Introduction
Bladder cancer is one of the major causes of
cancer-associated mortality, with a high incidence (1). The majority of patients with bladder
cancer initially present with non-muscle-invasive disease; however,
disease recurrence is observed in between 30 and 56% of patients
undergoing surgery (2). Radical
cystectomy with urinary diversion is currently the standard
treatment for those patients with refractory non-muscle-invasive
and muscle-invasive bladder cancer (3). Although there has been recent progress
in the development of treatments, the prognosis for patients with
advanced bladder cancer following cystectomy is poor (4,5).
Therefore, identification of effective molecular therapeutic
targets to treat advanced bladder cancer is urgently required.
Curcumin is a dietary antioxidant derived from
turmeric, which may possess anti-inflammatory, anti-proliferative
and pro-apoptotic properties (6,7). Curcumin
induces the activation of pro-apoptotic and anti-tumorigenic
signaling pathways (8). However, the
therapeutic potential of curcumin against various cancer cells, its
effects, and the molecular pathways involved in suppression of
bladder cancer growth and metastasis remain unclear. In the present
study, the anticancer effects of curcumin on bladder cancer in
vitro were examined. It was investigated whether curcumin
induces bladder cancer cell apoptosis and inhibits bladder cancer
cell survival and invasion.
Materials and methods
Reagents and cell culture
Curcumin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was prepared by dissolving it in dimethylsulfoxide (DMSO)
at a stock concentration of 5,000 mM and stored at −20°C. Serial
dilutions were prepared in culture medium. Human urinary bladder
transitional cell carcinoma (T24 and 5637) cells were obtained from
the Chinese Academy of Science (Shanghai, China). T24 and 5637 cell
lines were maintained in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine
serum (FBS; Biological Industries, Cromwell, CT, USA). All media
contained 100 units/ml penicillin and 100 µg/ml streptomycin. All
cell lines were maintained at 37°C in a humidified incubator
containing 5% CO2. The cells that entered the exponential growth
period were selected for experiments.
Proliferation assay (MTT assay)
T24 and 5637 cells in the exponential growth phase
were inoculated on 96-well plates at a density of
4×103/well, and sterile PBS was added to the edge well
as a control. After 24 h at 37°C, curcumin was added at the
concentrations of 5, 10, 20, 30 and 40 µmol/l. The experimental
control group (0.1% DMSO) and zero group were set up, and each
group contained 3 wells. Following treatment, 10 µl MTT solution
was added to each well for 2 h at 37°C, prior to dissolving the
formazan product using DMSO, and cell viability was determined by
measuring the absorbance at 540 nm using a microplate
spectrophotometer (Molecular Devices, LCC, Sunnyvale, CA, USA). The
mean percentage of viable cells ± standard deviation generated from
three independent experiments are reported.
Determination of cell apoptosis by
flow cytometry
Following treatment, apoptosis was detected using
the Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection
kit (BD Biosciences, Franklin Lakes, NJ, USA). T24 and 5637 cells
were detached by trypsinization and washed three times in PBS,
centrifuged at 1,000 × g for 5 min at 4°C and resuspended in 195 µl
Annexin V-FITC binding buffer. A 5 µl volume of Annexin V-FITC was
added and mixed. The cells were then stained with Annexin V-FITC
binding buffer in the dark for 10 min at room temperature.
Subsequently, cells were centrifuged at 1,000 × g for 5 min at 4°C
and resuspended in 190 µl Annexin V-FITC binding buffer. Finally,
10 µl propidium iodide (PI) staining solution was added and mixed.
The cells were kept on ice for 30 min in the dark and immediately
subjected to flow cytometry analysis. The data were analyzed using
Cell Quest BD FACStation™ v6.0x (BD Biosciences,
Franklin Lakes, NJ, USA). The experiment was repeated three
times.
Caspase 3/7 enzyme activity assay
Caspase 3/7 enzyme activity was determined using the
Caspase-Glo 3/7 assay (Promega Corporation, Madison, WI, USA),
according to the manufacturer's protocol. Cells (104
cells/well) were plated in a 96-well plate in 100 µl culture medium
in the absence or presence of 5, 10 and 20 µmol/l curcumin.
