Introduction
Gastric cancer is one of the most common
gastrointestinal cancers worldwide, ranking second in terms of
cancer-associated mortality rates (1,2), and
causing marked morbidity and mortality in China (3). Early detection is important in the
treatment of this type of cancer, as advanced-stage diagnosis
results in a poor prognosis (4,5). Western
medicine prioritizes surgical removal and other conventional
treatment methods, which directly kill tumor cells or inhibit their
proliferative and metastatic capabilities. However, these
treatments cannot completely eliminate the tumor cells, and cause
serious sideeffects in normal cells (5).
The treatment of tumors using traditional Chinese
medicine has unique advantages (6,7). The use
of traditional Chinese medicine in the treatment of cancer is able
to significantly improve the quality of life and survival rate of
patients (8). Omphalia
lapidescens Schroet is widely used as a traditional
anthelmintic in China. Previous studies have demonstrated that
O. lapidescens Schroet effectively induces tumor necrosis;
consequently, the China Food and Drug Administration has approved
Lei Wan Pian and Lei Wan Jiao Nang as antitumor auxiliary drugs
(9,10). Research has revealed that the
antitumor effects of the active ingredients from O.
lapidescens Schroet are associated with certain polysaccharides
and proteins (11–14), with mechanisms that include the direct
killing of tumor cells through induction of apoptosis, and the
enhancement of immune and anti-inflammatory responses. In a
previous study (14), purified
Omphalia lapidescens protein (pPeOp) was extracted
from dried sclerotoids with polyvinylpyrrolidone (PVP) extraction
buffer, and it was verified that a single protein band was retained
following isolation of the major fraction using molecular-sieve
chromatography. The major constituent, pPeOp, was identified
with relatively high chromatographic purity. pPeOp increased
apoptosis in human gastric tumor cells compared with conventional
Western treatments, which induce tumor cell (MC-4 and SGC-7901)
death and apoptosis significantly, but also trigger minor apoptosis
of normal gastric cells (MC-1) (14).
To further understand the underlying molecular mechanisms of the
antitumor activities of pPeOp, its effects on tumor cell
migration and cell cycle progression were investigated.
Materials and methods
Drugs and reagents
O. lapidescens Schroet powder was purchased
from Fang Hui Chun Tang (Hangzhou, Zhejiang, China); the protein
pPeOp was extracted from dried sclerotoids of O.
lapidescens Schroet using PVP extraction buffer [15% 1.0 M
Tris-HCl (pH 8.0), 2% PVP and 25% glycerol], with 100 µg/ml
5-fluorouracil (5-FU) (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) as a positive control. Antibodies against cyclin-dependent
kinase (CDK) 2 (cat. no. 2546T), cyclin B (cat. no. 4138T), CDK4
(cat. no. 12790T) and cyclin D1 (cat. no. 2922S) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies
against CDK1 (cat. no. ab131450), cyclin A (cat. no. ab181591), and
MMP-2 (cat. no. ab37150) and −9 (cat. no. ab73734) were purchased
from Abcam (Cambridge, UK). Anti-β-actin was used as a control and
horseradish peroxidase (HRP)-conjugated goat anti-rabbit
immunoglobulin G (IgG) was used as a secondary antibody. Both
antibodies were purchased from Beyotime Institute of Biotechnology
(Shanghai, China). Antibodies against CDK2, cyclin B, CDK4, cyclin
D1, CDK1, MMP-2, MMP-9 and β-actin were diluted 1:1,000 in TBST
containing 3% BSA, and antibody against cyclin A was diluted
1:2,000 for use.
Cell lines and cell culture
The human gastric cancer cell line MC-4 was obtained
from the Zhejiang Provincial Center for Disease Control and
Prevention (Hangzhou, China). MC-4 cells were cultured in RPMI-1640
medium (Genome Biotechnology, Hangzhou, China) supplemented with
10% (v/v) fetal bovine serum (Zhejiang Tianhang Biotechnology Co.
Ltd., Hangzhou, China), 100 units/ml penicillin and 100 units/ml
streptomycin (Genome Biotechnology) at 37°C in a humidified
atmosphere containing 5% CO2. Every 1–2 days, cells were used when
>80% cells were in the exponential growth phase. The control
group was untreated MC-4 cells, MC-4 cells treated with 90 µg/ml
PVP were used as a negative control and MC-4 cells treated with 100
µg/ml 5-FU were used as a positive control.
Cell counting kit (CCK)-8 assay
Exponential growth phase MC-4 cells were seeded in a
96-well plate at a density of 2×105 cells/ml/well, and
were placed in an incubator at 37°C overnight to allow for
attachment and recovery. Cells were pretreated with pPeOp
(30, 60 or 90 µg/ml), 90 µg/ml PVP or 100 µg/ml 5-FU (each drug was
dissolved in RPMI-1640 medium), with 6 wells for each treatment and
a total volume of 200 µl/well. Following incubation at 37°C for 24
h in a humidified atmosphere containing 5% CO2, 20 µl CCK-8 reagent
was added to each well, prior to reincubating for 2 h. The optical
density of each well was measured at 490 nm using a multimode plate
reader, with the viability of cells from three independent
biological replicates calculated for each experiment.
