Introduction
Meningioma accounts for ~25% of all primary
intracranial neoplasms, increasing to 40% if autopsy data are
included, indicating that a number of tumors remain clinically
silent (1,2). The rising incidence of this tumor with
age, in addition to higher life expectancy and more frequent use of
diagnostic imaging, has resulted in increased diagnosis of
meningioma in the elderly (1). In
particular, brain screening with computed tomography (CT) and
magnetic resonance imaging (MRI) is easily performed for
nonspecific complaints and ‘neurological checkups’ are performed
frequently in Japan (3). Thus, an
increasing number of intracranial meningiomas are identified
incidentally (4,5). However, few studies have reported the
outcome of surgical resection of intracranial meningiomas in the
elderly (6–10). Clinically, it is often difficult for
physicians to determine whether traditional surgical resection is
the optimal management strategy for meningioma in elderly patients:
Due to their aging physiology and multiple comorbidities, elderly
patients are potentially at risk of unexpected or even
life-threatening surgical complications (7,8). Recent
studies reported an increased risk of mortality and morbidity in
older patients who underwent surgical treatment for intracranial
meningioma (1,11,12),
whereas other studies have demonstrated similar mortality and
morbidity rates in old and young patients (8,13,14). Thus, patient selection and optimal
treatment strategies for intracranial meningiomas in the elderly,
which must consider patient lifestyle, survival benefits vs. side
effects and the potential complications of surgery, including
neurological deficits, continue to be debated (6,7,10,15). To
standardize the surgical indications for intracranial meningiomas
in the elderly, a number of studies have proposed grading systems,
which include the clinical-radiological grading system (CRGS)
(15), the geriatric scoring system
(GSS) (16), Karnofsky performance
scale (KPS) score, the American Society of Anesthesiology (ASA)
score and the Location of Tumor and Peritumoral Edema grading
system (SKALE) (17).
In the present study, the clinical features of
intracranial meningiomas in elderly patients who underwent surgical
treatment at the affiliated hospital of University of Occupational
and Environmental Health (Kitakyushu, Japan) were assessed, and
patient selection and the surgical management of intracranial
meningioma in the elderly was discussed.
Patients and methods
This study was approved by the institutional review
board of the University of Occupational and Environmental Health.
Patient informed consent was waived due to the retrospective nature
of the study. A total of 70 consecutive patients with intracranial
meningioma who underwent craniotomy for resection of meningiomas
between April 2007 and December 2013 were included. All patients
were newly diagnosed with intracranial meningiomas. Recurrent
cases, previous treatment of the brain with radiotherapy, and
patients under 18 years of age were excluded from the study. The
clinical diagnosis and treatment decision for all patients were
based on the results of CT and MRI. Surgery was indicated for
symptomatic patients, as well as asymptomatic patients that
exhibited evidence of tumor progression on CT and/or MRI. Patients
with no history of epileptic seizures, with the exception of those
with posterior fossa tumors, were administered prophylactic
anticonvulsant therapy (valproate, 600–800 mg/day or carbamazepine,
100–200 mg/day) for 2–4 weeks, and this treatment was prolonged for
those with a history of epileptic seizures for ≥2 years. During the
surgical procedures, a neuronavigation system (Kolibri; BrainLAB,
Heimstetten, Germany) and an electrophysiological monitoring system
(Neuromaster MEE-1216; Nihon Kohden Corporation, Tokyo, Japan) were
used for microsurgical tumor resection. All patients received
postoperative care in the intensive care unit at the University of
Occupational and Environmental Health and rehabilitation therapy
commenced on the first postoperative day.
Patient data was obtained by reviewing admission,
surgical and anesthesia records, and patients' postoperative status
was determined by reviewing outpatient clinical charts. The
following patient data were collected: Age at diagnosis, gender,
preoperative factors (patient symptoms, tumor location, maximum
tumor size, peritumoral brain edema, KPS score and ASA score), and
postoperative factors [pathological diagnosis, tumor proliferation
index (Ki-67), tumor resection rate (Simpson grade), length of
hospital stay and discharge destinations]. Patient outcomes were
assessed 6 months after surgery. Patient data was then
retrospectively compared between patients aged ≥75 years (n=16;
elderly group) and those aged <75 years (n=54; younger group).
Tumor location was divided into four groups: Convexity, falx,
parasagittal and skull base. The maximum tumor size was measured on
a contrast-enhanced T1-weighted image prior to surgery. Peritumoral
brain edema was measured on preoperative T2-weight images, as
described previously (17). Briefly,
severe brain edema was defined when the ratio of maximum diameter
of edema to the maximum diameter of the tumor was >1. Moderate
brain edema was defined when this ratio was ≤1. Tumor resection
rate was determined according to the Simpson grade (18) and tumors were pathologically graded
according to the World Health Organization (WHO) classification
(19). In addition, proposed grading
scoring systems, including CRGS, GSS, and SKALE, were used to
calculate scores for each patient using admission data, according
to previous studies (9,15,17) and
the correlation between these scores and surgical outcomes was
assessed.
Statistical analysis
Differences in perioperative characteristics between
the elderly and younger groups were compared using an unpaired
t-test for binomial data and the Fisher exact test and Mann-Whitney
U test were used for the comparison of nonparametric data. Multiple
logistic regression analysis was performed to determine the
association between the various risk factors for perioperative
surgical complications [age (≥75 years), tumor location (skull
base), maximum tumor size (≥5 cm diameter), preoperative KPS (≥70%)
and tumor resection rate (Simpson grade, ≥III)]. Odds ratios and
95% confidence intervals were calculated for each risk factor.