Caspase-Glo 3/7 reagent (100 µl) was added to each well and the
plates were incubated at room temperature for an additional 1 h.
Finally, the luminescence of each sample was measured by a
luminometer (Beckman Coulter, Inc., Brea, CA, USA; DTX 880
Multimode Reader).
Cell migration assay
Transwell inserts with a pore size of 8 mm from
Corning Incorporated (Corning, NY, USA) were used to determine
tumor cell migration capacity. Following culture for 24 h at 37°C,
T24 and 5637 cells were starved in medium without FBS for 24 h, and
5×104 cells were then resuspended in the FBS-free medium
and placed in the upper chambers in triplicate. The cells remaining
on the upper membrane were removed with cotton wool, whereas the
cells that had migrated to the bottom of the membrane were fixed
with 95% ethanol and stained with 0.1% crystal violet. Five visual
fields of each insert were randomly selected and images were
captured under a light microscope at ×200 magnification. All
experiments were performed in triplicate.
Western blot analysis
Cells were inoculated into the culture flask at
1×104 cells/cm2. Following adherence, cells
were treated with 10 µmol/l curcumin or 0.1% DMSO (negative control
group). Following incubation for 24 h at 37°C, cells were lysed
using the mammalian protein extraction reagent,
radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology, Haimen, China), supplemented with a protease
inhibitor cocktail (Roche Diagnostics, Basel, Switzerland) and PMSF
(Roche Diagnostics). Protein (30 µg) were separated by SDS-PAGE
(10% gels), transferred to a polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). and blocked with 5% skimmed
milk at room temperature for 1 h. Cells were washed with
TBS-Tween-20 (TBST) three times for 5 min. The primary antibodies
against matrix metalloproteinase (MMP)-2 (rabbit mAb; cat no.,
87809; dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA), MMP-9 (rabbit mAb; cat. no., 13667; dilution, 1:1,000;
