Introduction
Immunogenic death is a cell death modality that
stimulates the innate and adaptive immune system against cell death
associated antigens, inducing tumor cell immunogenicity.
Immunogenic cell death (ICD) is characterized by the exposure or
release of immunogenic molecules by dying tumor cells (1–3), termed
damage-associated molecular patterns (DAMPs). Certain DAMPs include
calreticulin (CRT) exposure on the outer surface of the plasma
membrane, which serves an important function as a phagocytic
signal, stimulating phagocytes to engulf dead tumor cells (3,4–8). The secretion of adenosine triphosphate
(ATP) by dying tumor cells is an important chemoattractant for
macrophages and dendritic cells to the site of tumor (3,9–13). The heat shock proteins (HSP) 70 and
HSP90, and non-histone chromatin binding protein high mobility
group box 1 (HMGB1) are released into the extracellular space and
promote the recognition of tumor cells by dendritic cells by
binding to receptors on the cell surface, leading to their
elimination by the immune system (14–19).
It has been suggested that only certain types of
cancer therapies induce immunogenic cell death in vitro
(20–23) and in vivo (5,24–29), and that these may be classified into
two groups. The targets of group I ICD inducers include DNA and
repair machinery proteins, cytosolic proteins, plasma membrane or
nucleic proteins, which are targeted by chemotherapeutic agents
including anthracyclines, oxaliplatin (OXP) and mitoxantrone;
cardiac glycosides, shikonin and ultraviolet C irradiation. Group
II ICD inducers target the endoplasmic reticulum, and include
photodynamic therapy with hypericin and Coxsackievirus B3 (8,30–34). Certain ICD agents with these
characteristics are considered to be anti-cancer vaccines, and as
therapies that prevent residual cancer.
IMMUNEPOTENT CRP (ICRP) is a dialysate of a
heterogeneous mixture of low-molecular-weight substances released
from the disintegrated leukocytes of the blood or lymphoid tissue
obtained from homogenized bovine spleens. ICRP exhibits in
vitro cytotoxic effects on different tumor cell lines and
modulates the immune response in vivo (35–40). The
aim of the present study was to determine whether ICRP or ICRP
combined with OXP induced ICD and prevented melanoma growth.
Materials and methods
Reagents and antibodies
OXP was obtained from Teva Pharmaceutical
Industries, Ltd. (Petah Tikva, Israel). IMMUNEPOTENT CRP was
produced by the Department of Immunology and Virology, Biological
Sciences Faculty, Autonomous University of Nuevo Leon (Nuevo Leon,
Mexico). Propidium iodide staining solution and allophycocyanin
(APC)-conjugated Annexin V was obtained from BD Pharmingen (BD
Biosciences, San Jose, CA, USA). Phycoerythin (PE)-conjugated CRT
monoclonal antibodies (cat. no. ADI-SPA-601PE-F) and IgG1 isotype
control monoclonal antibodies (cat. no. ADI-SAB-600PE-D) were
obtained from Enzo Life Sciences (Farmingdale, NY, USA). Mouse
monoclonal antibodies targeting HSP70 (cat. no. sc-24), HMGB1 (cat.
no. sc-56698), β-actin (cat. no. sc-69879), rabbit polyclonal IgG
antibody targeting HSP90 α/β (cat. no. sc-7947), and secondary
antibodies including mouse anti-rabbit (cat. no. sc-2357) and goat
anti-mouse (cat. no. sc-2005) IgGs conjugated to horseradish
peroxidase were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Complete Halt Protease inhibitor cocktail (100X)
was obtained from Thermo Fisher Scientific, Inc. (cat. no. 87786;
Waltham, MA, USA). The ENLITEN ATP Assay System Bioluminescence
Detection kit for ATP measurement was obtained from Promega
Corporation (Madison, WI, USA). The HMGB1 BioAssay ELISA kit
(mouse; cat. no. 194487) was purchased from US Biological Life
Sciences (Salem, MA, USA).
Cell line and culture conditions
The murine melanoma B16F10 cell line was obtained
from American Type Tissue Collection (Manassas, VA, USA) and was
maintained in Dulbecco's modified Eagle's medium/F-12 medium 1:1
containing 2.50 mM L-Glutamine, 15 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer medium
(cat. no. SH30023.FS; all HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine
serum (cat. no. 10082147) and 100 U/ml penicillin/streptomycin
(cat. no. 15140122; both Gibco; Thermo Fisher Scientific, Inc.).
The cell line was incubated in a humidified atmosphere with 5% CO2
at 37°C.
Cell death assays
B16F10 cells (1×105) were seeded into
12-well plates and cultured overnight in 5% CO2 at 37°C. Cells were
treated with ICRP (1 U/ml), OXP (800 µM) or a combination of ICRP
(1 U/ml) + OXP (800 µM) for 24, 48 and 72 h. Following treatment,
cells were collected and washed with phosphate-buffered saline
(PBS) and resuspended in 100 µl of 1X binding buffer (0.1 M Hepes
pH 7.4, 1.4 M NaCl and 25 mM CaCl2; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) supplemented with APC-conjugated Annexin
V (5 µl/sample) and propidium iodide (1 µl/sample), incubated on
ice and kept in the dark for 15 min. Flow cytometry analysis was
performed using an Accuri C6 cytometer; BD Accuri C6 Software
version 1.0.264.21 was used for data analysis (both BD Biosciences,
San Jose, CA, USA).
Analysis of CRT on the cell
surface
Flow cytometry was used to determine the level of
CRT exposure induced by the treatments. B16F10 cells
(1×105) were treated with ICRP (1 U/ml), OXP (800 µM) or
the combination of ICRP (1 U/ml) + OXP (800 µM) for 6, 12, 24, 48
or 72 h. The cells were harvested, suspended in 1X PBS with 1%
fetal bovine serum and incubated for 1 h at room temperature in the
dark with a CRT monoclonal antibody (dilution, 1:100), then
analyzed.
Western blot analysis
B16F10 cells (5×106) cells were treated
with ICRP (1 U/ml), OXP (800 µM) or a combination of ICRP (1 U/ml)
+ OXP (800 µM) for 24, 48 and 72 h. Following this, supernatants
and cells were collected and centrifuged at 260 × g for 10 min at
room temperature. The cells were washed with PBS and homogenized
using the SET 2X lysis buffer (20 mM Tris pH 6.8, 2 mM EDTA pH 8.0,
300 mM NaCl and 4% SDS; Sigma-Aldrich; Merck KGaA) supplemented
with the Complete Halt protease inhibitor cocktail. Protein
quantification was performed using a detergent compatible Lowry
protocol protein assay (cat. no. 5000112; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Equal amounts of soluble proteins (50 µg)
were resolved by 12% SDS-PAGE and transferred to a nitrocellulose
membrane. Non-specific binding sites were blocked by incubating the
membrane for 1 h at room temperature in TBS-Tween-20 supplemented
with 5% non-fat powdered milk followed by overnight incubation at
4°C with primary antibodies, including anti-HSP90 or -β-actin (the
internal standard) at a dilution of 1:500, or anti-HSP70 or -HMGB1
at a dilution of 1:400. The antibodies were diluted in 10 ml of 1X
TBS-0.1% Tween-20 buffer supplemented with 5% w/v BSA. Primary
antibodies were detected by incubation with mouse anti-rabbit or
goat anti-mouse IgGs conjugated to horseradish peroxidase, diluted
to 1:2,000, for 2 h at room temperature. The protein bands were
visualized using an enhanced chemiluminescence western blotting
detection kit and high performance chemiluminescence film (both GE
Healthcare Life Sciences).