Similarly, risk factors for surgical complications, including
proposed grading scoring systems, were evaluated by multiple
logistic analyses. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using StatView 5.0 statistical software (SAS Institute,
Cary, NC, USA).
Results
Patients
Patient characteristics, preoperative factors and
outcomes are shown in Table I. The
mean (±standard deviation) ages of the elderly and younger patient
groups were 81.1±5.3 years (range, 75–92 years) and 60.0±9.6 years
(range, 35–73 years), respectively. The elderly patient group
consisted of 4 male and 12 female patients, while the younger group
consisted of 10 males and 44 females. Elderly patients most
frequently presented with dementia as their initial symptom
(31.3%), however, this was rare in the younger patient group
(3.7%). Younger patients most commonly presented with visual
disturbances (20.4%) and cranial nerve disturbances (20.4%). A
total of 19 patients (35.2%) in the younger group were asymptomatic
compared with 1 patient (6.3%) in the elderly group.
 | Table I.Clinicopathological characteristics,
perioperative factors and outcome of 70 intracranial meningioma
patients. |
Table I.
Clinicopathological characteristics,
perioperative factors and outcome of 70 intracranial meningioma
patients.
| Parameter | Elderly patient
group, n (%) | Younger patient
group, n (%) | P-value |
|---|
| Age, years |
|
| <0.0001 |
| Mean ±
SD | 81.1±5.3 | 60.0±9.6 |
|
|
Range | 75–92 | 35–73 |
| Gender |
|
| 0.7226 |
|
Male | 4
(25.0) | 10 (18.5) |
|
|
Female | 12 (75.0) | 44 (81.5) |
|
| Symptoms |
| Visual
disturbances | 2
(12.5) | 11 (20.4) |
|
| Cranial
nervesa | 2
(12.5) | 11 (20.4) |
|
| Headache | 1 (6.3) | 3 (5.6) |
|
|
Dementia | 5
(31.3) | 2 (3.7) |
|
|
Hemiparesis | 3
(18.8) | 2 (3.7) |
|
|
Ataxia | 2
(12.5) | 4 (7.4) |
|
|
Epilepsy | 0 (0.0) | 2 (3.7) |
|
|
Asymptomatic | 1 (6.3) | 19 (35.2) |
|
| Preoperative KPS,
% |
|
| <0.0001 |
|
≥80 | 9
(56.2) | 52
(96.2) |
|
|
60–70 | 1 (6.3) | 1 (1.9) |
|
|
<50 | 6
(37.5) | 1 (1.9) |
|
| Preoperative ASA
score |
|
| 0.0108 |
| Class
1 | 1
(6.3) | 15 (27.7) |
|
| Class
2 | 12 (75) | 38 (70.4) |
|
| Class
3 | 3
(18.7) | 1 (1.9) |
|
| Class
4–5 | 0
(0.0) | 0 (0.0) |
|
| Tumor location |
|
| 0.0316 |
|
Convexity | 4
(25.0) | 9
(16.7) |
|
|
Falx | 1 (6.3) | 6
(11.1) |
|
|
Parasagittal | 6
(37.5) | 1 (1.9) |
|
| Skull
base | 5
(31.2) | 38 (70.3) |
|
| Maximum tumor size,
cm |
|
| 0.0004 |
| Mean ±
SD | 50.1±13.3 | 34.9±14.7 |
|
| Peritumoral
edemab |
|
| 0.0001 |
|
None | 3 (18.8) | 37 (68.5) |
|
|
Moderate | 8 (50.0) | 15 (27.8) |
|
|
Severe | 5 (31.2) | 2 (3.7) |
|
| Pathological
diagnosis |
|
| 0.0147 |
|
Meningothelial | 6
(37.5) | 41 (75.9) |
|
|
Fibrous | 3
(18.8) | 0 (0.0) |
|
|
Transitional | 0 (0.0) | 3 (5.6) |
|
|
Psammomatous | 5
(31.2) | 8
(14.8) |
|
|
Otherb | 2
(12.5) | 2 (3.7) |
|
| Ki-67, % |
|
| 0.0336 |
| Mean ±
SD | 2.3±2.5 | 1.4±1.0 |
|
|
Simpson's grade |
|
| 0.0792 |
| I | 6 (37.5) | 15 (27.8) |
|
| II | 8 (50.0) | 19 (35.1) |
|
|
III | 0 (0.0) | 4 (7.4) |
|
| IV | 2 (12.5) | 15 (27.8) |
|
| V | 0 (0.0) | 1 (1.9) |
|
| Length of hospital
stay, days |
|
| 0.5823 |
| Mean ±
SD | 25.7±8.7 | 23.6±14.1 |
|
|
Median | 25 | 17 |
|
| Discharge
destination |
|
| 0.0420 |
|
Home | 11 (68.8) | 49 (90.7) |
|
|
Rehabilitation Center | 5
(31.2) | 5 (9.3) |
|
| Postoperative
mortalitiesc | 0
(0.0) | 0 (0.0) |
|
| Postoperative
complications |
|
| 0.1641 |
| Cranial
nerve palsy | 1
(6.3) | 7
(13.0) |
|
|
Hemiparesis | 0
(0.0) | 3 (5.6) |
|
| Speech
disturbance | 0
(0.0) | 1 (1.9) |
|
| Wound
infection | 0
(0.0) | 2 (3.7) |
|
|
None | 15 (93.8) | 41 (75.9) |
Preoperative KPS scores were significantly lower in
the elderly group compared with the younger group (P<0.0001).