Cell Signaling Technology, Inc.) and tissue inhibitor of
metalloproteinase-2 (TIMP-2; rabbit mAb; cat. no., 5738; dilution,
1:1,000; Cell Signaling Technology, Inc.) were added to the
membrane prior to incubation at 4°C overnight. The membrane was
washed with TBST three times for 5 min. The secondary antibody
(1:10,000; anti-rabbit IgG, horseradish peroxidase-linked antibody;
cat. no., 7074; Cell Signaling Technology, Inc.) was added to the
membrane and agitated for 1 h at room temperature prior to washing
three times with TBST for 5 min. The blot was imaged using an
enhanced chemiluminescent imaging system (Pierce; Thermo Fisher
Scientific, Inc.).
Statistical analysis
All values are presented as the mean + standard
error of the mean. For the MTT assay, the results are presented as
the mean ± standard deviation. Statistical significance was
determined using Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Curcumin inhibits the proliferation of
bladder cancer cells
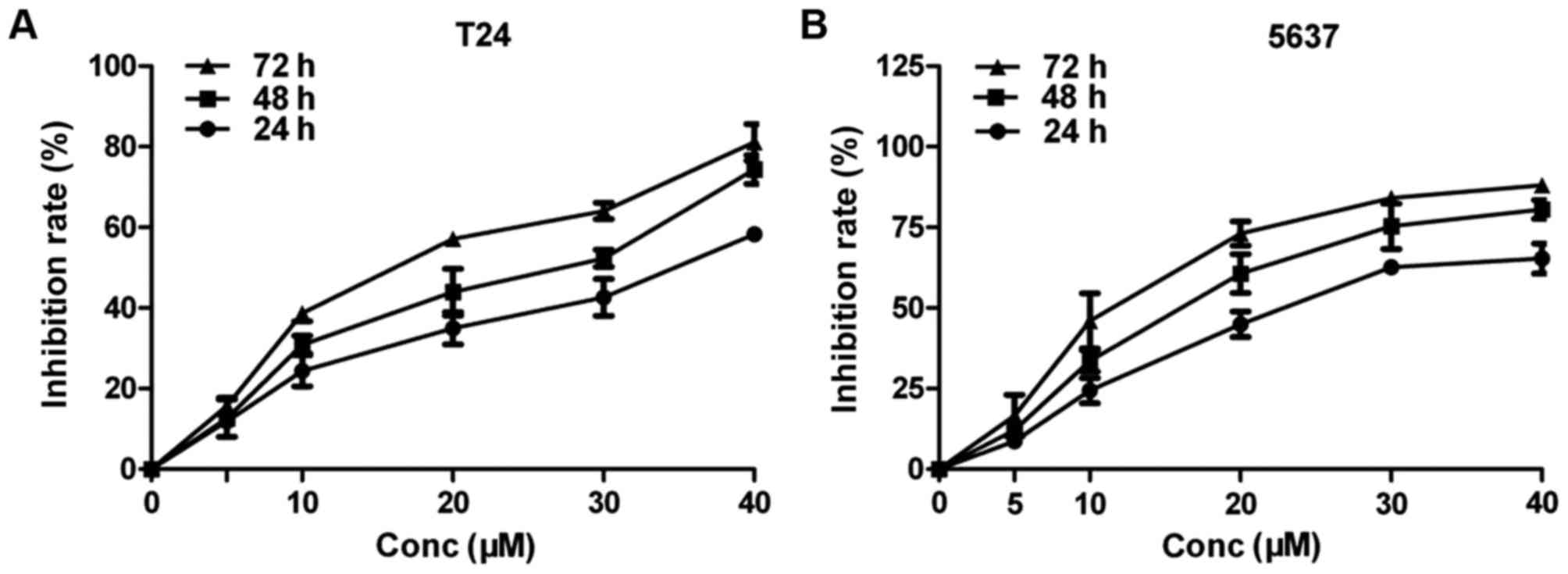
To determine the inhibitory effect of curcumin on
bladder cancer cells, an MTT assay was performed. The concentration
of curcumin used was 5, 10, 20, 30 and 40 µmol/l. When the
concentration of curcumin was >10 µmol/l, the action time was
>24 h, the growth of cells was inhibited in a time- and
dose-dependent manner and the difference was statistically
significant (P<0.001; Fig. 1A and
B). These results indicated that curcumin exhibited a strong
inhibitory effect on the survival rates of T24 and 5637 cells. In
the subsequent tests, 5, 10 and 20 µmol/l curcumin were selected to
investigate the effects of curcumin on bladder cancer cells and
explore the underlying molecular mechanisms.
Effect of curcumin on apoptosis of
bladder cancer cells
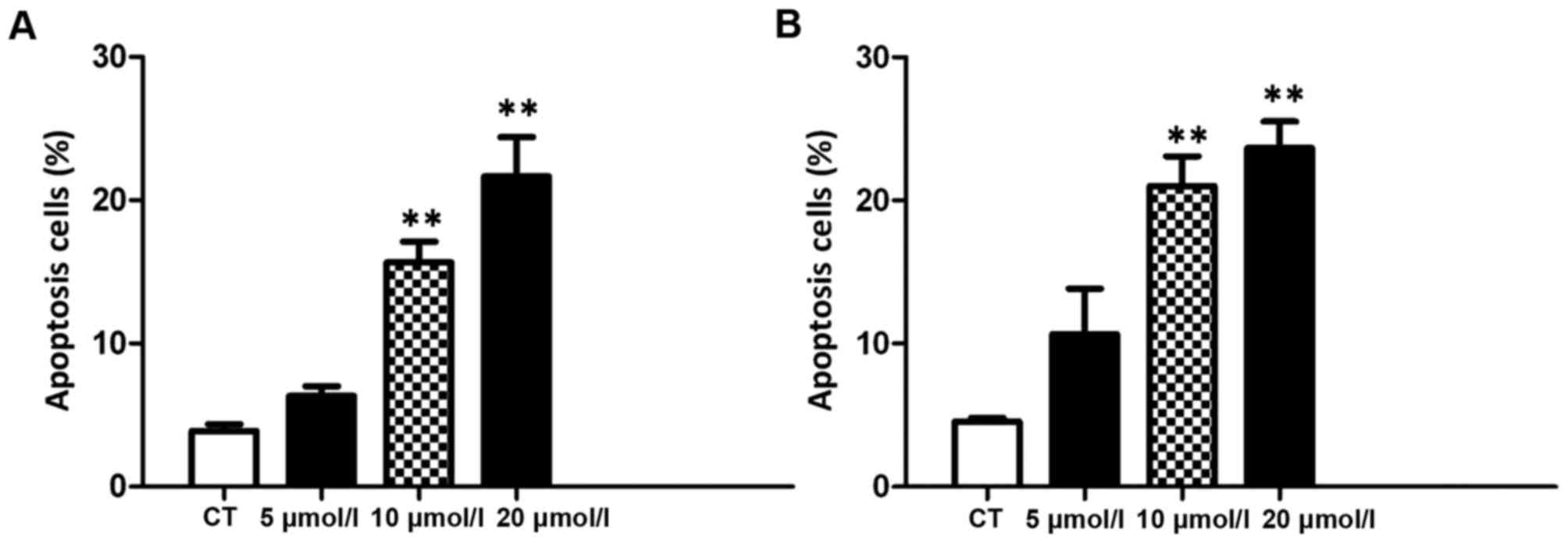
To determine whether curcumin induces apoptosis of
T24 and 5637 cells, Annexin V FITC/PI staining was performed.
