629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yokota T, Nishio H, Kubota Y and Mizoguchi
M: The inhibitory effect of glabridin from licorice extracts on
melanogenesis and inflammation. Pigment Cell Res. 11:355–361. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inoue H, Mori T, Shibata S and Koshihara
Y: Modulation by glycyrrhetinic acid derivatives of TPA-induced
mouse ear oedema. Br J Pharmacol. 96:204–210. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gerber B, Scholz C, Reimer T, Briese V and
Janni W: Complementary and alternative therapeutic approaches in
patients with early breast cancer: A systematic review. Breast
Cancer Res Treat. 95:199–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kushman ME, Kabler SL, Ahmad S, Doehmer J,
Morrow CS and Townsend AJ: Protective efficacy of hGSTM1-1 against
B[a]P and (+)- or (−)-B[a]P-7,8-dihydrodiol cytotoxicity,
mutagenicity, and macromolecular adducts in V79 cells coexpressing
hCYP1A1. Toxicol Sci. 99:51–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soriano J, García-Díaz M, Mora M, Sagristá
ML, Nonell S, Villanueva A, Stockert JC and Cañete M: Liposomal
temocene (m-THPPo) photodynamic treatment induces cell death by
mitochondria-independent apoptosis. Biochim Biophys Acta.
1830:4611–4620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blix ES, Irish JM, Husebekk A, Delabie J,
Forfang L, Tierens AM, Myklebust JH and Kolstad A: Phospho-specific
flow cytometry identifies aberrant signaling in indolent B-cell
lymphoma. BMC Cancer. 12:4782012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang T, Song X, Zhang Z, Guo M, Jiang H,
Wang W, Cao Y, Zhu L and Zhang N: Stevioside inhibits inflammation
and apoptosis by regulating TLR2 and TLR2-related proteins in S.
aureus-infected mouse mammary epithelial cells. Int
Immunopharmacol. 22:192–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Zhang B, Yuan X, Yang F, Liu J,
Zhao H, Liu L, Wang Y, Wang Z and Zheng Q:
Isoliquiritigenin-induced differentiation in mouse melanoma B16F0
cell line. Oxid Med Cell Longev. 2012:5349342012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsu YL, Kuo PL, Lin LT and Lin CC:
Isoliquiritigenin inhibits cell proliferation and induces apoptosis
in human hepatoma cells. Planta Med. 71:130–134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim DC, Ramachandran S, Baek SH, Kwon SH,
Kwon KY, Cha SD, Bae I and Cho CH: Induction of growth inhibition
and apoptosis in human uterine leiomyoma cells by
isoliquiritigenin. Reprod Sci. 15:552–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jung JI, Chung E, Seon MR, Shin HK, Kim
EJ, Lim SS, Chung WY, Park KK and Park JH: Isoliquiritigenin (ISL)
inhibits ErbB3 signaling in prostate cancer cells. Biofactors.
28:159–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takahashi T, Takasuka N, Iigo M, Baba M,
Nishino H, Tsuda H and Okuyama T: Isoliquiritigenin, a flavonoid
from licorice, reduces prostaglandin E2 and nitric oxide, causes
apoptosis, and suppresses aberrant crypt foci development. Cancer
Sci. 95:448–453. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou GS, Song LJ and Yang B:
Isoliquiritigenin inhibits proliferation and induces apoptosis of
U87 human glioma cells in vitro. Mol Med Rep. 7:531–536.
2013.PubMed/NCBI
|
|
38
|
Choi JS, Shin S, Jin YH, Yim H, Koo KT,
Chun KH, Oh YT, Lee WH and Lee SK: Cyclin-dependent protein kinase
2 activity is required for mitochondrial translocation of Bax and
disruption of mitochondrial transmembrane potential during
etoposide-induced apoptosis. Apoptosis. 12:1229–1241. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Darvin P, Baeg SJ, Joung YH, Sp N, Kang
DY, Byun HJ, Park JU and Yang YM: Tannic acid inhibits the
Jak2/STAT3 pathway and induces G1/S arrest and mitochondrial
apoptosis in YD-38 gingival cancer cells. Int J Oncol.
47:1111–1120. 2015.PubMed/NCBI
|
|
40
|
Harbour JW, Luo RX, Dei Santi A, Postigo
AA and Dean DC: Cdk phosphorylation triggers sequential
intramolecular interactions that progressively block Rb functions
as cells move through G1. Cell. 98:859–869. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adon AM, Zeng X, Harrison MK, Sannem S,
Kiyokawa H, Kaldis P and Saavedra H: Cdk2 and Cdk4 regulate the
centrosome cycle and are critical mediators of centrosome
amplification in p53-null cells. Mol Cell Biol. 30:694–710. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu L, Ma J, Han J, Wang B, Chen X, Gao C,
Li D and Zheng Q: Licochalcone B arrests cell cycle progression and
induces apoptosis in human breast cancer MCF-7 Cells. Recent Pat
Anticancer Drug Discov. 11:444–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang J, Lv C, Hu M and Zhong G: The
mitochondria-mediate apoptosis of Lepidopteran cells induced by
azadirachtin. PLoS One. 8:e584992013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Priyadarsini RV, Murugan RS, Sripriya P,
Karunagaran D and Nagini S: The neem limonoids azadirachtin and
nimbolide induce cell cycle arrest and mitochondria-mediated
apoptosis in human cervical cancer (HeLa) cells. Free Radic Res.
44:624–634. 2010. View Article : Google Scholar : PubMed/NCBI
|