Cell cycle analysis
Cells were quantified using a Cell Cycle and
Apoptosis Analysis kit (cat. no. C1052; Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. MC-4
cells were pretreated with pPeOp (30, 60 or 90 µg/ml), 90
µg/ml PVP or 100 µg/ml 5-FU prior to culturing at 37°C for 24 h in
a humidified atmosphere containing 5% CO2. The cells were
subsequently washed with phosphate-buffered saline (PBS) three
times and fixed with 70% ice-cold ethanol at 4°C overnight. The
cells were centrifuged, washed with PBS, treated with 20 µl RNase A
in a water bath at 37°C for 30 min, and placed in the dark at 4°C
for 30 min with 300–500 µl propidium iodide solution. The cell
cycle distribution of the cells was determined using a Beckman
FC500 flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) with
red fluorescent light at a wavelength of 488 nm.
Wound healing assay
MC-4 cells at a density of 2×105 cells/ml
were seeded into 6-well plates. The cells were cultured at 37°C in
an atmosphere containing 5% CO2 for ~24 h, at which time they were
~80% confluent. Cells layers were scratched with a 200 µl pipette
tip. The cells were treated with 30, 60 or 90 µg/ml pPeOp,
90 µg/ml PVP, or 100 µg/ml 5-FU and cultured at 37°C in a
humidified atmosphere containing 5% CO2 for 24 h to allow for
migration into the cell-free area. Images of the cells were
captured with a light microscope (magnification, ×40) 24 h
following treatment.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from the MC-4 cells treated with
pPeOp (30, 60 or 90 µg/ml), 90 µg/ml PVP or 100 µg/ml 5-FU
was extracted using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. cDNA was synthesized using a Maxima First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The thermocycling
conditions were as follows: 42°C for 60 min for the reverse
transcription reaction; 70°C for 5 min to terminate the reaction;
and finally immediate cooling on ice. The cDNA reaction solution
was used as a template for subsequent qPCR. Primers used for qPCR
are presented in Table I and were
purchased from Sangon Biotech (Shanghai, China). qPCR was performed
using a MasterCycler RealPlex4 Real-Time PCR instrument
(Eppendorf, Hamburg, Germany). β-actin was used as an internal
normalization control. The PCR amplification conditions using a
two-step method were as follows: 1 cycle at 95°C for 2 min
(pre-denaturing); 40 cycles of 95°C for 15 sec (denaturing); and
62.9°C (β-actin), 57.8°C (MMP2, MMP9, cyclin A, cyclin B, cyclin
D1) or 61°C (CDK1, 2 and 4) for 1 min (annealing). At the end of
the PCR cycle, a dissociation curve was created to confirm
amplification of a single product. The results are represented as
the fold change in gene expression relative to that of β-actin
(2−ΔΔCq) (15).
 | Table I.Primers used in reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used in reverse
transcription-quantitative polymerase chain reaction analysis.
| Target | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| β-actin |
ACCTGCAGCAATACCATTGAC |
AAGGTGAGGGACTCAAACTGC |
| CDK1 |
TGCTGGGGTCAGCTCGTTACTCA |
TGGGATGCTAGGCTTCCTGGTT |
| CDK2 |
GTGGGCCCGGCAAGATTTTAG |
GCCGAAATCCGCTTGTTAGGG |
| CDK4 |
CTTGATCTGAGAATGGCTACCTCT |
CATGAAGGAAATCTAGGCCTCTTA |
| Cyclin D1 |
CATCTCTGTACTTTGCTTGCTCAT |
CGCTATTTCCTACACCTATTGGAC |
| Cyclin B |
TCGAGCAACATACTTTGG |
GCAAAAAGCTCCTGCTGC |
| Cyclin A |
AGACCCTGCATTTGGCTGTG |
ACAAACTCTGCTACTTCTGG |
| MMP-2 |
TGATGGTGTCTGCTGGAAAG |
GACACGTGAAAAGTGCCTTG |
| MMP-9 |
GGAGACCTGAGAACCAATCTC |
TCCAATAGGTGATGTTGTGG |
Western blotting
MC-4 cells were collected following treatment with
pPeOp (30, 60 or 90 µg/ml), 90 µg/ml PVP or 100 µg/ml 5-FU,
subsequently lysed with radioimmunoprecipitation lysis buffer
(Beyotime Institute of Biotechnology) for 30 min on ice. The
lysates were separated by centrifugation at 12,000 × g for 15 min
at 4°C. The total protein concentration in the supernatants was
determined using a bicinchoninic acid assay kit (Nanjing KeyGen
Biotech Co., Ltd., Nangjing, China), according to the
manufacturer's protocol. SDS-PAGE was performed using 10% gels with
~20 µg protein per lane. Proteins were subsequently transferred
onto polyvinylidene fluoride membranes, which were blocked with 5%
dried skimmed milk in Tris-buffered saline containing 1% Tween-20
(TBST) and incubated with primary antibodies (described above)
overnight at 4°C. Membranes were washed three times with TBST and
incubated with HRP-conjugated goat anti-rabbit IgG at room
temperature for 2 h, followed by washing three times with TBST.