Annexin V and PI staining results indicated that, with an increase
in curcumin concentration after 24 h, the number of apoptotic cells
was also markedly increased (P<0.01; Fig. 2A and B). These results indicated that
curcumin induces increased apoptosis in T24 and 5637 cells.
Caspase 3/7 enzyme activity in
response to curcumin in bladder cancer cells
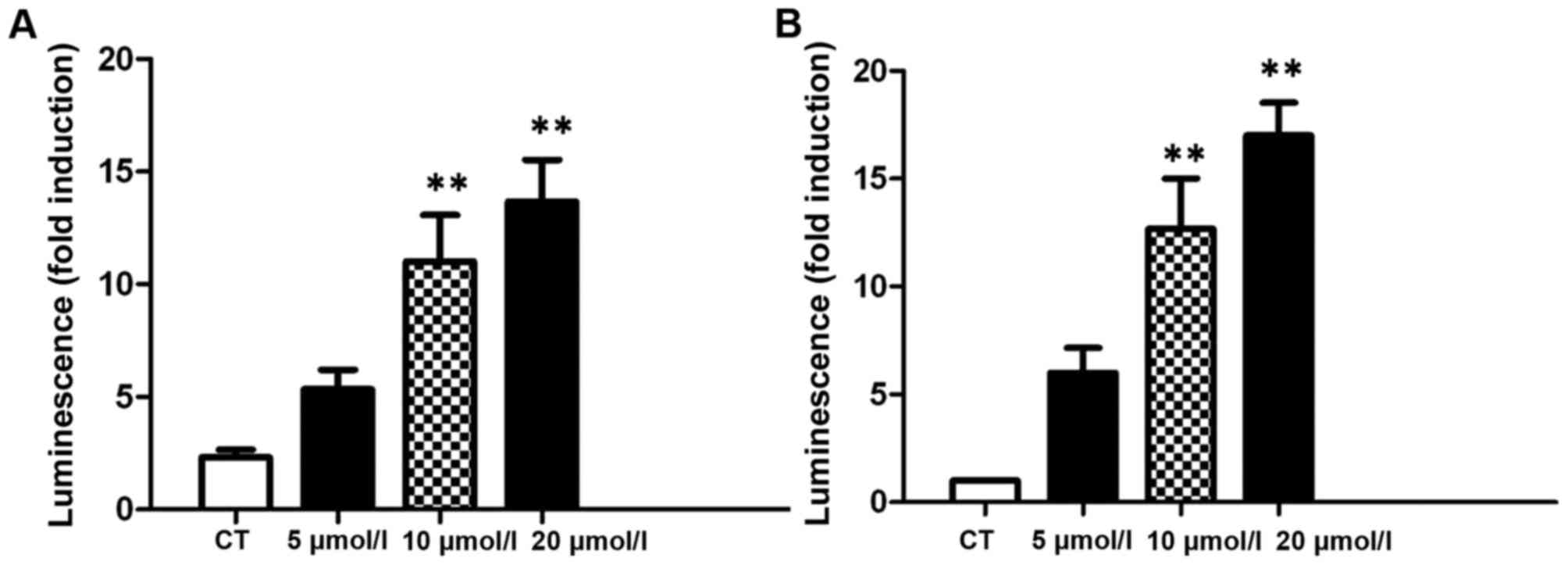
In order to evaluate whether caspases serve roles in
curcumin-induced apoptosis of bladder cancer cells, the levels of
caspase 3/7 were measured following treatment with various
concentrations of curcumin for 24 h. The results revealed that
there was a dose-dependent increase in caspase 3/7 enzyme activity
in curcumin in T24 and 5637 cells (Fig.