Similarly, preoperative ASA scores were also significantly lower in
the elderly group than the younger group (P=0.0108). Regarding
tumor location, parasagittal meningiomas were the most common in
elderly patients (37.5%), however, only 1 case of parasagittal
meningioma was observed in the younger patient group (1.9%). The
majority of tumors in younger patients were located at the skull
base (70.3%). Notably, tumor size in the elderly group was
significantly larger than that in the younger group (P=0.008). In
addition, peritumoral brain edema was significantly more severe in
the elderly group when compared with the younger group (P=0.0001;
Table I).
Pathology
Pathologically, certain differences were observed
between the two groups. Elderly patients frequently presented with
meningothelial (37.5%) and psammomatous (31.2%) tumor types with a
significantly higher proliferation index (Ki-67) than the younger
group (P=0.05). In addition, 1 meningioma (WHO grade II; atypical
type) case (6.3%) was observed in the elderly group. By contrast,
the majority of meningiomas in the younger group were classified as
the meningothelial type (75.9%). One meningioma case (WHO grade II;
clear cell) was observed in the younger patient group, the
incidence was low (1.9%) compared with that in the elderly patient
group (Table I). However, no
significant difference in the incidence of meningiomas was
identified between the groups (P=0.4075).
Surgery and outcome
Gross total resection (Simpson grade I + II) was
performed in 87.5 and 62.9% of the patients in the elderly and
younger groups, respectively. No significant difference in the
tumor resection rate was identified between the groups (P=0.0792).
Although no significant difference in the length of hospital stay
was identified between the groups, elderly patients were more
likely to visit a rehabilitation center/convalescence hospital
following discharge when compared with younger patients (P=0.042).
No postoperative mortality was observed in either group.
Surgical complications in the elderly were limited
to one case of facial palsy (7.7%). A total of 13 patients (25.6%)
in the younger group exhibited surgical complications, which
included the following: Cranial nerve palsy [oculomotor (n=1),
trochlear (n=2), abducens (n=1), lower cranial (n=3)], hemiparesis
(n=3), speech disturbance (n=1) and wound infection (n=2). One case
of facial palsy in the elderly group, 2 cases of cranial palsy and
1 case of hemiparesis in the younger group persisted for >1 year
after surgery. However, no significant difference in the incidence
of surgical complications was identified between the two groups
(P=0.1641). Among the 21 cases of Simpson grade III–IV meningiomas,
19 cases (90%) were skull base lesions and 2 cases (10%) were falx
lesions. Among these, surgical complications affected 9 cases,
including 6 cases of cranial palsy, 2 cases of hemiparesis and 1
case of speech disturbance. The 6 cases of cranial palsy were
associated with manipulation of the cranial nerve, which was
encased within or had adhered to tumors during the surgery. One
case of hemiparesis and 1 case of speech disturbance were
associated with postoperative brain edema in young patients. One
case of hemiparesis was associated with postoperative cerebral
infarction in the area of the lenticulostriate artery that was
encased within the tumor. No cases of symptomatic intracavitary
hematoma were observed following surgery.
Among the 2 cases of Simpson grade III–IV
meningiomas in the elderly group, 1 patient developed postoperative
facial palsy due to nerve manipulation during surgery. Multivariate
logistic regression analysis identified a significant association
between Simpson grade (III–V) and surgical complications. For
patients with meningiomas with a low resection rate (Simpson grade
III–V), the risk of experiencing surgical complications was 5.662
times higher than that of patients with a higher resection rate
(Simpson grade, I–II) tumors (odds ratio, 5.662, 95% confidence
interval, 1.323 to 24.236, P=0.0194) (Table II). Age, tumor location, maximum
tumor size and preoperative KPS score were not associated with
surgical complications.
 | Table II.Multivariate logistic regression
analysis of factors associated with surgical complications. |
Table II.
Multivariate logistic regression
analysis of factors associated with surgical complications.
| Parameter | Surgical
complication OR | 95% CI | P-value |
|---|
| Age (≥75
years) | 0.265 | 0.017–4.071 | 0.3406 |
| Tumor location
(skull base) | 1.504 | 0.244–9.274 | 0.6598 |
| Maximum tumor size
(≥5 cm) | 4.507 | 0.793–25.603 | 0.0893 |
| Preoperative KPS
(≤70%) | 0.814 | 0.050–13.311 | 0.8854 |
| Severe peritumoral
edema | 0.857 | 0.059–12.474 | 0.9103 |
| Simpson's grade
(III–V) | 5.680 | 1.321–24.420 | 0.0196 |
CRGS, GSS, SKALE scores
Patient characteristics with corresponding CRGS, GSS
and SKALE scores, are shown in Table
III. Previous studies demonstrated that a poor outcome
following surgical treatment for intracranial meningiomas in the
elderly was associated with a score ≤9, ≤15 and ≤7 for the CRGS,
GSS and SKALE, respectively (15–17). The
cut-off point for postoperative complications was ≤9, ≤15, and ≤7
for CRGS, GSS, and SKALE, respectively (9,15,17). Multivariate logistic regression
analysis revealed no significant associations between patient age
(≥75 years), lower CRGS (≤9), GSS (≤15), and SKALE scores (≤7) and
surgical complications (P=0.0992, P=0.7935, P=0.1414 and P=0.6441,
respectively) (Table IV). In
addition, among elderly patients (≥75 years), multivariate logistic
regression analysis also revealed no significant difference between
lower CRGS, GSS, and SKALE scores and surgical complications
(P=0.9797, P>0.9999 and P=0.9883, respectively).
 | Table III.CRGS, GSS and SKALE scores of 70
intracranial meningioma patients according to clinicopathological
factors. |
Table III.
