Introduction
Gastric cancer is one of the most frequent causes of
cancer-associated mortality worldwide, with ~951,600 new cases and
723,100 mortalities occurring in 2012 (1). Although developments in early diagnosis,
surgery, adjuvant chemotherapy and targeted therapies have
improved, the prognosis of patients with gastric cancer and their
long-term survival remains unsatisfactory (2). Numerous patients are diagnosed at a late
stage and have a poor prognosis. Therefore, it is important and
urgent to distinguish novel biomarkers in patients with gastric
cancer for early diagnosis and to search for potential therapeutic
targets.
Long non-coding RNAs (lncRNAs) are defined as
transcripts containing >200 nucleotides without the capacity for
coding proteins (3,4). A number of lncRNAs have been
demonstrated to serve important functions in a wide range of
diseases, including neurodegenerative diseases, chronic obstructive
pulmonary disease and cancers (5,6). LncRNAs
may function as oncogenes or tumor suppressor genes, and may be
involved in the development or progression of cancers. Thus,
lncRNAs may be used as cancer biomarkers for early diagnosis, as
potential therapeutic targets and to predict cancer prognosis.
Among these, homeobox (HOX)-associated lncRNAs are biologically
important. For instance, HOX transcript antisense RNA (HOTAIR) and
HOXA transcript at the distal tip (HOTTIP) are the most frequently
studied in this area. HOTAIR is a lncRNA of 2,158 nucleotides in
length, which is expressed in the HOXC locus of chromosome 12
(7). It has been reported to be a
pro-oncogenic factor and a negative prognostic factor in several
types of cancer, including breast, pancreatic, gastric, colorectal
and bladder cancer (8–12).
Another lncRNA, HOTTIP, is an antisense non-coding
transcript located at the 5′ end of the HOXA gene cluster. It has
been reported to directly bind the adaptor protein WD
repeat-containing protein 5 (WDR5) and to target WDR5/mixed lineage
leukemia complexes, driving histone H3 lysine 4 trimethylation and
gene transcription of distal HOXA genes (13). Several previous studies have reported
that, compared with normal adjacent tissues (NATs), HOTTIP
expression was significantly increased in skin, hepatocellular,
pancreatic, lung and tongue squamous cell cancer tissues (14–20).
HOTTIP may be involved in the progression of these cancers.
However, the association between HOTTIP and gastric cancer remains
unknown.
In the present study, the expression of HOTTIP was
explored in gastric cancer tissues, and the associations between
HOTTIP expression and clinicopathological characteristics were
investigated.
Materials and methods
Patients and tissue samples
A total of 94 fresh gastric cancer tissues and
matched NATs were obtained from patients who underwent radical
resection for gastric cancer at the First Hospital of China Medical
University (Shenyang, China) between May 2009 and July 2010. The
matched NATs were obtained from tissues that were at least 5 cm
from the edge of the cancer tissue. All tissues were frozen
immediately in liquid nitrogen following resection and stored at
−80°C prior to use. The tumor histological grade was classified
according to the 7th edition of the Tumor-Node-Metastasis staging
system (21).
The present study was approved by the Research
Ethics Committee of China Medical University (Shenyang, China).
Written informed consent was obtained from all patients.
Cell culture
The human gastric cancer cell lines MGC-803,
BGC-823, SGC-7901 and HGC-27 were obtained from the Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The human gastric cancer AGS cell line was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). MGC-803, BGC-823, SGC-7901 and HGC-27 were
cultured in RPMI-1640 medium (Biological Industries, Kibbutz
Beit-Haemek, Israel). AGS was cultured in F-12K Medium (ATCC).
Media were supplemented with 10% fetal bovine serum (Clark
Bioscience, Claymont, DE, USA). Cell lines were cultured in an
incubator at 37°C in a humidified atmosphere containing 5% CO2.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples and five
cell lines using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The concentration and purity
of total RNA was determined by using a Nano-Photometer UV/Vis
spectrophotometer (Implen GmbH, München, Germany). A value of
A260/A280>1.9 indicated good purity. Reverse transcription was
performed using the PrimeScript RT reagent kit with gDNA eraser
(Takara Biotechnology Co., Ltd., Dalian, China). qPCR analyses were
performed using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.)