3A and B). These results demonstrated that there was a
dose-dependent increase in caspase 3/7 enzyme activation in
curcumin-treated T24 and 5637 cells which induced significantly
increased apoptosis compared with the control.
Effect of curcumin on the migratory
ability of bladder cancer cells
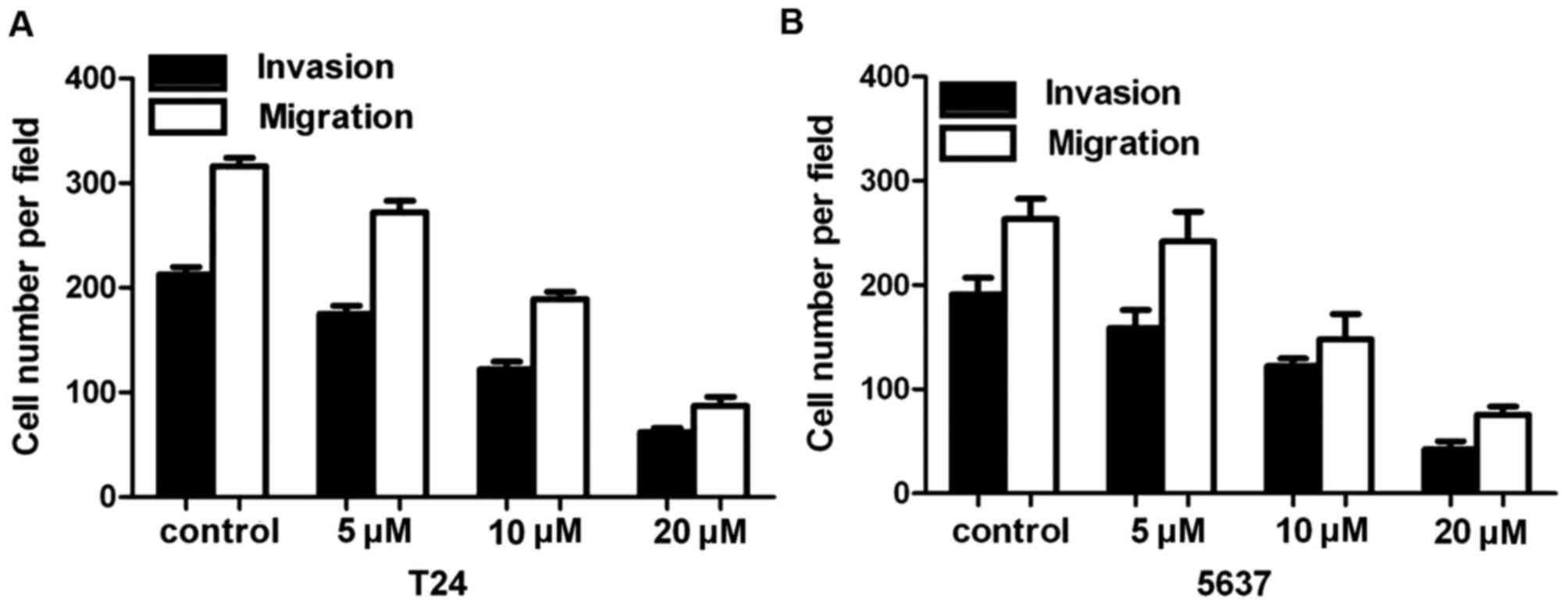
The Transwell invasion chamber experiments
demonstrated that the number of T24 and 5637 cells passing through
the polycarbonate membrane in the high concentration group was
significantly less compared with that in the control group and the
low concentration group (P<0.01; Fig.