CRGS, GSS and SKALE scores of 70
intracranial meningioma patients according to clinicopathological
factors.
| A, CRGS score |
|---|
|
|---|
|
| CRGS score |
|---|
|
|
|
|---|
| Clinicopathological
factor | 1, n (%) | 2, n (%) | 3, n (%) |
|---|
| Size of tumor | 8 (11) | 24 (34) | 38 (55) |
| Location of
lesion | 42 (60) | 6 (9) | 22 (31) |
| Presence of
edema | 15 (21) | 15 (21) | 40 (58) |
| Neurological
condition | 2 (3) | 48 (69) | 20 (28) |
| Concomitant
disease | 4 (6) | 46 (66) | 20 (28) |
| KPS score | 7 (10) | 25 (36) | 38 (54) |
|
| B, GSS score |
|
|
| GSS score |
|
|
|
| Clinicopathological
factor | 1, n (%) | 2, n (%) | 3, n (%) |
|
| Tumor size | 18 (26) | 25 (36) | 27 (38) |
| Neurological
deficit | 47 (67) | 2 (3) | 21 (30) |
| KPS score | 7 (10) | 25 (36) | 38 (54) |
| Tumor location | 13 (19) | 17 (24) | 40 (57) |
| Peritumoral
edema | 15 (21) | 15 (21) | 40 (58) |
| Diabetes
mellitus | 1 (1) | 4 (6) | 65 (93) |
| Hypertension | 0 (0) | 38 (54) | 32 (46) |
| Pulmonary
disease | 0 (0) | 4 (6) | 66 (94) |
|
| C, SKALE score |
|
|
| SKALE score |
|
|
|
| Clinicopathological
factor | 0, n (%) | 2, n (%) | 4, n (%) |
|
| Gender | 14 (20) | 56 (80) | – |
| KPS score | 7 (10) | 12 (17) | 51 (73) |
| ASA score | 0 (0) | 3 (4) | 67 (96) |
| Tumor location | 47 (67) | 23 (33) | – |
| Presence of
edema | 7 (10) | 23 (33) | 40 (57) |
 | Table IV.Multivariate logistic regression
analysis of the association between proposed grading system scores
and surgical complications. |
Table IV.
Multivariate logistic regression
analysis of the association between proposed grading system scores
and surgical complications.
| Variables | Surgical
complications OR | 95% CI | P-value |
|---|
| Age (≥75
years) | 0.107 | 0.007–1.527 | 0.0992 |
| CRGS score | 1.300 | 0.182–9.258 | 0.7935 |
| GSS score | 7.875 | 0.503–123.276 | 0.1414 |
| SKALE score | 2.690 | 0.040–179.157 | 0.6441 |
A case of left frontal convexity
meningioma in elderly
An 87-year-old healthy and independent woman
consulted her local hospital for an examination of a slight hearing
disturbance, and a brain tumor was incidentally detected on a CT
scan in November 2009. Therefore, the patient consulted the