ATP release assays
Extracellular ATP levels were measured in the
supernatant of B16F10 cells treated with ICRP (1 U/ml), OXP (800
µM) or a combination of ICRP (1 U/ml) + OXP (800 µM) for 24, 48 and
72 h by luciferin-based ENLITEN ATP assay (Promega Corporation)
according to the manufacturer's protocol. Chemiluminescence was
recorded with a BioTek Synergy microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
HMGB1 release assays
HMGB1 concentration was measured in the supernatant
of untreated or treated B16F10 cells with ICRP (1 U/ml), OXP (800
µM) and the combination of ICRP (1 U/ml) + OXP (800 µM) for 24, 48
and 72 h using the HMGB1 ELISA kit according to the manufacturer's
protocol.
Animals
A total of 20 female C57BL/6 mice were purchased
from Harlan Laboratories (Mexico City, Mexico). The body weight of
mice was 23 (±2) g and they were between 6 and 10 weeks of age. The
mice were housed at 25–29°C with 45% humidity and a 12 h light: 12
h dark cycle. Mice were provided food and water ad libitum.
According to experimental protocols that were approved by the
Ethics Review Committee for Animal Experimentation of the
Biological Sciences Faculty, Autonomous University of Nuevo Leon
(San Nicolas de los Garza, Mexico).
In vivo anti-tumor vaccination
experiments
A total of 5×106 B16F10 cells were
treated with ICRP (3 U/ml), OXP (12,600 µM) or a combination of
ICRP (1.2 U/ml) + OXP (900 µM) for 48 h in vitro. Following
this, the cells were centrifuged at 260 × g for 10 min and washed
twice with PBS. Finally, cells were resuspended in 200 µl PBS and
inoculated subcutaneously into the left flank of the mice. After 7
days, mice were challenged with 5×105 live B16F10 cells
resuspended in 200 µl of PBS via subcutaneous injection into the
right flank. Tumor incidence and growth were measured every day at
the two injection sites for 60 days with a digital caliper. Tumor
volume was calculated using the formula: V=(W (2) × L)/2, where V is tumor volume, W is
tumor width and L is tumor length.
Humane end-points were used to avoid
unnecessary suffering
Mice were sacrificed when the width of tumors
reached 20 mm. Effort was made to minimize environmental stress.
The control group was sacrificed at 20 days, the OXP group at 30
days, and the ICRP and ICRP + OXP groups at 60 days.
Statistical analysis
The experiments were performed in triplicate and
statistical analysis was performed using a one-way analysis of
variance followed by Dunnett's test. P<0.05 was considered to
indicate a statistically significant difference. SPSS version 17.0
(IBM Corp., Armonk, NY, USA) was used to perform the analysis.
Results
Cell death is induced by ICRP, OXP or
ICRP + OXP treatments in the B16F10 cell line
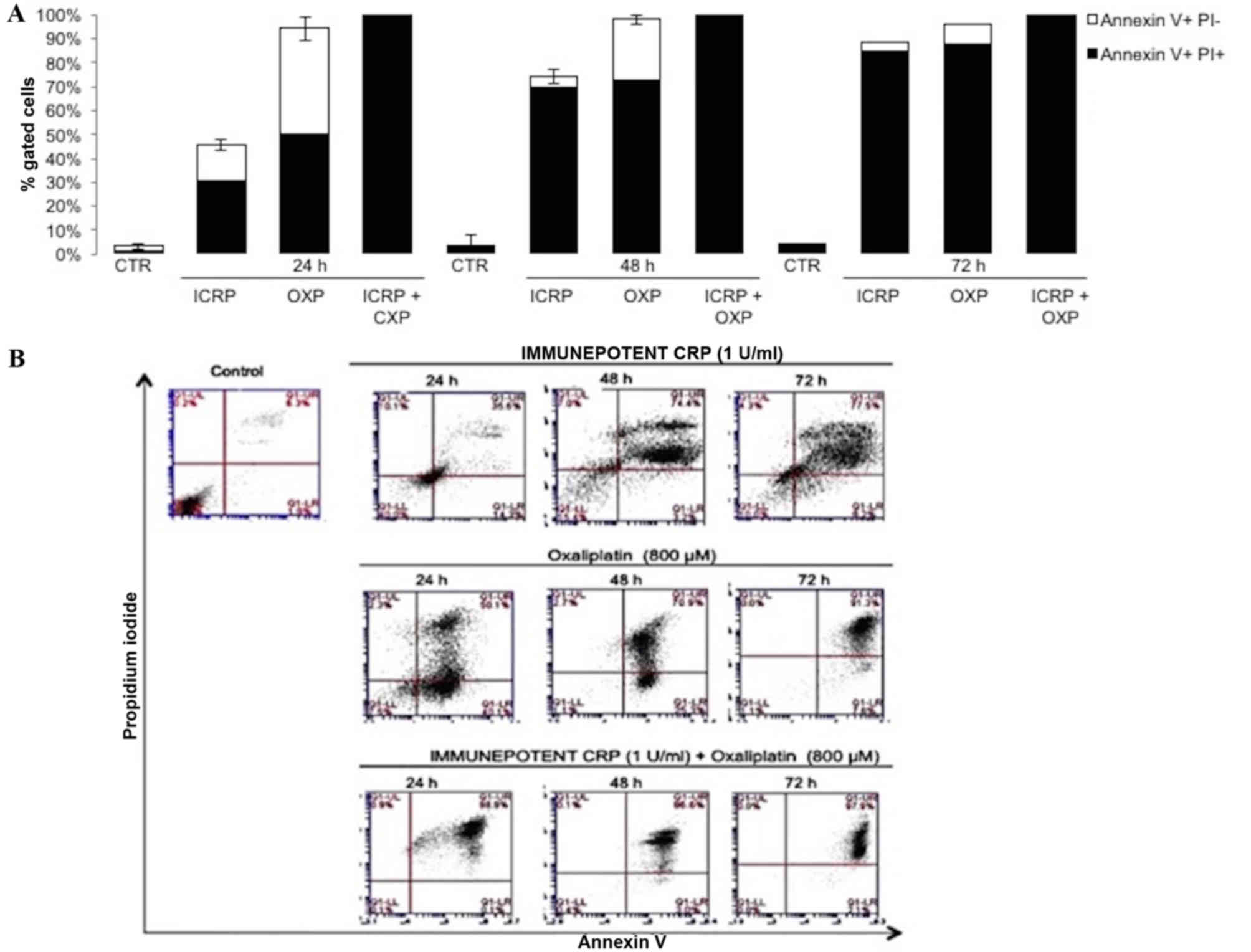
Treatment with ICRP [24 h (30.6%), 48 h (69.6%), and
72 h (85%)] and OXP [24 h (50.5%), 48 h (72.6%), and 72 h (88%)]
induced cell death in a time-dependent manner (Fig. 1A). Treatment with the combination of
ICRP + OXP induced a cytotoxic effect at all time-points evaluated
[24 h (98.8%), 48 h (100%), and 72 h (100%); Fig. 1A]. Apoptosis was indicated by staining
with Annexin V-APC, which binds to phosphatidylserine residues on
the surface of dying cells, and propidium iodide, which penetrates
only into dead cells (Fig. 1B). In
the untreated B16F10 cells, cell viability was not affected.