on a light cycler 480 II real-time PCR system (Roche Diagnostics,
Basel, Switzerland). Each 25-µl PCR reaction mixture contained 0.3
µl forward and 0.3 µl reverse primers, 12.5 µl SYBR-Green mix, 2 µl
gene cDNA and 9.9 µl RNase-free water. The reaction was amplified
in 45 cycles of 95°C for 5 sec, 60°C for 30 sec and 72°C for 30
sec. Specific primers for HOTTIP were based on the first and fifth
splice variant. The sequences of the primers used were as follows:
HOTTIP forward, 5′-CGTAGAGACACAGGCAGCAG-3′ and reverse,
5′-CAGCCGAACAGAGTCAGAGG-3′; GAPDH, forward
5′-CGGATTTGGTCGTATTGGG-3′ and reverse, 5′-CTGGAAGATGGTGATGGGATT-3′.
The RT-qPCR reactions were performed in triplicate. All reagents
were used according to the manufacturer's protocol. The expression
level of HOTTIP in gastric cancer tissues compared with NATs was
calculated using the 2−ΔΔCq method (22). If the 2−ΔΔCq value was
<1, there was low expression in cancer tissues and cancer cell
lines compared with NATs.
Statistical analysis
Statistical analysis was performed using SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). The
associations between HOTTIP expression and clinicopathological
characteristics were tested using non-parametric tests: The
Mann-Whitney U test for 2 groups and the Kruskal-Wallis test for ≥3
groups. Survival rates, including overall survival and disease-free
survival were calculated using the Kaplan-Meier method with the
log-rank test applied for comparison. A receiver operating
characteristic (ROC) curve was used to evaluate the diagnostic
value of HOTTIP expression levels. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of HOTTIP in gastric
cancer
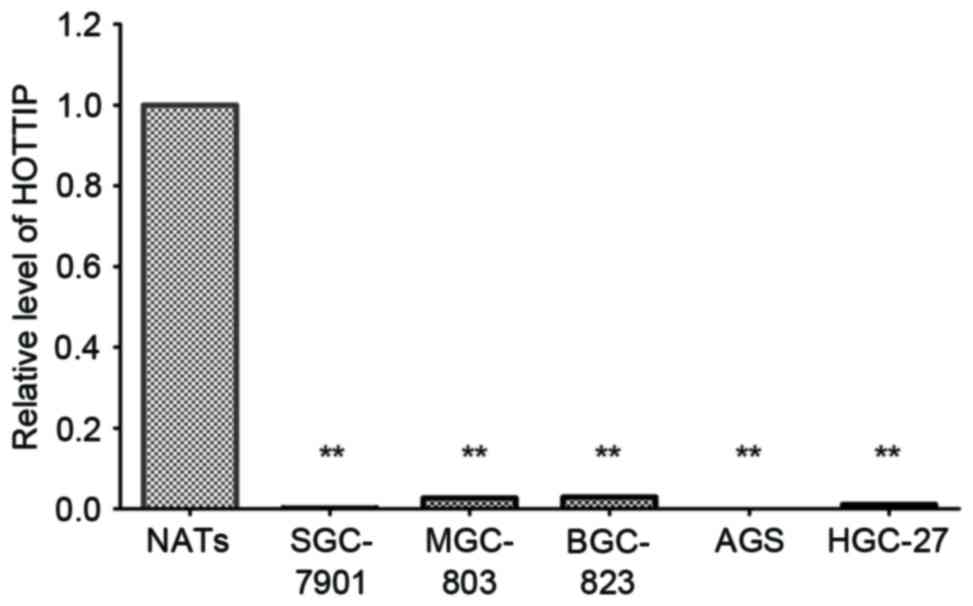
Using RT-qPCR, significantly decreased HOTTIP
expression levels were observed in MGC-803 cells, BGC-823 cells,
SGC-7901 cells, HGC-27 and AGS cells compared with three NATs
randomly selected from the patients (P<0.001; Fig. 1).