affiliated hospital of University of Occupational and Environmental
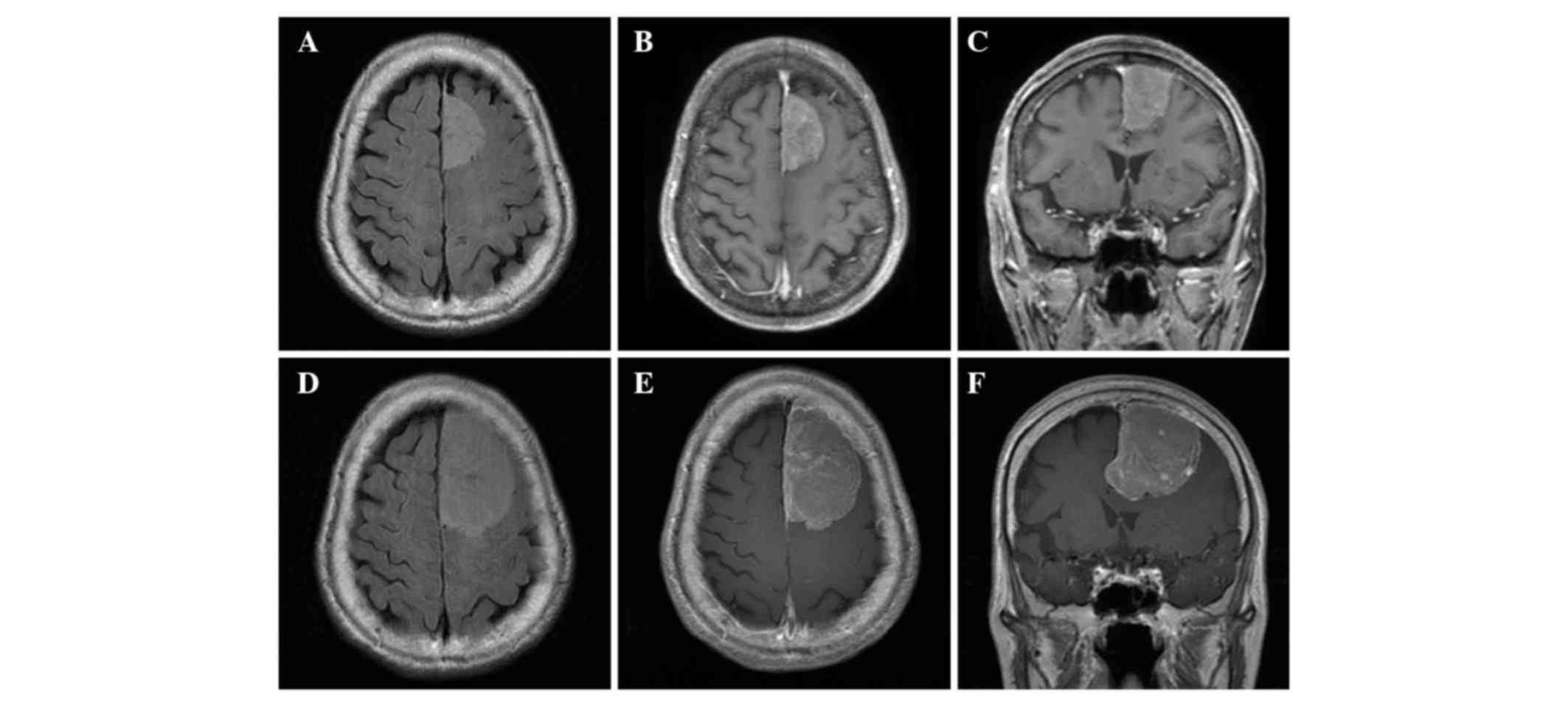
Health. Neurological examination revealed no deficit. MRI
demonstrated an enhanced extra-axial mass without peritumoral brain
edema, corresponding to a parasagittal meningioma (Fig. 1A-C). Conservative treatment was
selected due to the asymptomatic nature of the meningioma and the
age of the patient. Thus, the patient was subjected to close
observation using MRI without prophylactic anticonvulsant therapy.
However, the patient discontinued undergoing follow-up MRI
examinations 1 year later. Two years after diagnosis, the patient's
condition deteriorated, and she consulted the affiliated hospital
of University of Occupational and Environmental Health again in
March 2011 with the assistance of her family, using a wheelchair.
The patient presented with severe dementia and mild right
hemiparesis (KPS, 20%; ASA score, 3). The patient's CRGS, GSS and
SKALE scores were 10, 13 and 8, respectively. MRI revealed tumor
progression with peritumoral brain edema (Fig. 1D-F). Subsequently, the patient
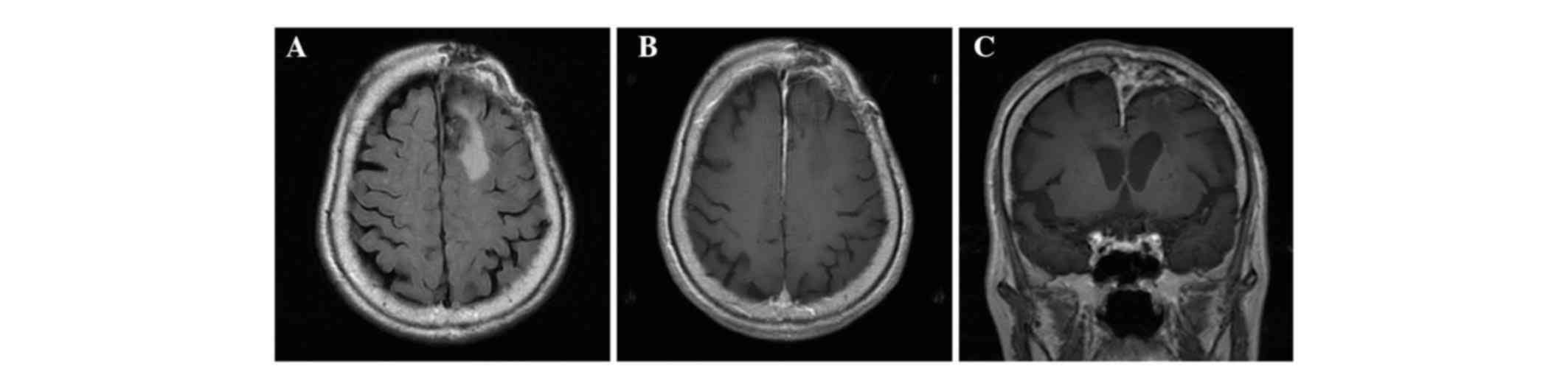
underwent gross total resection of the tumor (Simpson grade II)
without any surgical complications (Fig.
2). The pathological diagnosis was transitional meningioma (WHO
grade I) (Ki-67, 2%). Following surgery, the patient's condition
gradually improved and she was able to walk at the time of
discharge. Follow-up MRI examination 1 year after surgery revealed
no evidence of tumor recurrence (Fig.
2A-C) and a KPS score of 90%.
Discussion
Based on the results of previous autopsy studies,
intracranial meningioma is likely to become an increasingly common
disease in the elderly population (20–22). The
annual incidence of intracranial meningioma among the elderly is
estimated to be 8.4/100,000 persons in Manitoba, Canada (23) compared with 1.2–3.1/100,000 persons
per year in the general population of Canada (23), USA (24), United Kingdom (25) and Japan (4), as assessed by epidemiological studies.
As a result, neurosurgeons are increasingly confronted with the
issue of intracranial meningioma management in the elderly.