CRT exposure is induced by treatment
with ICRP, OXP or ICRP + OXP in the B16F10 cell line
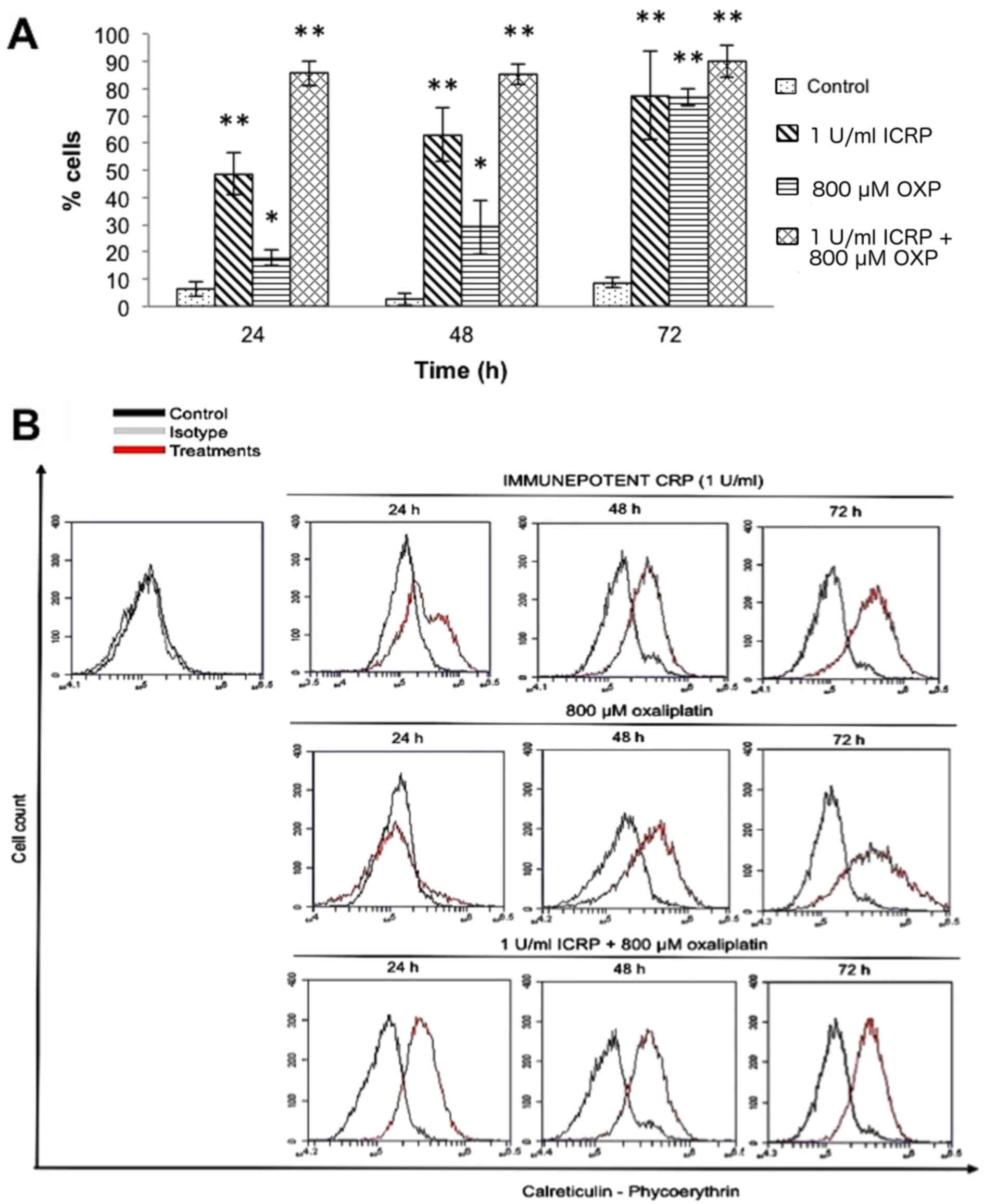
Treatment with ICRP or OXP induced CRT exposure in a
time-dependent manner (ICRP: 6 h, 9.1%; 12 h, 23%; 24 h, 48.6%; 48
h, 63%; and 72 h, 77.4%; P<0.001; OXP: 6 h, 2.6%; 12 h, 9.2%; 24
h, 17.7%; 48 h, 29%; and 72 h, 76.8%; P<0.05; Fig. 2). Treatment with a combination of ICRP
+ OXP induced the highest exposure of CRT following treatment for
24 h, however, following combined treatment for 6 h, CRT exposure
was higher compared with the individual treatments (6 h, 12.7%; 12
h, 58.2%; 24 h, 85.4%; 48 h, 85.10%; and 72 h, 90%; P<0.001;
Fig. 2).