4A and B). Compared with the negative control group, the number
of transmembrane cells gradually decreased as the drug
concentration of curcumin increased, and the differences between
each group were statistically significant (P<0.01).
Effect of curcumin on expression of
MMP-2, MMP-9 and TIMP-2 in T24 cells
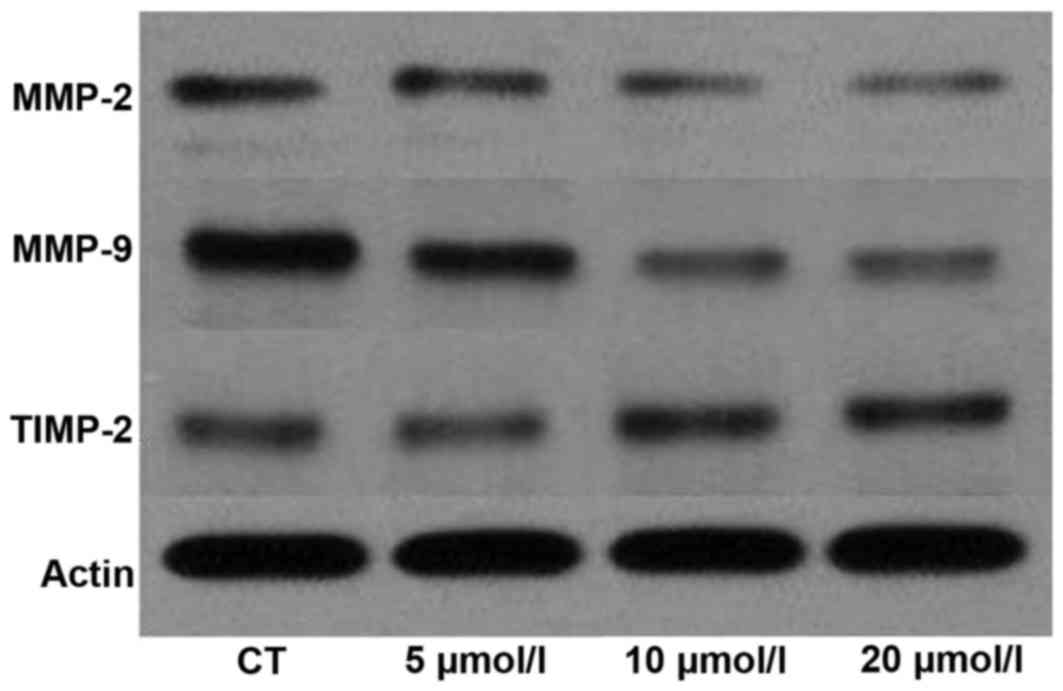
The expression of MMP-2, MMP-9 and TIMP-2 was
examined using western blotting in T24 cells (Fig. 5). The results indicated that the
expression of MMP-2 and MMP-9 proteins was significantly decreased
(P<0.01), and that of the TIMP-2 protein was significantly
increased, in the 10 µM curcumin group compared with the control
group. These results confirmed that curcumin inhibits cell
metastatic potential via MMP pathways.
Discussion
Bladder cancer is one of the most frequent
urological malignancies, which is characterized by increasing
incidence and mortality (9). It has
been proposed that chemotherapy represents important modality for
patients with bladder cancer (10).
However, the prognosis of bladder cancer remains poor (11). Early diagnosis and effective treatment
are essential in order to increase the life expectancy of patients
with bladder cancer.
Curcumin is a polyphenol compound extracted from
turmeric that has been identified to regulate tumor progression
(12,13). Previous studies have focused on
curcumin due to its anticancer properties (14,15).
However, there have been few studies on the effect of curcumin on
the survival and invasion of bladder cancer cells. In the present
study, the underlying molecular mechanism of curcumin in bladder
cancer was investigated by studying the effect of curcumin on
proliferation and apoptosis of the human bladder cancer cell lines
T24 and 5637.
Several effects of curcumin have been identified.