Surgical treatment of intracranial meningiomas in the elderly may
be performed, depending on the patient's age and physiology, with
consideration of the technical and ethical issues. However, the
risk of surgical treatment must be balanced against the morbidity
due to tumor growth that is associated with a conservative
treatment.
Previous studies have investigated the natural
history of intracranial meningiomas (26–30). The
growth rate of intracranial meningiomas has been reported as
2.4–5.3 mm per year (27–29). Other studies have reported that
hyperintensity on T2-weighted imaging, a non-skull base location
and the absence of calcification on imaging are considered positive
indicators of tumor growth in intracranial meningiomas (30,31). In
addition, a non-skull base location and the absence of
calcification correlate with a high tumor proliferation index
(MIB-1) in intracranial meningiomas (32–35). To
determine the MIB-1 staining index, the number of cells stained
positively with MIB-1 and the total number of cells were counted in
several representative fields containing >1,000 cells, and the
ratio was defined as the MIB-1 staining index. Similarly, in the
present study, it was demonstrated that intracranial meningiomas in
elderly patients frequently exhibited a non-skull base location,
significantly large tumor size and a high proliferation index
(Ki-67) when compared with younger meningioma patients.
Furthermore, peritumoral edema in intracranial meningiomas was more
severe in elderly patients than younger patients. By contrast,
previous studies have reported that untreated meningiomas in
elderly patients were associated with a significantly lower
incidence of tumor growth compared with that in younger patients
(29,31,36).
Conservative treatment is usually recommended for
asymptomatic meningiomas in the elderly. However, the Brain Tumor
Registry of Japan reported that the incidence of surgical WHO grade
II and III meningioma cases is 13.3 and 9.4% in elderly (≥75 years
old) and younger patients (<75 years old), respectively
(37). The present study demonstrated
that meningiomas in the elderly exhibit significant histological
variations and a higher incidence of WHO grade II (6.5%)
meningiomas when compared with younger meningioma patients. Taken
together, these results indicate that surgically treated cases of
intracranial meningioma in elderly patients may be biologically
different when compared with untreated cases. Therefore, it is
suggested that not only symptomatic tumors, but also asymptomatic
cases that exhibit tumor growth during follow-up, should be
considered for surgical treatment.
Whether there is an increased surgical risk for
meningioma in elderly patients remains controversial (6,7,10,11,17). In
the present study, the 1-year postoperative mortality rate of
meningioma patients was 0% and the rate of postoperative
complications in elderly patients was 7.7%. Multivariate logistic
regression analysis indicated that the resection rate (Simpson
grade III–V) was an important predictor of postoperative
complications. By contrast, surgical complications did not
correlate with age (>75 years), tumor location (skull base),
tumor size (>5 cm), preoperative KPS score (≤70%) or
preoperative ASA score (>class 3). Among the Simpson grade
III–IV meningioma cases, surgical complications were observed in
only 9 cases. One case was observed in the elderly patient group,
whereby a facial nerve was encased in perto-clival meningioma.
These complications were the result of surgical procedures,
including nerve manipulation, postoperative brain edema and
postoperative cerebral infarction due to brain retraction and
coagulation of perforating arteries. No cases exhibited critical
physical complications, such as severe cardiopulmonary dysfunction.
Regardless of patient age, surgical difficulties during tumor
resection in intracranial meningiomas may lead to postoperative
complications and consequently result in a low rate of tumor
resection (Simpson grade III–IV).
Previous studies have reported that the 30 day
postoperative mortality rate is 0–10.8% in elderly meningioma
patients (10,15,17,38). A
recent prospective study of surgical resection of intracranial
meningiomas reported that elderly patients (>70 years) exhibited
significantly higher 30-day postoperative mortality rates (12.0%)
than younger patients (4.6%) (6). In
addition, multiple regression analysis identified age (>70
years), functional health status (including ASA score),
preoperative disseminated cancer and tumor location
(infratentorial) as important predictors of 30-day postoperative
mortality (6). In particular, the
risk of mortality in elderly patients (>70 years) was 3 times
higher than that of younger patients (6). By contrast, a recent systematic
meta-analysis indicated that the 1-year mortality rate following
meningioma resection in elderly patients was 6–16% compared with
2–18% in untreated cohorts (7,39,40). In addition, the survival of elderly
patients following meningioma resection was similar to that of the
general population (7,39,40).
In a previous study, age was not an independent
factor for predicting surgical outcome (7,10,15); in the present study it was
demonstrated that age was not associated with increased surgical
risk in elderly patients with intracranial meningioma, which was
consistent with previous studies (7,10,15). Several studies have assessed
predictors for surgical outcome in elderly meningioma patients
using CRGS, GSS and SKALE scoring systems that include tumor size,
gender, KPS score, ASA score, tumor location, peritumoral edema and
concomitant disease (9,10,16). The
CRGS, GSS and SKALE scoring systems have been proposed for use in
patients aged over 70, 65 and 80 years, respectively. Although
these scores may provide useful information for determining the
optimal treatment for intracranial meningiomas in the elderly, in
actual practice, the difficulties encountered during surgery for
intracranial meningiomas (including tumor vascularity, venous
drainage, tumor attachment, involvement of cranial nerves and
degree of brain stem adhesion or compression) and the surgeon's
experience are more likely to affect the surgical outcome (40–42).
A previous study investigated postoperative outcomes
in intracranial meningiomas, extending the CRGS and SKALE scoring
systems to younger patients (≥65 years old) (10). However, they were unable to reproduce
the utility of the two proposed grading systems (17). The present study also evaluated
surgical complications, using these scoring systems, including GSS.