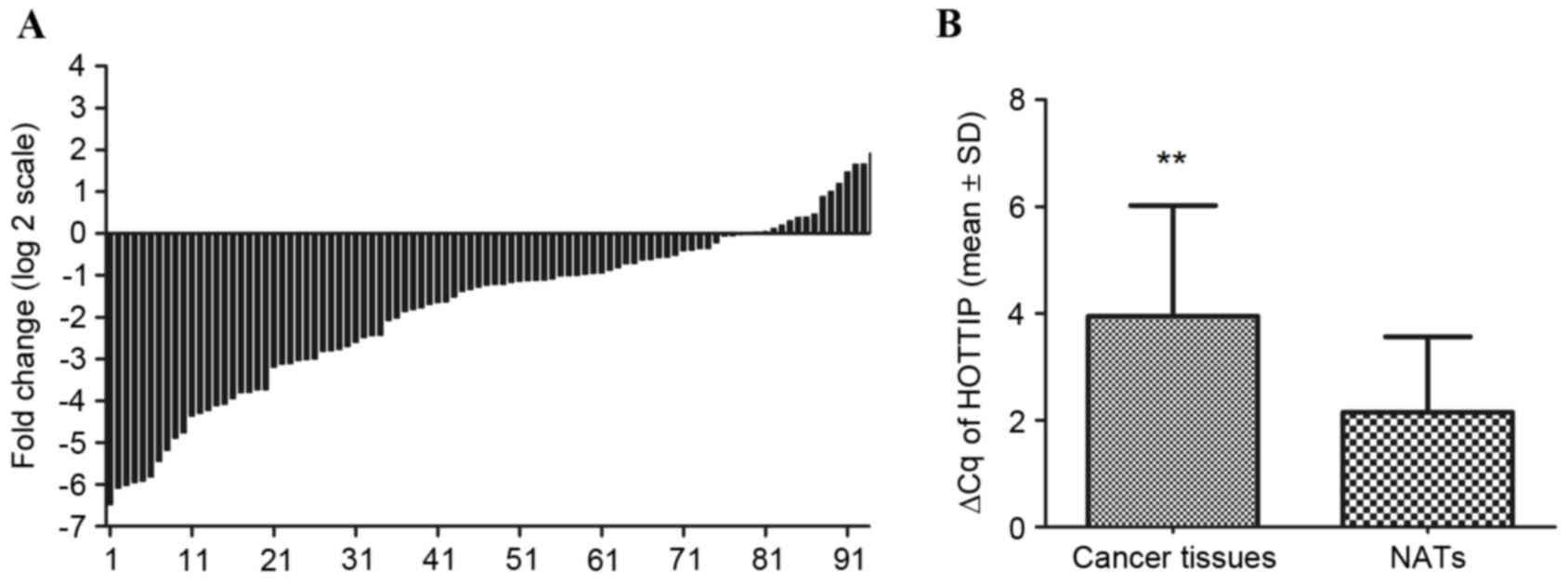
In addition, HOTTIP was detected in all 94 gastric
cancer tissues and their paired NATs. Among the 94 patients with
gastric cancer, 83% (78/94) of cases indicated that the expression
of HOTTIP was decreased in gastric cancer tissues compared with
their paired NATs (Fig. 2A).
Furthermore, HOTTIP expression was significantly decreased in
cancer tissues compared with NATs (P<0.001; Fig. 2B).
Associations between HOTTIP expression
and clinicopathological characteristics
The present study also investigated the associations
between HOTTIP expression and clinicopathological parameters using
non-parametric tests. No statistically significant association was
observed between HOTTIP expression and any clinicopathological
characteristic (Table I).
 | Table I.Associations between the expression of
HOTTIP and clinicopathological characteristics in 94 patients with
gastric cancer. |
Table I.
Associations between the expression of
HOTTIP and clinicopathological characteristics in 94 patients with
gastric cancer.