One of them is the effect of curcumin on coronary heart disease
(15,16). In addition, curcumin has been safely
demonstrated to function as an antitumor drug (17,18). The
efficacy of curcumin on the proliferation of bladder cancer in
vitro was examined, and the results demonstrated that curcumin
inhibits cell proliferation in a time- and dose-dependent manner.
The results indicated that curcumin is a potential anticancer drug
for the treatment of bladder cancer. The results of the present
study identified that curcumin may cause significant growth
inhibition in bladder cancer cell lines, which was in accordance
with the aforementioned previous studies (19,20).
Curcumin exhibited the ability to effectively
modulate the apoptotic effect of cancer cells (20,21). The
results of the present study identified that the number of
apoptotic cells increased markedly with an increase in curcumin
concentration, indicating that curcumin induces apoptosis in two
human urinary bladder transitional cell carcinoma cells. In
addition, caspase 3/7 activity was examined, and the results
indicated that curcumin stimulated a marked increase in caspase
activity beginning at concentrations of 10 and 20 µmol/l.
A previous study has demonstrated that curcumin may
inhibit the metastasis of melanoma cells in vitro (22). The results of the present study are in
agreement with previous studies (23,24);
curcumin inhibited the migration of T24 and 5637 cells. To date,
few studies have investigated the molecular mechanisms through
which curcumin inhibits metastasis (25). Curcumin may impede cell metastasis via
downregulation of Src and focal adhesion kinase activity, two
important factors in integrin signal transduction (26). In the present study, curcumin
inhibited the migration of bladder cancer cells by inhibiting MMP
signaling pathways. MMPs belong to a family of
Zn2+-dependent endogenous proteolytic enzymes. MMPs
degrade almost all components of the extracellular matrix, with the
exception of polysaccharides, and are involved in invasion and
metastasis of tumors. The results of western blot analysis
indicated that the expression of MMP-2 and MMP-9 proteins was
significantly decreased in the 10 µM curcumin group compared with
the control group, which confirmed that curcumin inhibits cell
metastatic potential via MMP signaling pathways.
In conclusion, the results of the present study
identified that the anticancer effects of curcumin inhibited cell
proliferation and migration, and promoted apoptosis of bladder
cancer cell through the suppression of MMP signaling pathways.
Additional studies are required to determine the in vivo
anticancer effects of curcumin in animal models, in order to
evaluate the therapeutic potential of curcumin on bladder cancer
cells and the potential benefits of curcumin for clinical practice
in the future.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin 2014. 64:9–29. 2014. View Article : Google Scholar
|
|
2
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shirodkar SP and Lokeshwar VB: Potential
new urinary markers in the early detection of bladder cancer. Curr
Opin Urol. 19:488–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim WJ and Bae SC: Molecular biomarkers in
urothelial bladder cancer. Cancer Sci. 99:646–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A;
European Association of Urology, : EAU guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2013
guidelines. Eur Urol. 65:778–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okudan N, Belviranlı M, Gökbel H, Oz M and
Kumak A: Protective effects of curcumin supplementation on
intestinal ischemia reperfusion injury. Phytomedicine. 20:844–848.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fan Z, Jing H, Yao J, Li Y, Hu X, Shao H,
Shen G, Pan J, Luo F and Tian X: The protective effects of curcumin
on experimental acute liver lesion induced by intestinal
ischemia-reperfusion through inhibiting the pathway of NF-κB in a
rat model. Oxid Med Cell Longev. 2014:1916242014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: An ‘old-age’
disease with an ‘age-old’ solution. Cancer Lett. 267:133–164. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kirkali Z, Chan T, Manoharan M, Algaba F,
Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM,
et al: Bladder cancer: Epidemiology, staging and grading, and
diagnosis. Urology. 66 6 Suppl 1:S4–S34. 2005. View Article : Google Scholar
|
|
10
|
Nargund VH, Tanabalan CK and Kabir MN:
Management of non-muscle-invasive (superficial) bladder cancer.