Similarly, no correlation was identified between these scoring
systems and surgical complications in all patients, including the
elderly. In the present study, only the tumor resection rate was
associated with postoperative complications. Thus, the difficulty
of meningioma resection may affect the tumor resection rate and
consequently lead to postoperative complications. Taken together,
for the surgical management of meningioma in the elderly,
individual patient health status and characteristics of the tumor
should be considered rather than patient age.
An important limitation of this study is that no
neuropsychological evaluations were performed. In this study, 5
patients presented with dementia as the initial symptom. The
incidence of large tumors, convexity, and falx meningioma was
increased in the elderly when compared with younger patients. In
elderly intracranial meningioma patients that develop dementia, it
is difficult to determine whether the disease is a result of
meningiomas or the natural aging process. Thus, prospective studies
of surgical management of intracranial meningiomas in the elderly
which investigate neuropsychological function, are required.
Neurosurgeons may be increasingly confronted with
the issue of intracranial meningioma management in the elderly,
which in the majority of cases is treated conservatively. Although
the sample number was limited in the present study, it was
demonstrated that only tumor resection rate, not patient age, was
associated with surgical outcome. If tumors in elderly patients are
symptomatic, or asymptomatic with tumor growth during follow-up,
specific treatment, including surgical resection, is required. The
present study demonstrated that regardless of patient age, the
decision to perform surgical resection should be made on an
individual basis whereby tumor characteristics and the general
health of the patient are considered.
Glossary
Abbreviations
Abbreviations:
|
ASA
|
American Society of Anesthesiology
|
|
CT
|
computed tomography
|
|
KPS
|
Karnofsky performance scale
|
|
MRI
|
magnetic resonance imaging
|
|
WHO
|
World Health Organization
|
References
|
1
|
Wiemels J, Wrensch M and Claus EB:
Epidemiology and etiology of meningioma. J Neurooncol. 99:307–314.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kurland LT, Schoenberg BS, Annegers JF,
Okazaki H and Molgaard CA: The incidence of primary intracranial
neoplasms in Rochester, Minnesota, 1935–1977. Ann NY Acad Sci.
381:6–16. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsutani M: Treatment for asymptomatic
meningioma. No To Shinkei. 53:327–330. 2001.(In Japanese).
PubMed/NCBI
|
|
4
|
Kuratsu J, Kochi M and Ushio Y: Incidence
and clinical features of asymptomatic meningiomas. J Neurosurg.
92:766–770. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Onizuka M, Suyama K, Shibayama A, Hiura T,
Horie N and Miyazaki H: Asymptomatic brain tumor detected at brain
check-up. Neurol Med Chir (Tokyo). 41:431–435. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patil CG, Veeravagu A, Lad SP and Boakye
M: Craniotomy for resection of meningioma in the elderly: A
multicentre, prospective analysis from the National Surgical
Quality Improvement Program. J Neurol Neurosurg Psychiatry.
81:502–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poon MT, Fung LH, Pu JK and Leung GK:
Outcome of elderly patients undergoing intracranial meningioma
resection - a systematic review and meta-analysis. Br J Neurosurg.
28:303–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bateman BT, Pile-Spellman J, Gutin PH and
Berman MF: Meningioma resection in the elderly: Nationwide
inpatient sample, 1998–2002. Neurosurgery. 57:866–872. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cohen-Inbar O, Soustiel JF and Zaaroor M:
Meningiomas in the elderly, the surgical benefit and a new scoring
system. Acta Neurochir (Wien). 152:87–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schul DB, Wolf S, Krammer MJ, Landscheidt
JF, Tomasino A and Lumenta CB: Meningioma surgery in the elderly:
Outcome and validation of 2 proposed grading score systems.
Neurosurgery. 70:555–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Black P, Kathiresan S and Chung W:
Meningioma surgery in the elderly: A case-control study assessing
morbidity and mortality. Acta Neurochir (Wien). 140:1013–1017.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maurice-Williams RS and Kitchen ND:
Intracranial tumours in the elderly: The effect of age on the
outcome of first time surgery for meningiomas. Br J Neurosurg.
6:131–137. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuratsu J and Ushio Y: Epidemiological
study of primary intracranial tumours in elderly people. J Neurol
Neurosurg Psychiatry. 63:116–118. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Papo I: Intracranial meningiomas in the
elderly in the CT scan era. Acta Neurochir (Wien). 67:195–204.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caroli M, Locatelli M, Prada F, Beretta F,
Martinelli-Boneschi F, Campanella R and Arienta C: Surgery for
intracranial meningiomas in the elderly: A clinical-radiological
grading system as a predictor of outcome. J Neurosurg. 102:290–294.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cohen-Inbar O, Sviri GE, Soustiel JF and
Zaaroor M: The Geriatric Scoring System (GSS) in meningioma
patients - validation. Acta Neurochir (Wien). 153:1501–1508. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sacko O, Sesay M, Roux FE, Riem T, Grenier
B, Liguoro D and Loiseau H: Intracranial meningioma surgery in the
ninth decade of life. Neurosurgery. 61:950–955. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simpson D: The recurrence of intracranial
meningiomas after surgical treatment. J Neurol Neurosurg
Psychiatry. 20:22–39. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perry A, Louis DN, Scheithauer BW, Budka H
and von Deimling A: MeningiomasWHO Classification of Tumours of the
Central Nervous System. Louis DN, Ohgaki H, Wiestler OD and Cavenee
WK: 4th. IARC Press; Lyon: pp. 164–172. 2007
|
|
20
|
Helseth A, Mørk SJ, Johansen A and Tretli
S: Neoplasms of the central nervous system in Norway. IV. A
population-based epidemiological study of meningiomas. APMIS.