| Clinicopathological
characteristic | n | HOTTIP
expressiona | P-value |
|---|
| Gender |
|
| 0.892 |
| Male | 68 | 0.389
(0.124–0.782) |
|
|
Female | 26 | 0.463
(0.096–0.675) |
|
| Age, years |
|
| 0.218 |
| ≥63 | 48 | 0.301
(0.117–0.654) |
|
|
<63 | 46 | 0.465
(0.121–0.967) |
|
| Tumor size, cm |
|
| 0.145 |
| ≥5.5 | 56 | 0.301
(0.084–0.744) |
|
|
<5.5 | 38 | 0.498
(0.175–0.760) |
|
| Macroscopic
typeb |
|
| 0.304 |
| Early
stage | 6 | 0.466
(0.029–0.598) |
|
| Borrmann
I–II | 7 | 0.520
(0.463–1.234) |
|
|
Borrmann III–IV | 81 | 0.382
(0.119–0.725) |
|
|
Differentiation |
|
| 0.397 |
|
Good | 3 | 0.292
(0.034–0.697) |
|
|
Moderate | 40 | 0.280
(0.062–0.774) |
|
|
Poor | 51 | 0.460
(0.154–0.760) |
|
| Lauren
typec |
|
| 0.228 |
|
Intestinal type | 32 | 0.256
(0.054–0.774) |
|
| Diffuse
type | 62 | 0.450
(0.152–0.695) |
|
| pT stage |
|
| 0.578 |
|
T1+T2 | 14 | 0.506
(0.315–0.977) |
|
| T3 | 16 | 0.335
(0.139–0.926) |
|
| T4 | 64 | 0.389
(0.111–0.739) |
|
| pN stage |
|
| 0.512 |
| N0 | 20 | 0.390
(0.083–0.516) |
|
| N1 | 17 | 0.609
(0.195–1.008) |
|