Release of ATP in the B16F10 cell line
following treatment with ICRP, OXP or ICRP + OXP
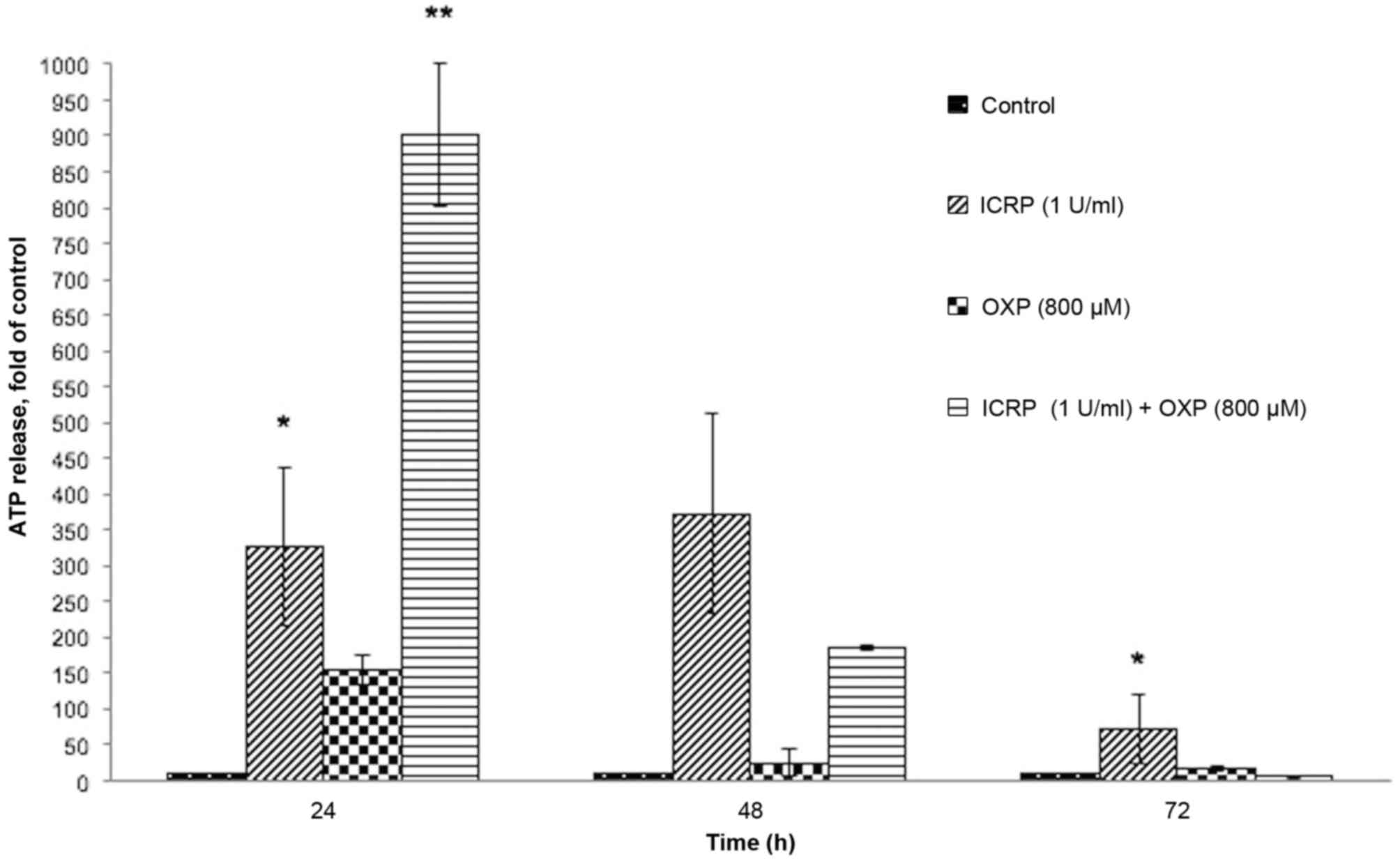
The release of ATP significantly increased following
24 h of treatment with ICRP (P<0.05; Fig. 3) and the combination of ICRP+OXP
(P<0.001; Fig. 3). Following 48 h
of treatment, ATP release was not detected except by ICRP treatment
at 72 h (P<0.01; Fig. 3). The
decreased ATP detection following 48 h of treatment may be because
extracellular ATP is not stable, due to the presence of various
enzymes that degrade ATP (ATPases) or the decomposition of ATP to
adenosine diphosphate, adenosine monophosphate, adenosine and
inorganic phosphate (10,13).
Release of HSP70, HSP90 and HMGB1
proteins in the B16F10 cell line treated with ICRP, OXP or
ICRP+OXP
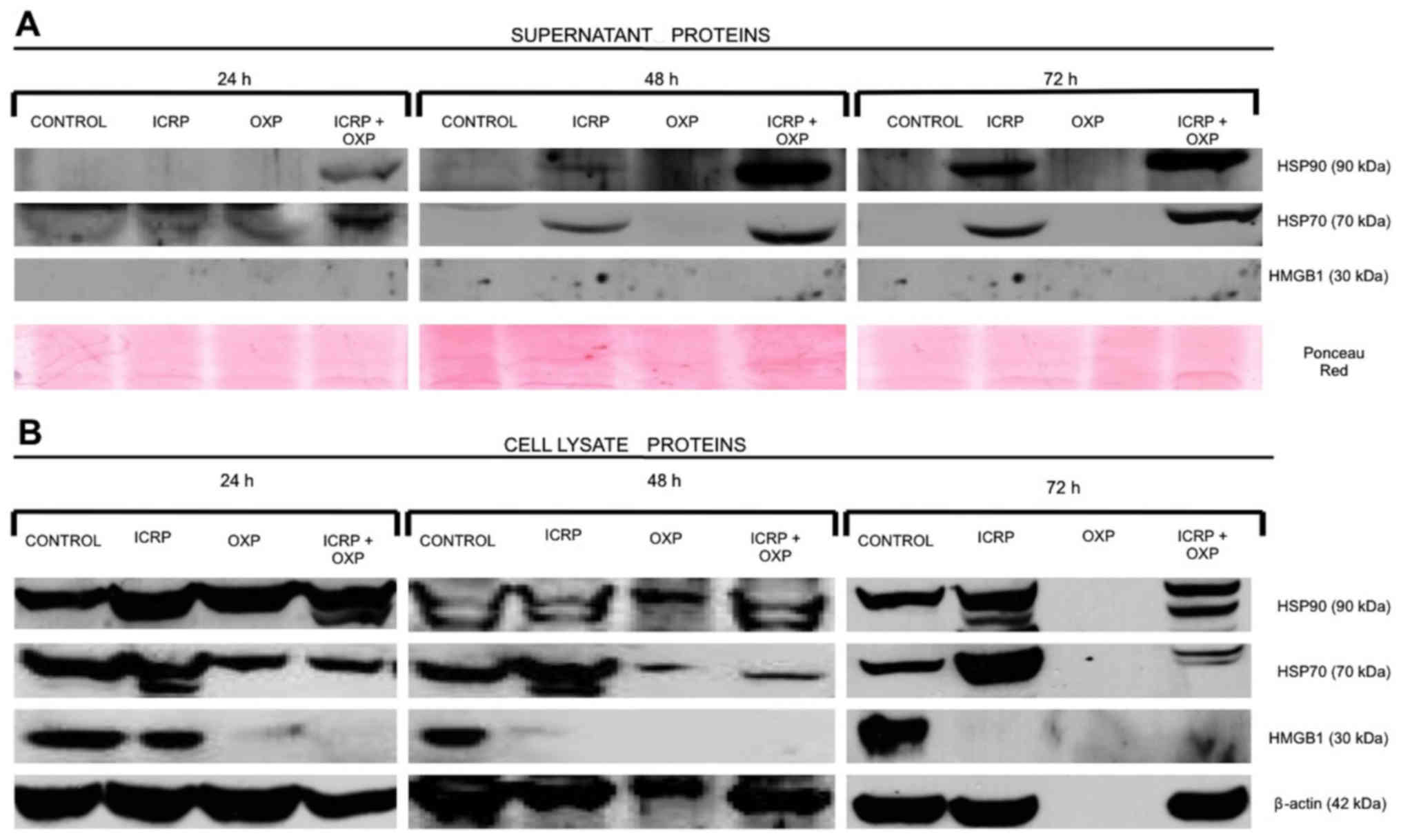
In the supernatant, treatment with ICRP + OXP
induced the release of HSP70 and HSP90 in the B16F10 cells at all
evaluated time-points, and their release was increased at 48 and 72
h relative to 24 h. ICRP treatment induced the release of these
proteins, and they were detected at 48 h, and their release was
increased in the supernatants at 72 h relative to 24 h. OXP did not
induce the release of HSP70 or HSP90 at any of the evaluated
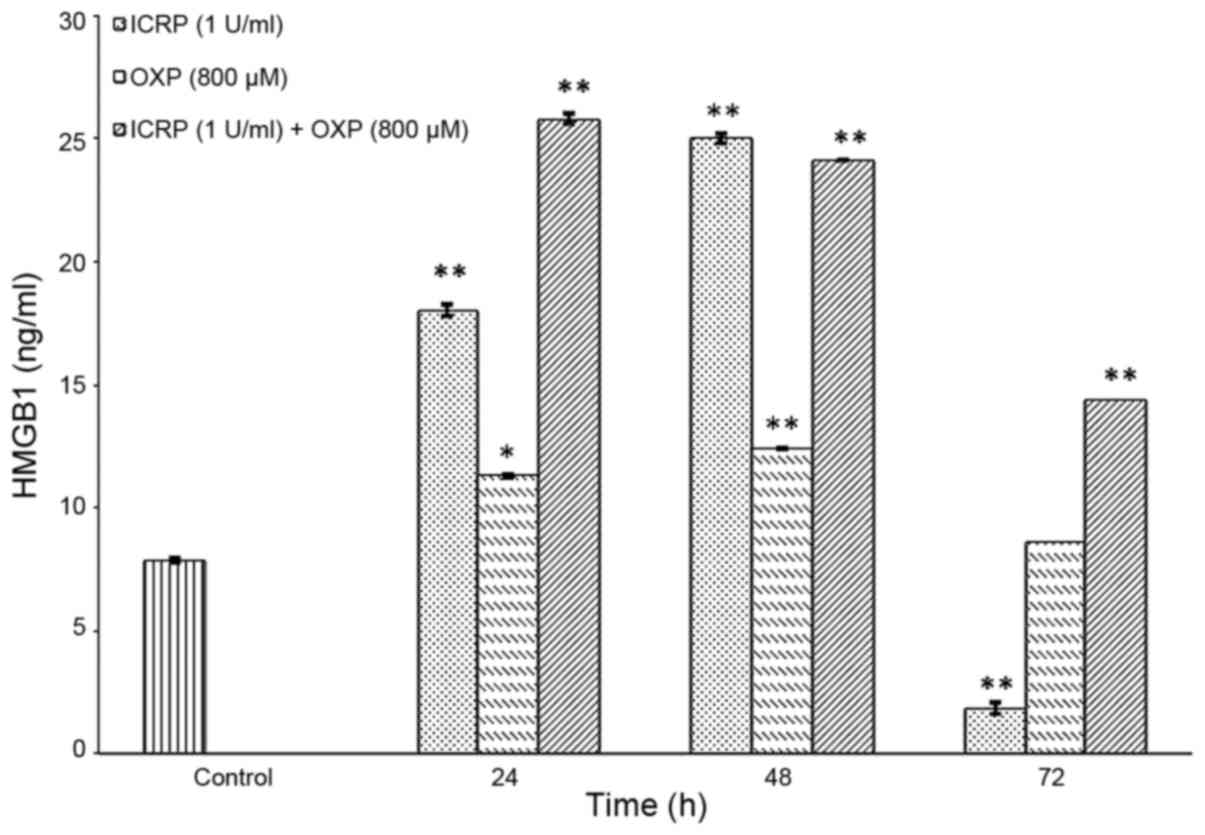
time-points. HMGB1 was not detected in any of the treatments by
western blotting (Fig. 4). Therefore,
an ELISA kit with high sensitivity was used for its detection, and