97:646–654. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakasu S, Hirano A, Shimura T and Llena
JF: Incidental meningiomas in autopsy study. Surg Neurol.
27:319–322. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sadetzki S, Modan B, Chetrit A and
Freedman L: An iatrogenic epidemic of benign meningioma. Am J
Epidemiol. 151:266–272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rohringer M, Sutherland GR, Louw DF and
Sima AA: Incidence and clinicopathological features of meningioma.
J Neurosurg. 71:665–672. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heshmat MY, Kovi J, Simpson C, Kennedy J
and Fan KJ: Neoplasms of the central nervous system. Incidence and
population selectivity in the Washington DC, metropolitan area.
Cancer. 38:2135–2142. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barker DJ, Weller RO and Garfield JS:
Epidemiology of primary tumours of the brain and spinal cord: A
regional survey in southern England. J Neurol Neurosurg Psychiatry.
39:290–296. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Claus EB, Bondy ML, Schildkraut JM,
Wiemels JL, Wrensch M and Black PM: Epidemiology of intracranial
meningioma. Neurosurgery. 57:1088–1095. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olivero WC, Lister JR and Elwood PW: The
natural history and growth rate of asymptomatic meningiomas: A
review of 60 patients. J Neurosurg. 83:222–224. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niiro M, Yatsushiro K, Nakamura K,
Kawahara Y and Kuratsu J: Natural history of elderly patients with
asymptomatic meningiomas. J Neurol Neurosurg Psychiatry. 68:25–28.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoneoka Y, Fujii Y and Tanaka R: Growth of
incidental meningiomas. Acta Neurochir (Wien). 142:507–511. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hashimoto N, Rabo CS, Okita Y, Kinoshita
M, Kagawa N, Fujimoto Y, Morii E, Kishima H, Maruno M, Kato A and
Yoshimine T: Slower growth of skull base meningiomas compared with
non-skull base meningiomas based on volumetric and biological
studies. J Neurosurg. 116:574–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hashiba T, Hashimoto N, Izumoto S, Suzuki
T, Kagawa N, Maruno M, Kato A and Yoshimine T: Serial volumetric
assessment of the natural history and growth pattern of
incidentally discovered meningiomas. J Neurosurg. 110:675–684.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Torp SH, Lindboe CF, Grønberg BH, Lydersen
S and Sundstrøm S: Prognostic significance of Ki-67/MIB-1
proliferation index in meningiomas. Clin Neuropathol. 24:170–174.
2005.PubMed/NCBI
|
|
33
|
Hus CY, Ho DM, Yang CF and Chiang H:
Interobserver reproducibility of MIB-1 labeling index in astrocytic
tumors using different counting methods. Mod Pathol. 16:951–957.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kasuya H, Kubo O, Tanaka M, Amano K, Kato
K and Hori T: Clinical and radiological features related to the
growth potential of meningioma. Neurosurg Rev. 29:293–297. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McGovern SL, Aldape KD, Munsell MF,
Mahajan A, DeMonte F and Woo SY: A comparison of World Health
Organization tumor grades at recurrence in patients with non-skull
base and skull base meningiomas. J Neurosurg. 112:925–933. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rubin G, Herscovici Z, Laviv Y, Jackson S
and Rappaport ZH: Outcome of untreated meningiomas. Isr Med Assoc
J. 13:157–160. 2011.PubMed/NCBI
|
|
37
|
Committee of Brain Tumor Registry of
Japan, . Report of Brain Tumor Registry of Japan (2001–2004), Vol.
13. Neurol Med Chir (Tokyo). 54:1–102. 2014.
|
|
38
|
D'Andrea G, Roperto R, Caroli E, Crispo F
and Ferrante L: Thirty-seven cases of intracranial meningiomas in
the ninth decade of life: Our experience and review of the
literature. Neurosurgery. 56:956–961. 2005.PubMed/NCBI
|
|
39
|
Cahill KS and Claus EB: Treatment and
survival of patients with nonmalignant intracranial meningioma:
Results from the Surveillance, Epidemiology and End Results Program
of the National Cancer Institute. Clinical article. J Neurosurg.
115:259–267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sankila R, Kallio M, Jääskeläinen J and
Hakulinen T: Long-term survival of 1986 patients with intracranial
meningioma diagnosed from 1953 to 1984 in Finland. Comparison of
the observed and expected survival rates in a population-based
series. Cancer. 70:1568–1576. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Adachi K, Kawase T, Yoshida K, Yazaki T
and Onozuka S: ABC Surgical risk scale for skull base meningioma: A
new scoring system for predicting the extent of tumor removal and
neurological outcome. Clinical article. J Neurosurg. 111:1053–1061.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamamoto J, Kakeda S, Takahashi M, Aoyama
Y, Soejima Y, Saito T, Akiba D, Korogi Y and Nishizawa S: Dural
attachment of intracranial meningiomas: Evaluation with
contrast-enhanced three-dimensional fast imaging with steady-state
acquisition (FIESTA) at 3 T. Neuroradiology. 53:413–423. 2011.
View Article : Google Scholar : PubMed/NCBI
|