| N2 | 20 | 0.270
(0.128–0.706) |
|
| N3 | 37 | 0.445
(0.117–0.972) |
|
| pTNM stage |
|
| 0.431 |
| I | 8 | 0.447
(0.019–0.508) |
|
| II | 19 | 0.499
(0.185–0.964) |
|
|
III | 67 | 0.396
(0.116–0.760) |
|
Association between HOTTIP expression
and patient survival time
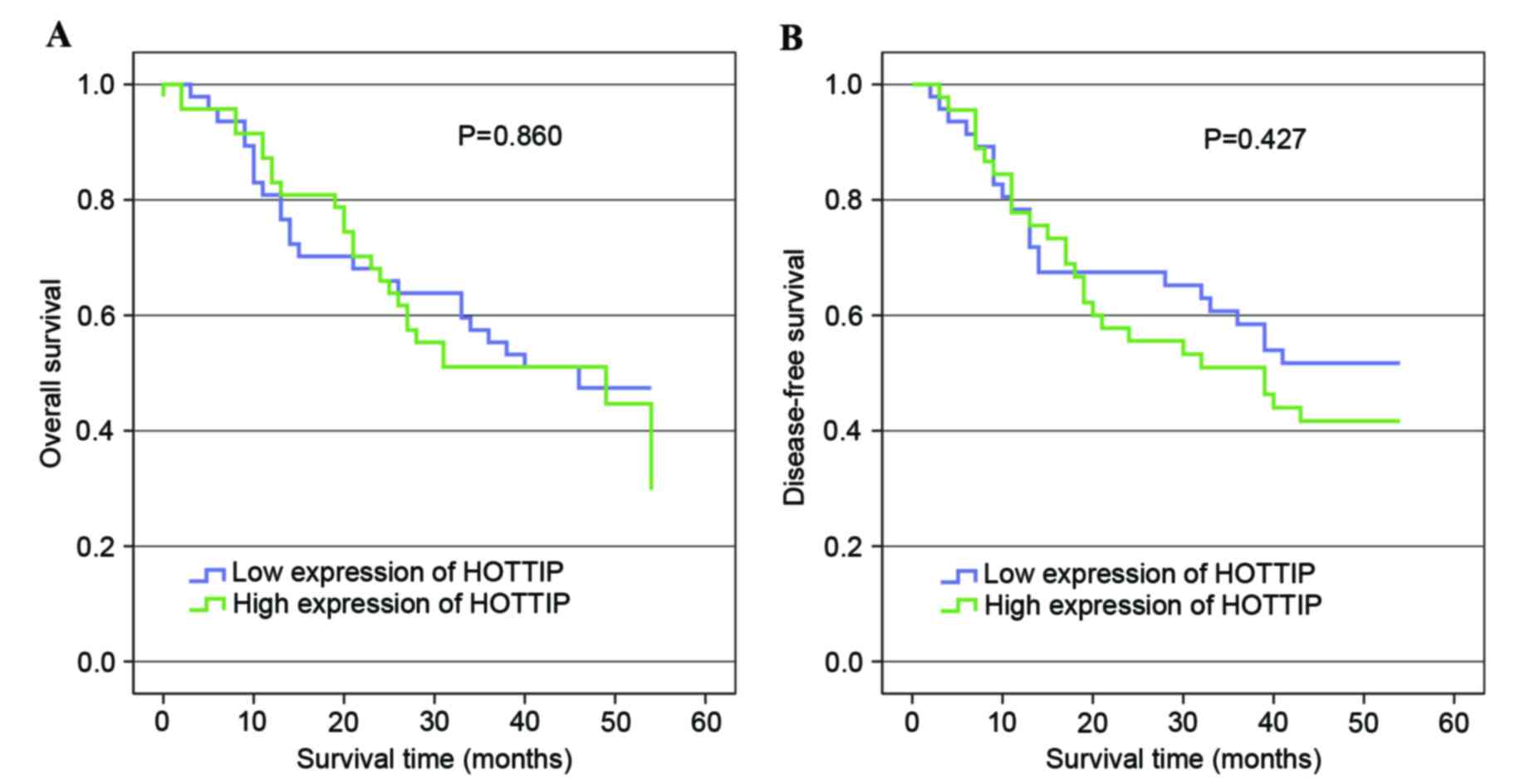
To investigate the association between HOTTIP
expression and the survival time of patients with gastric cancer,
Kaplan-Meier analysis was performed. The median relative expression
levels of HOTTIP were used to divide patients into high and low
expression groups. Kaplan-Meier analyses indicated there was no
significant prognostic difference between patients with high and
low HOTTIP expression (Fig. 3).
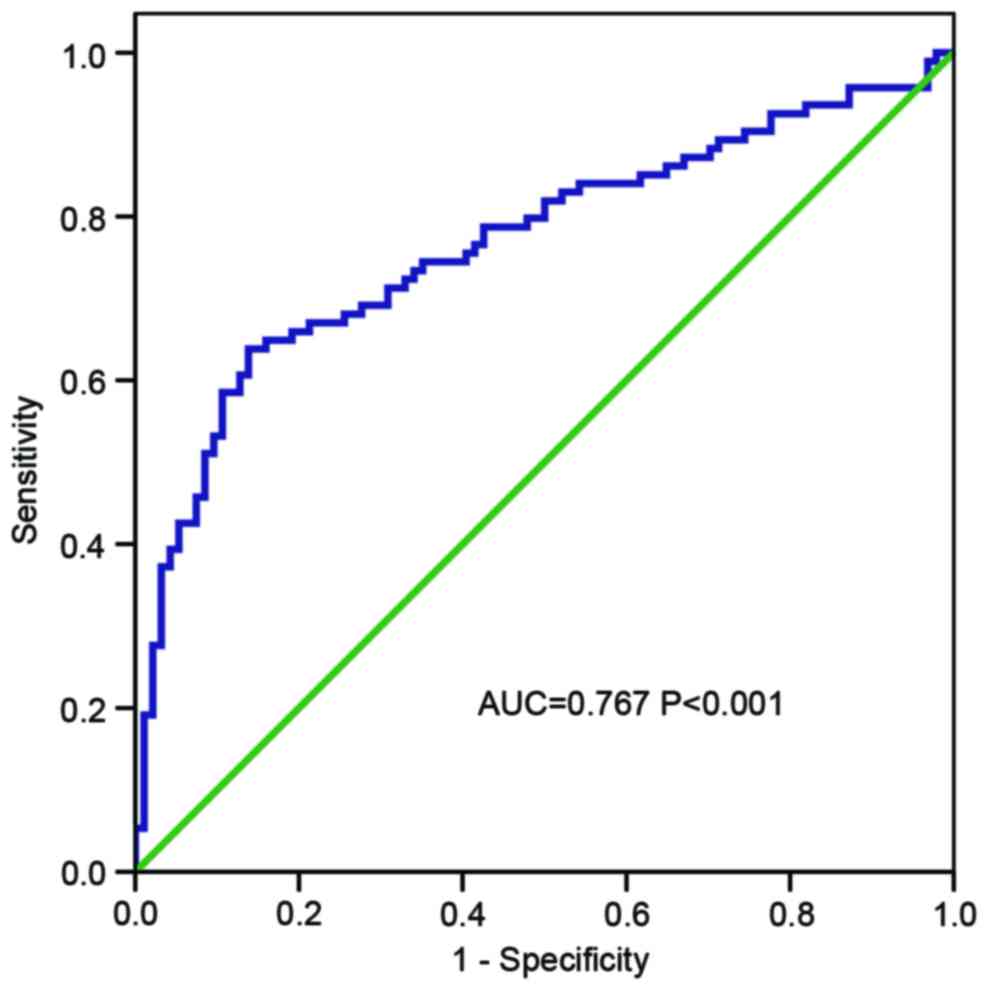
The diagnostic value of using HOTTIP
as a biomarker
To evaluate the diagnostic value of HOTTIP for
distinguishing gastric cancer tissue from normal tissue, a ROC
curve was constructed. The area under the ROC curve (AUC) was 0.767
(P<0.001; Fig. 4), which indicated
that HOTTIP is a potential biomarker for gastric cancer.