it was revealed that all ICRP, OXP or ICRP + OXP treatments
increased HMGB1 release at 24 and 48 h compared with the control
(P<0.001; Fig. 5). Treatment with
ICRP significantly increased the release of HMGB1 at 24 h
(P<0.001) and 48 h (P<0.001) compared with the control;
however, it decreased at 72 h (P<0.001; Fig. 5). Treatment with OXP significantly
increased the release of HMGB1 at 24 h (P<0.05) and 48 h
(P<0.001) but decreased the release at 72 h compared with the
control (Fig. 5). The treatment with
ICRP + OXP significantly increased the release of HMGB1 at all the
times evaluated (P<0.001) compared with the control. In the cell
lysates, the presence of HSP70, HSP90 and HMGB1 was detected in the
control treatment at all the time-points evaluated. HSP70 and HSP90
were detected in cells exposed to ICRP or ICRP + OXP treatments at
24, 48 and 72 h, but were not detected in cells exposed to OXP
treatment at 72 h. HMGB1 only was detected in cells exposed to ICRP
treatment at 24 h (Fig. 4).
In vivo effects of tumor-derived
DAMP-rich cell lysates derived from B16F10 cells treated with ICRP,
OXP or ICRP + OXP in the prevention of melanoma
In vitro experiments demonstrated the
cytotoxic effect and induction of DAMPs, which are characteristic
of immunogenic cell death, following treatments with ICRP, OXP, or
a combination of ICRP + OXP. To evaluate the immunogenicity of the
immunogenic cell death induced by the treatments in vivo,
anti-tumor vaccination experiments were performed in a mouse model.