Semin Oncol. 39:559–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chaffer CL, Brennan JP, Slavin JL, Blick
T, Thompson EW and Williams ED: Mesenchymal-to-epithelial
transition facilitates bladder cancer metastasis: Role of
fibroblast growth factor receptor-2. Cancer Res. 66:11271–11278.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jain SK, Gill MS, Pawar HS and Suresh S:
Novel curcumin diclofenac conjugate enhanced curcumin
bioavailability and efficacy in streptococcal cell wall-induced
arthritis. Indian J Pharm Sci. 76:415–22. 2014.PubMed/NCBI
|
|
13
|
Shetty D, Kim YJ, Shim H and Snyder JP:
Eliminating the heart from the curcumin molecule: Monocarbonyl
curcumin mimics (MACs). Molecules. 20:249–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manju V and Nalini N: Chemopreventive
efficacy of ginger, a naturally occurring anticarcinogen during the
initiation, post-initiation stages of 1, 2
dimethylhydrazine-induced colon cancer. Clin Chim Acta. 358:60–67.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang CL, Liu YY, Ma YG, Xue YX, Liu DG,
Ren Y, Liu XB, Li Y and Li Z: Curcumin blocks small cell lung
cancer cells migration, invasion, angiogenesis, cell cycle and
neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One.
7:e379602012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
García-Niño WR, Tapia E, Zazueta C,
Zatarain-Barrón ZL, Hernández-Pando R, Vega-García CC and
Pedraza-Chaverrí J: Curcumin pretreatment prevents potassium
dichromate-induced hepatotoxicity, oxidative stress, decreased
respiratory complex I activity, and membrane permeability
transition pore opening. Evid Based Complement Alternat Med.
2013:4246922013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jana S, Rudra DS, Paul S and Snehasikta S:
Curcumin delays endometriosis development by inhibiting MMP-2
activity. Indian J Biochem Biophys. 49:342–348. 2012.PubMed/NCBI
|
|
18
|
Jana S, Paul S and Warnakar SS: Curcumin
as anti-endometriotic agent: Implication of MMP-3 and intrinsic
apoptotic pathway. Biochem Pharmacol. 83:797–804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang Y, Hu W, Bai E, Zheng H, Liu Z, Wu J,
Jin R, Zhao C and Liang G: Curcumin sensitizes human gastric cancer
cells to 5-fluorouracil through inhibition of the NFκB
survival-signaling pathway. Onco Targets Ther. 9:7373–7384. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tong W, Wang Q, Sun D and Suo J: Curcumin
suppresses colon cancer cell invasion via AMPK-induced inhibition
of NF-κB, uPA activator and MMP9. Oncol Lett. 12:4139–4146.
2016.PubMed/NCBI
|
|
21
|
Gong G, Pan Q, Wang K, Wu R, Sun Y and Lu
Y: Curcumin-incorporated albumin nanoparticles and its tumor image.
Nanotechnology. 26:0456032015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang GM, Xie WY, Wang HS, Du J, Wu BP, Xu
W, Liu HF, Xiao P, Liu ZG, Li HY, et al: Curcumin combined with
FAPαc vaccine elicits effective antitumor response by targeting
indolamine-2,3-dioxygenase and inhibiting EMT induced by TNF-α in
melanoma. Oncotarget. 6:25932–25942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao F, Liu T, Xu Y, Xu D and Feng S:
Curcumin inhibits cell proliferation and promotes apoptosis in
human osteoclastoma cell through MMP-9, NF-κB and JNK signaling
pathways. Int J Clin Exp Pathol. 8:6037–6045. 2015.PubMed/NCBI
|
|
24
|
Li ZC, Zhang LM, Wang HB, Ma JX and Sun
JZ: Curcumin inhibits lung cancer progression and metastasis
through induction of FOXO1. Tumor Biol. 35:111–116. 2014.
View Article : Google Scholar
|
|
25
|
Wang Q, Qu C, Xie F, Chen L, Liu L, Liang
X, Wu X, Wang P and Meng Z: Curcumin suppresses
epithelial-to-mesenchymal transition and metastasis of pancreatic
cancer cells by inhibiting cancer-associated fibroblasts. Am J
Cancer Res. 7:125–133. 2017.PubMed/NCBI
|
|
26
|
Leu TH, Su SL, Chuang YC and Maa MC:
Direct inhibitory effect of curcumin on Src and focal adhesion
kinase activity. Biochem Pharmacol. 66:2323–2331. 2003. View Article : Google Scholar : PubMed/NCBI
|