Discussion
The prognosis of gastric cancer remains poor as the
majority of patients are diagnosed at a late stage, when treatments
are less effective (2,23). Thus, cancer screening and early
diagnosis serve an important function in the survival of patients
with gastric cancer. The identification of novel and specific
biomarkers is of great clinical value for the diagnosis and
treatment of gastric cancer. Multiple previous studies have
reported that lncRNAs are involved in cancer pathogenesis and may
provide novel insight into the biology of this disease (5,6), where
they may act as oncogenes or tumor suppressors. A number of
previous studies have reported that HOTAIR may possess
pro-oncogenic functions and that it is involved in multiple cancers
(8–12). Similarly, a number of studies have
demonstrated that HOTTIP may serve as a critical regulator in
certain cancers. For example, Quagliata et al (15) reported that HOTTIP and HOXA13 were
highly expressed in patients with hepatocellular carcinoma, where
they were associated with metastasis and poor survival. Li et
al (17) reported that HOTTIP was
upregulated in pancreatic cancer and may promote cancer cell
proliferation, invasion and chemoresistance by regulating HOXA13.
However, another previous study reported that in pancreatic cancer
cells, HOTTIP did not regulate HOXA13 but was involved in the
regulation of certain HOX genes, including HOXA1, HOXA9, HOXA10,
HOXA11 and HOXB2 (16). Deng et
al (19) reported that HOTTIP
promoted tumor growth and inhibited cell apoptosis in lung cancer.
In conclusion, dysregulated expression of HOTTIP has been
associated with a wide variety of biological characteristics of
tumors, and exists in multiple cancers.
To the best of our knowledge, this is the first
report to investigate the value of HOTTIP in gastric cancer. The
present results indicated that HOTTIP expression was significantly
downregulated in gastric cancer tissues compared with NATs among 94
patients (P<0.001). Furthermore, a ROC curve was constructed to
evaluate the diagnostic value of HOTTIP in gastric cancer. The
results revealed that the AUC was 0.767, suggesting that HOTTIP had
potential diagnostic value in gastric cancer. Previously, multiple
studies reported that in different types of cancer there was
abnormal expression of lncRNA, indicating that the same lncRNA may
serve different functions in different types of cancer. For
instance, a lncRNA named SPRY4 intronic transcript 1 (SPRY4-IT1)
was reported to be overexpressed in melanoma, renal cell carcinoma,
esophageal squamous cell carcinoma, breast cancer, bladder cancer
and glioma, and associated with poor prognosis and promotion of
tumor growth (24–29). Certain studies also demonstrated that
SPRY4-IT1 expression was decreased in non-small-cell lung cancer
and gastric cancer, and acted with significant antitumor function
in these two types of cancer (30,31).
Similarly, HOTTIP may exhibit a tissue-specific expression pattern
and serve a different function in different types of cancer, as
with SPRY4-IT.
A number of studies have reported that lncRNAs may
act on their neighboring protein-coding genes in a cis-manner
(32,33). HOTTIP is located the 5′ end of the
HOXA gene cluster, and Wang et al (13) demonstrated that chromosomal looping
may make HOTTIP in close proximity to gene targets, thus, HOTTIP
may be involved in the regulation of its neighboring HOXA genes. It
was reported that HOTTIP may regulate the expression of HOX genes,
including HOXA1, HOXA9, HOXA10, HOXA11 and HOXA13, in liver cancer
and pancreatic cancer (15–17). In addition, numerous studies have
reported that aberrant expression of HOXA genes was associated with
a number of biological characteristics of gastric cancer (34–37).
HOTTIP may be critical in regulating HOXA genes in gastric cancer,
but the underlying mechanism remains unknown and requires
additional study.