The tumor-derived DAMP-rich cell lysates derived from previous
treatments of B16F10 cells with ICRP, OXP or a combination of ICRP
+ OXP were administered to mice prior to inoculation with live
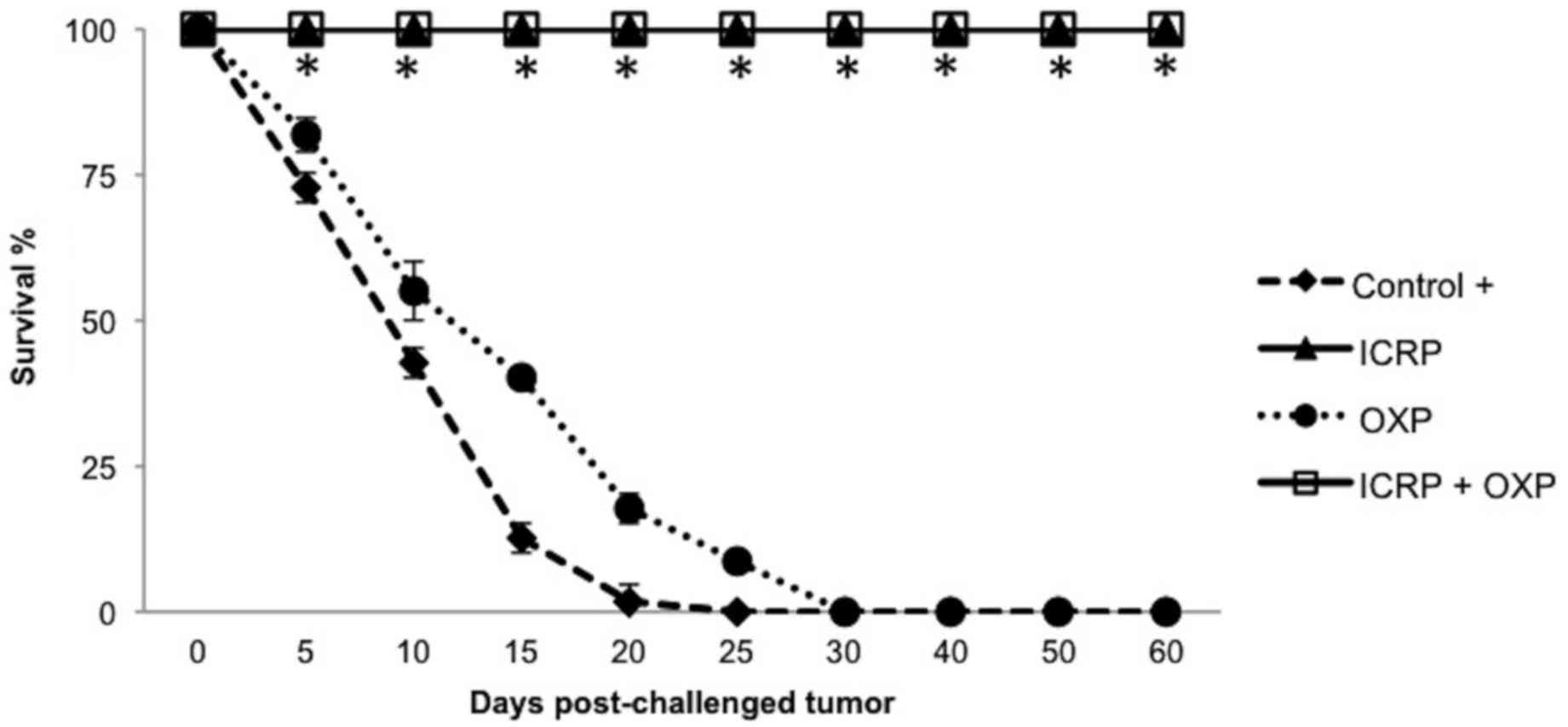
B16F10 cells. The results demonstrated that the ICRP and ICRP + OXP
treatments prevented the development of melanoma growth. The
tumor-derived DAMP-rich cell lysates from the OXP treatment did not
protect against melanoma growth, but delayed mortality in the mice
(30 days) compared with the control (20 days; Fig. 6).
Discussion
There has been increasing interest in the
optimization of old and the identification of novel therapeutic
agents with the capacity to generate anticancer immunity.
Immunogenic cell death generated from antitumor treatments is one
of the mechanisms through which these treatments elicit their
tumor-targeting immune responses (41). The present study aimed to determine
the potential of ICRP used in combination with chemotherapy to
increase cytotoxicity against tumor cells and to induce molecules
associated with immunogenic death, as novel therapeutic regimens
that focus on a combination of strategies to trigger these
mechanisms are in development.
The results of the present study demonstrated that
treatment with ICRP was cytotoxic in B16F10 melanoma cells, and
that the combination of ICRP treatment with OXP increased the rate
of cell death. This was similar to results concerning the cytotoxic
effects on cancer cells of ICRP treatment alone, which were
identified previously by our group (37). It is important to note that in the
present study, the treatments with ICRP or ICRP + OXP induced the
release of several immunogenic molecules (CRT, ATP, HSP70, HSP90
and HMGB1) in vitro. The presence of these molecules, when
induced by anthracycline treatment in colon cancer CT26 cells,
melanoma B16F10 cells or fibrosarcoma MCA205 cells, has been
associated with the prevention of tumor growth (42–44). HMGB1
was not detected in the supernatant by western blot assay,
potentially due to the sensitivity of the antibody used; but when
examined with an ELISA kit with high sensitivity the release of
HMGB1 was detected. Similar results for human HMBG1 were
demonstrated by Nowak et al (45), where concentrations of 150 ng/ml were
detected by ELISA and western blot, but lower concentrations (1 or
15 ng/ml) were not detected by western blot; only by ELISA.
OXP treatment has been suggested to induce the
release of DAMPs (CRT, ATP, HMGB1 and type I interferon) in several
cancer cell lines, and is considered to be an inductor agent of ICD
(27,31). To the best of our knowledge, studies
examining the effect of immunogenic cell death induced by OXP on
the B16F10 cancer cell line, which is poorly immunogenic, had not
yet been performed. The results of the present study indicated that
oxaliplatin induced the exposure of CRT and the release of ATP and
HMGB1, but did not induce the release of HSP70 and HSP90 in the
B16F10 cancer cell line. Compounds with the capability to induce
the release of HSP70, HSP90, ATP and HMGB1 have been demonstrated
to prevent tumor growth (30–34,46,47). In
addition, Chen et al (24)
suggested that treatment with shikonin in B16F10 cells induced
exposure of CRT and the release of HSP70, HSP90, GRP78 and HMGB1
in vitro, and allowed the maturation of dendritic cells; and
shikonin tumor-derived cell lysate-loaded dendritic cell vaccines
were indicated to induce retardation of tumor growth and to
increase the survival rate of mice. Similar results were obtained
in the present study, where tumor cell lysates derived from B16F10
cells treated with ICRP or ICRP + OXP administered to mice were
demonstrated to prevent melanoma growth induced by injection with
live B16F10 cells, indicating the potential of these treatments to
induce immunogenic cell death. OXP-induced immunogenic death of
colon cancer cells has been demonstrated in murine and human cell
lines, and OXP prevented the formation of tumors (4,27).
However; in the present study this was not observed, suggesting
that it is necessary for OXP to induce the additional release of
HSP70 and HSP90 in B16F10 cells in order to generate immunogenic
cell death, similar to the aforementioned treatments (30–34,48,49).
Depending on the type of cell death inducer involved, tumor cells
may expose or release factors that affect their uptake (CRT),
maturation (HSP90) or antigen presentation by dendritic cells
(HMGB1) (46). Studies investigating
the induction of immunogenic cell death by anthracyclines, OXP or
ionizing radiation suggest that these effects require the presence
of DAMPs and their corresponding receptors on antigen-presenting
cells for complete therapeutic success (46).
It has been demonstrated that in cancer patients or
whole tumor cells, treatment with drugs including oxaliplatin or
doxorubicin (30–34) or physical procedures (47,50–52) may
induce a specific immune response through tumor antigens that have
been exposed to dendritic cells, together with DAMP priming and the
activation of naïve T cells to target tumors (53).