In summary, the results of the present study
indicated that lncRNA HOTTIP expression was decreased in patients
with gastric cancer, and that HOTTIP may be a predictive biomarker
in gastric cancer. However, the underlying molecular mechanisms
through which HOTTIP is involved in gastric cancer require further
study.
Acknowledgements
The authors would like to thank the Department of
Surgical Oncology and General Surgery, First Hospital of China
Medical University (Shenyang, China) for providing the human
gastric tissue samples. The authors would also like to thank the
College of China Medical University (Shenyang, China) for technical
assistance in experiments. The present study was supported by the
National Science Foundation of China (grant nos. 81201888, 81372549
and 81172370), the Natural Science Foundation of Liaoning Province
(grant no. 2014029201) and the Key Technologies Research and
Development Program of Liaoning Province (grant no.
2012225008).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen L, Shan YS, Hu HM, Price TJ, Sirohi
B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, et al: Management of
gastric cancer in Asia: Resource-stratified guidelines. Lancet
Oncol. 14:e535–e547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Flynn RA and Chang HY: Long noncoding RNAs
in cell-fate programming and reprogramming. Cell Stem Cell.
14:752–761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao W, Dong S, Duan B, Chen P, Shi L, Gao
H and Qi H: HOTAIR is a predictive and prognostic biomarker for
patients with advanced gastric adenocarcinoma receiving
fluorouracil and platinum combination chemotherapy. Am J Transl
Res. 7:1295–1302. 2015.PubMed/NCBI
|
|
9
|
Xue Y, Gu D, Ma G, Zhu L, Hua Q, Chu H,
Tong N, Chen J, Zhang Z and Wang M: Genetic variants in lncRNA
HOTAIR are associated with risk of colorectal cancer. Mutagenesis.
30:303–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun X, Du P, Yuan W, Du Z, Yu M, Yu X and
Hu T: Long non-coding RNA HOTAIR regulates cyclin J via inhibition
of microRNA-205 expression in bladder cancer. Cell Death Dis.
6:e19072015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang KC, Yang YW, Liu B, Sanyal A,
Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta
RA, et al: A long noncoding RNA maintains active chromatin to
coordinate homeotic gene expression. Nature. 472:120–124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang YJ and Bikle DD: LncRNA profiling
reveals new mechanism for VDR protection against skin cancer
formation. J Steroid Biochem Mol Biol. 144:87–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quagliata L, Matter MS, Piscuoglio S,
Arabi L, Ruiz C, Procino A, Kovac M, Moretti F, Makowska Z,
Boldanova T, et al: Long noncoding RNA HOTTIP/HOXA13 expression is
associated with disease progression and predicts outcome in
hepatocellular carcinoma patients. Hepatology. 59:911–923. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng Y, Jutooru I, Chadalapaka G, Corton
JC and Safe S: The long non-coding RNA HOTTIP enhances pancreatic
cancer cell proliferation, survival and migration. Oncotarget.
6:10840–10852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Z, Zhao X, Zhou Y, Liu Y, Zhou Q, Ye H,
Wang Y, Zeng J, Song Y, Gao W, et al: The long non-coding RNA
HOTTIP promotes progression and gemcitabine resistance by
regulating HOXA13 in pancreatic cancer. J Transl Med. 13:842015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsang FH, Au SL, Wei L, Fan DN, Lee JM,
Wong CC, Ng IO and Wong CM: Long non-coding RNA HOTTIP is
frequently up-regulated in hepatocellular carcinoma and is targeted
by tumour suppressive miR-125b. Liver Int. 35:1597–1606. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng HP, Chen L, Fan T, Zhang B, Xu Y and
Geng Q: Long non-coding RNA HOTTIP promotes tumor growth and
inhibits cell apoptosis in lung cancer. Cell Mol Biol
(Noisy-le-grand). 61:34–40. 2015.PubMed/NCBI
|
|
20
|
Zhang H, Zhao L, Wang YX, Xi M, Liu SL and
Luo LL: Long non-coding RNA HOTTIP is correlated with progression
and prognosis in tongue squamous cell carcinoma. Tumour Biol.