Although additional studies are necessary to
understand the mechanisms underlying the prevention of melanoma
growth, the present study demonstrated that IMMUNEPOTENT CRP,
currently used in Mexico as an adjuvant to the immune system, may
be used in combination with chemotherapy as a potential agent to
increase the action of antitumor drugs by inducing immunogenic cell
death to eliminate residual cancer cells in patients, and may
generate the development of a whole tumor vaccine.
Acknowledgements
The present study was supported by the Laboratory of
Immunology and Virology, Biological Sciences Faculty, Autonomous
University of Nuevo Leon, in collaboration with ‘Red temática de
Inmunología en Cancer y Enfermedades Infecciosas’ (grant no.
253053; CONACYT).
References
|
1
|
Tesniere A, Panaretakis T, Kepp O, Apetoh
L, Ghiringhelli F, Zitvogel L and Kroemer G: Molecular
characteristics of immunogenic cancer cell death. Cell Death
Differ. 15:3–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tesniere A, Apetoh L, Ghiringhelli F, Joza
N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L and Kroemer G:
Immunogenic cancer cell death: A key-lock paradigm. Curr Opin
Immunol. 20:504–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kroemer G, Galluzzi L, Kepp O and Zitvogel
L: Immunogenic cell death in cancer therapy. Annu Rev Immunol.
31:51–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Obeid M, Tesniere A, Ghiringhelli F, Fimia
GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T,
Casares N, et al: Calreticulin exposure dictates the immunogenicity
of cancer cell death. Nat Med. 13:54–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Obeid M, Panaretakis T, Joza N, Tufi R,
Tesniere A, van Endert P, Zitvogel L and Kroemer G: Calreticulin
exposure is required for the immunogenicity of gamma-irradiation
and UVC light-induced apoptosis. Cell Death Differ. 14:1848–1850.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panaretakis T, Joza N, Modjtahedi N,
Tesniere A, Vitale I, Durchschlag M, Fimia GM, Kepp O, Piacentini
M, Froehlich KU, et al: The co-translocation of ERp57 and
calreticulin determines the immunogenicity of cell death. Cell
Death Differ. 15:1499–1509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Panaretakis T, Kepp O, Brockmeier U,
Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N,
Pierron G, van Endert P, et al: Mechanisms of pre-apoptotic
calreticulin exposure in immunogenic cell death. EMBO J.
28:578–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krysko DV, Garg AD, Kaczmarek A, Krysko O,
Agostinis P and Vandenabeele P: Immunogenic cell death and DAMPs in
cancer therapy. Nat Rev Cancer. 12:860–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aymeric L, Apetoh L, Ghiringhelli F,
Tesniere A, Martins I, Kroemer G, Smyth MJ and Zitvogel L: Tumor
Cell Death and ATP release prime dendritic cells and efficient
anticancer immunity. Cancer Res. 70:855–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghiringhelli F, Apetoh L, Tesniere A,
Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G,
Ullrich E, et al: Activation of the NLRP3 inflammasome in dendritic
cells induces IL-1beta-dependent adaptive immunity against tumors.
Nat Med. 15:1170–1178. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garg AD, Krysko DV, Verfaillie T,
Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu
C, Roebroek AJ, et al: A novel pathway combining calreticulin
exposure and ATP secretion in immunogenic cancer cell death. EMBO
J. 31:1062–1079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martins I, Tesniere A, Kepp O, Michaud M,
Schlemmer F, Senovilla L, Séror C, Métivier D, Perfettini JL,
Zitvogel L and Kroemer G: Chemotherapy induces ATP release from
tumor cells. Cell Cycle. 8:3723–3728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martins I, Wang Y, Michaud M, Ma Y,
Sukkurwala AQ, Shen S, Kepp O, Métivier D, Galluzzi L, Perfettini
JL, et al: Molecular mechanisms of ATP secretion during immunogenic
cell death. Cell Death Differ. 21:79–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srivastava P: Interaction of heat shock
proteins with peptides and antigen presenting cells: Chaperoning of
the innate and adaptive immune responses. Annu Rev Immunol.
20:395–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Srivastava P: Roles of heat-shock proteins
in innate and adaptive immunity. Nat Rev Immunol. 2:185–194. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schmitt E, Gehrmann M, Brunet M, Multhoff
G and Garrido C: Intracellular and extracellular functions of heat
shock proteins: Repercussions in cancer therapy. J Leukoc Biol.
81:15–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kepp O, Tesniere A, Schlemmer F, Michaud
M, Senovilla L, Zitvogel L and Kroemer G: Immunogenic cell death
modalities and their impact on cancer treatment. Apoptosis.
14:364–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bianchi ME and Manfredi AA: High-mobility
group box 1 (HMGB1) protein at the crossroads between innate and
adaptive immunity. Immunol Rev. 220:35–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamazaki T, Hannani D, Poirier-Colame V,
Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP,
Freudenberg M, et al: Defective immunogenic cell death of
HMGB1-deficient tumors: Compensatory therapy with TLR4 agonists.
Cell Death Differ. 21:69–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fredly H, Ersvær E, Gjertsen BT and
Bruserud O: Immunogenic apoptosis in human acute myeloid leukemia
(AML): Primary human AML cells expose calreticulin and release heat
shock protein (HSP) 70 and HSP90 during apoptosis. Oncol Rep.
25:1549–1556. 2011.PubMed/NCBI
|
|
21
|
D'Eliseo D, Manzi L and Velotti F:
Capsaicin as an inducer of damage-associated molecular patterns
(DAMPs) of immunogenic cell death (ICD) in human bladder cancer
cells. Cell Stress Chaperones. 18:801–808. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garg AD, Krysko DV, Vandenabeele P and
Agostinis P: Hypericin-based photodynamic therapy induces surface
exposure of damage-associated molecular patterns like HSP70 and
calreticulin. Cancer Immunol Immunother. 61:215–221. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fucikova J, Kralikova P, Fialova A,
Brtnicky T, Rob L, Bartunkova J and Spísek R: Human tumor cells
killed by anthracyclines induce a tumor-specific immune response.
Cancer Res. 71:4821–4833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen HM, Wang PH, Chen SS, Wen CC, Chen
YH, Yang WC and Yang NS: Shikonin induces immunogenic cell death in
tumor cells and enhances dendritic cell-based cancer vaccine.
Cancer Immunol Immunother. 61:1989–2002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao T, Ren H, Jia L, Chen J, Xin W, Yan
F, Li J, Wang X, Gao S, Qian D, et al: Inhibition of HIF-1α by
PX-478 enhances the anti-tumor effect of gemcitabine by inducing
immunogenic cell death in pancreatic ductal adenocarcinoma.
Oncotarget. 6:2250–2262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Menger L, Vacchelli E, Adjemian S, Martins
I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, et
al: Cardiac glycosides exert anticancer effects by inducing
immunogenic cell death. Sci Transl Med. 4:143ra992012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tesniere A, Schlemmer F, Boige V, Kepp O,
Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault
L, et al: Immunogenic death of colon cancer cells treated with
oxaliplatin. Oncogene. 29:482–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calvet CY, Famin D, André FM and Mir LM:
Electro-chemotherapy with bleomycin induces hallmarks of
immunogenic cell death in murine colon cancer cells.