36:8805–8809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: American Joint Committee on Cancer (AJCC)
Cancer Staging Handbook. 7th. Springer-Verlag; New York: 2010
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khaitan D, Dinger ME, Mazar J, Crawford J,
Smith MA, Mattick JS and Perera RJ: The melanoma-upregulated long
noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer
Res. 71:3852–3862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie HW, Wu QQ, Zhu B, Chen FJ, Ji L, Li
SQ, Wang CM, Tong YS, Tuo L, Wu M, et al: Long noncoding RNA
SPRY4-IT1 is upregulated in esophageal squamous cell carcinoma and
associated with poor prognosis. Tumour Biol. 35:7743–7754. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang HM, Yang FQ, Yan Y, Che JP and Zheng
JH: High expression of long non-coding RNA SPRY4-IT1 predicts poor
prognosis of clear cell renal cell carcinoma. Int J Clin Exp
Pathol. 7:5801–5809. 2014.PubMed/NCBI
|
|
27
|
Liu H, Lv Z and Guo E: Knockdown of long
noncoding RNA SPRY4-IT1 suppresses glioma cell proliferation,
metastasis and epithelial-mesenchymal transition. Int J Clin Exp
Pathol. 8:9140–9146. 2015.PubMed/NCBI
|
|
28
|
Shi Y, Li J, Liu Y, Ding J, Fan Y, Tian Y,
Wang L, Lian Y, Wang K and Shu Y: The long noncoding RNA SPRY4-IT1
increases the proliferation of human breast cancer cells by
upregulating ZNF703 expression. Mol Cancer. 14:512015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao XL, Zhao ZH, Xu WC, Hou JQ and Du XY:
Increased expression of SPRY4-IT1 predicts poor prognosis and
promotes tumor growth and metastasis in bladder cancer. Int J Clin
Exp Pathol. 8:1954–1960. 2015.PubMed/NCBI
|
|
30
|
Sun M, Liu XH, Lu KH, Nie FQ, Xia R, Kong
R, Yang JS, Xu TP, Liu YW, Zou YF, et al: EZH2-mediated epigenetic
suppression of long noncoding RNA SPRY4-IT1 promotes NSCLC cell
proliferation and metastasis by affecting the
epithelial-mesenchymal transition. Cell Death Dis. 5:e12982014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie M, Nie FQ, Sun M, Xia R, Liu YW, Zhou
P, De W and Liu XH: Decreased long noncoding RNA SPRY4-IT1
contributing to gastric cancer cell metastasis partly via affecting
epithelial-mesenchymal transition. J Transl Med. 13:2502015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kelsey AD, Yang C, Leung D, Minks J,
Dixon-McDougall T, Baldry SE, Bogutz AB, Lefebvre L and Brown CJ:
Impact of flanking chromosomal sequences on localization and
silencing by the human non-coding RNA XIST. Genome Biol.
16:2082015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guil S and Esteller M: Cis-acting
noncoding RNAs: Friends and foes. Nat Struct Mol Biol.
19:1068–1075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bai Y, Fang N, Gu T, Kang Y, Wu J, Yang D,
Zhang H, Suo Z and Ji S: HOXA11 gene is hypermethylation and
aberrant expression in gastric cancer. Cancer Cell Int. 14:792014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han Y, Tu WW, Wen YG, Li DP, Qiu GQ, Tang
HM, Peng ZH and Zhou CZ: Identification and validation that
up-expression of HOXA13 is a novel independent prognostic marker of
a worse outcome in gastric cancer based on immunohistochemistry.
Med Oncol. 30:5642013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Degl' Innocenti D Rossi, Castiglione F,
Buccoliero AM, Bechi P, Taddei GL, Freschi G and Taddei A:
Quantitative expression of the homeobox and integrin genes in human
gastric carcinoma. Int J Mol Med. 20:621–629. 2007.PubMed/NCBI
|
|
37
|
Sentani K, Oue N, Naito Y, Sakamoto N,
Anami K, Oo HZ, Uraoka N, Aoyagi K, Sasaki H and Yasui W:
Upregulation of HOXA10 in gastric cancer with the intestinal mucin
phenotype: Reduction during tumor progression and favorable
prognosis. Carcinogenesis. 33:1081–1088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Borrmann R: Geshwulste des magens und
duodenumsHenke F and Lubarsch O: Handbuch der speziellen
pathologischen anatomie und histologie. Springer-Verlag; Berlin:
pp. 8651926
|
|
39
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|