Oncoimmunology. 3:e281312014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Obeid M, Tesniere A, Panaretakis T, Tufi
R, Joza N, van Endert P, Ghiringhelli F, Apetoh L, Chaput N,
Flament C, et al: Ecto-calreticulin in immunogenic chemotherapy.
Immunol Rev. 220:22–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dudek AM, Garg AD, Krysko DV, De Ruysscher
D and Agostinis P: Inducers of immunogenic cancer cell death.
Cytokine Growth Factor Rev. 24:319–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bezu L, Gomes-de-Silva LC, Dewitte H,
Breckpot K, Fucikova J, Spisek R, Galluzzi L, Kepp O and Kroemer G:
Combinatorial strategies for the induction of immunogenic cell
death. Front Immunol. 6:1872015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garg AD, Galluzzi L, Apetoh L, Baert T,
Birge RB, Bravo-San Pedro JM, Breckpot K, Brough D, Chaurio R,
Cirone M, et al: Molecular and translational classifications of
DAMPS in immunogenic cell death. Front Immunol. 6:5882015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pol J, Vacchelli E, Aranda F, Castoldi F,
Eggermont A, Cremer I, Sautès-Fridman C, Fucikova J, Galon J,
Spisek R, et al: Trial Watch: Immunogenic cell death inducers for
anticancer chemotherapy. Onco Immunology. 4:e10088662015.
|
|
34
|
Inoue H and Tani K: Multimodal immunogenic
cancer cell death as a consequence of anticancer cytotoxic
treatments. Cell Death Differ. 21:39–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Franco-Molina MA, Mendoza-Gamboa E,
Zapata-Benavides P, Vera-García ME, Castillo-Tello P, García de la
Fuente A, Mendoza RD, Garza RG, Támez-Guerra RS and
Rodríguez-Padilla C: IMMUNEPOTENT CRP (bovine dialyzable leukocyte
extract) adjuvant immunotherapy: A phase I study in non-small cell
lung cancer patients. Cytotherapy. 10:490–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Franco-Molina MA, Mendoza-Gamboa E,
Miranda-Hernández D, Zapata-Benavides P, Castillo-León L,
Isaza-Brando C, Tamez-Guerra RS and Rodríguez-Padilla C: In vitro
effects of bovine dialyzable leukocyte extract (bDLE) in cancer
cells. Cytotherapy. 8:408–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Franco-Molina MA, Mendoza-Gamboa E,
Zapata-Benavides P, Castillo-Tello P, Isaza-Brando CE, Zamora-Avila
D, Rivera-Morales LG, Miranda-Hernández DF, Sierra-Rivera CA,
Vera-García ME, et al: Antiangiogenic and antitumor effects of
IMMUNEPOTENT CRP in murine melanoma. Immunopharmacol Immunotoxicol.
32:637–646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Franco-Molina MA, Mendoza-Gamboa E,
Castillo-León L, Tamez-Guerra RS and Rodríguez-Padilla C: Bovine
dialyzable leukocyte extract modulates the nitric oxide and
pro-inflammatory cytokine production in
lipopolysaccharide-stimulated murine peritoneal macrophages in
vitro. J Med Food. 8:20–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mendoza-Gamboa E, Franco-Molina MA,
Zapata-Benavides P, Castillo-Tello P, Vera-García ME, Tamez-Guerra
RS and Rodríguez-Padilla C: Bovine dialyzable leukocyte extract
modulates AP-1 DNA-binding activity and nuclear transcription
factor expression in MCF-7 breast cancer cells. Cytotherapy.
10:212–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Franco-Molina MA, Mendoza-Gamboa E,
Castillo-León L, Tamez-Guerra RS and Rodríguez-Padilla C: Bovine
dialyzable leukocyte extract protects against LPS-induced, murine
endotoxic shock. Int Immunopharmacol. 4:1577–1586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zitvogel L, Kepp O, Senovilla L, Menger L,
Chaput N and Kroemer G: Immunogenic tumor cell death for optimal
anticancer therapy: The calreticulin exposure pathway. Clin Cancer
Res. 16:3100–3104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao C, Han Y, Ren Y and Wang Y:
Mitoxantrone-mediated apoptotic B16-F1 cells induce specific
anti-tumor immune response. Cell Mol Immunol. 6:469–475. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tongu M, Harashima N, Yamada T, Harada T
and Harada M: Immunogenic chemotherapy with cyclophosphamide and
doxorubicin against established murine carcinoma. Cancer Immunol
Immunother. 59:769–777. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Perez CA, Fu A, Onishko H, Hallahan DE and
Geng L: Radiation induces an antitumor immune response to mouse
melanoma. Int J Radiat Biol. 85:1126–1136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nowak P, Abdurahman S, Lindkvist A,
Troseid M and Sönnerborg A: Impact of HMGB1/TLR ligand complexes on
HIV-1 replication: Possible role for flagellin during HIV-1
infection. Int J Microbiol. 2012:2638362012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Skitzki JJ, Repasky EA and Evans SS:
Hyperthermia as an immunotherapy strategy for cancer. Curr Opin
Investig Drugs. 10:550–558. 2009.PubMed/NCBI
|
|
47
|
Shi H, Cao T, Connolly JE, Monnet L,
Bennett L, Chapel S, Bagnis C, Mannoni P, Davoust J, Palucka AK and
Banchereau J: Hyperthermia enhances CTL cross-priming. J Immunol.
176:2134–2141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tamura Y, Peng P, Liu K, Daou M and
Srivastava PK: Immunotherapy of tumors with autologous
tumor-derived heat shock protein preparations. Science.
278:117–120. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vanaja DK, Grossmann ME, Celis E and Young
CY: Tumor prevention and antitumor immunity with heat shock protein
70 induced by 15-deoxy-delta12,14-prostaglandin J2 in transgenic
adenocarcinoma of mouse prostate cells. Cancer Res. 60:4714–4718.
2000.PubMed/NCBI
|
|
50
|
Chiang CL, Ledermann JA, Rad AN, Katz DR
and Chain BM: Hypochlorous acid enhances immunogenicity and uptake
of allogeneic ovarian tumor cells by dendritic cells to cross-prime
tumor-specific T cells. Cancer Immunol Immunother. 55:1384–1395.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chiang CL, Ledermann JA, Aitkens E,
Benjamin E, Katz DR and Chain BM: Oxidation of ovarian epithelial
cancer cells by hypochlorous acid enhances immunogenicity and
stimulates t cells that recognize autologous primary tumor. Clin
Cancer Res. 14:4898–4907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chiang CL, Coukos G and Kandalaft LE:
Whole tumor antigen vaccines: where are we? Vaccines (Basel).
3:344–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zitvogel L, Apetoch L, Ghiringhelli F and
Kroemer G: Immunological aspects of cancer chemotherapy. Nat Rev
Immunol. 8:59–73. 2008. View Article : Google Scholar : PubMed/NCBI
|