Images of the blots were captured on film in a darkroom using
BeyoECL Plus Substrate kit (Beyotime Institute of Biotechnology)
and were scanned and quantified using the Quantity One 1-D image
analysis software (version 4.6.2; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
All experiments were conducted in triplicate.
Results are presented as the mean ± standard error of the mean.
Statistical analyses were performed using SPSS software for Windows
(version 16.0; SPSS, Inc., Chicago, IL, USA). Statistical
differences were assessed using the Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
pPeOp inhibits MC-4 cell
proliferation
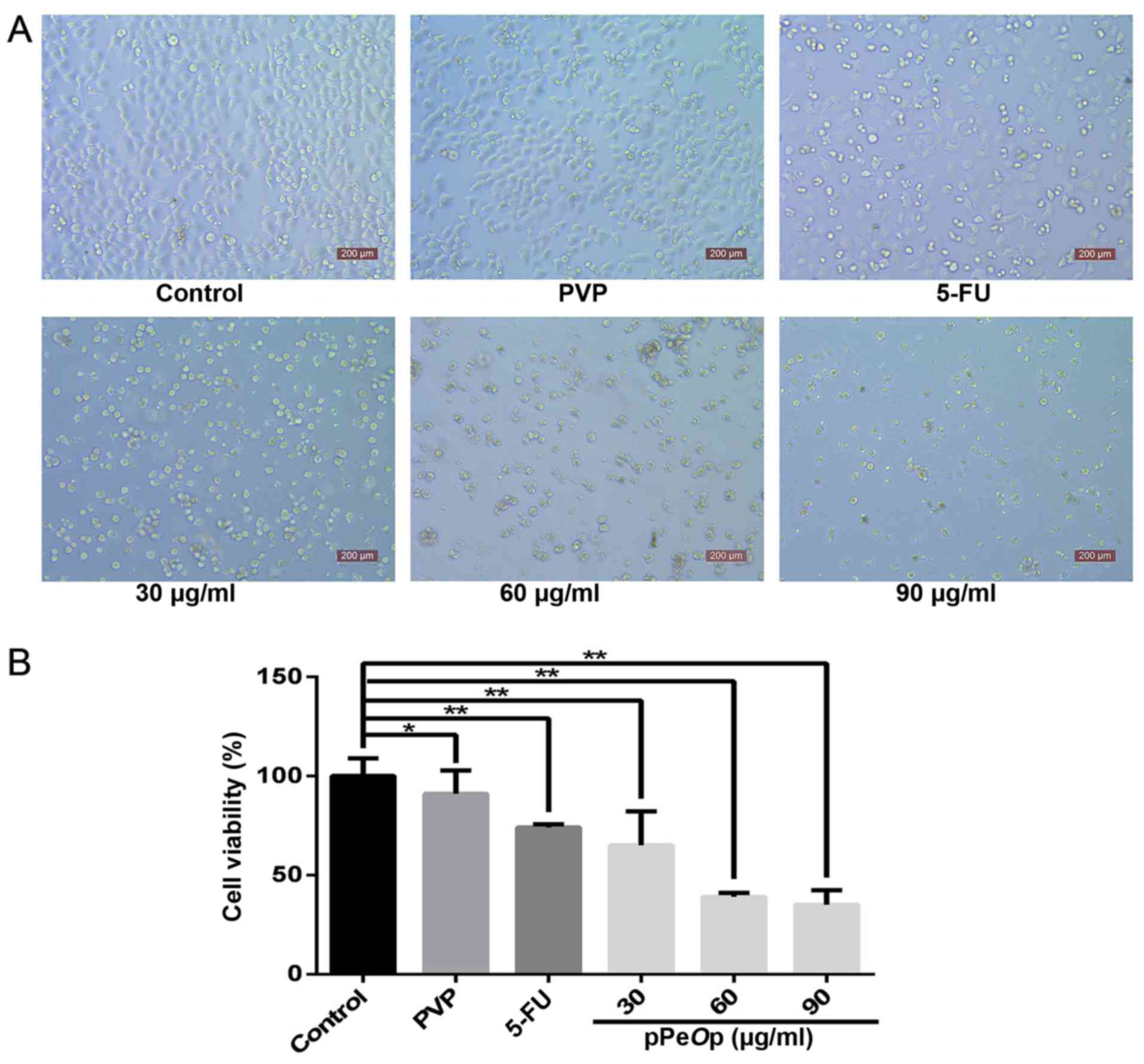
As presented in Fig.
1A, cell proliferation was decreased in MC-4 cells 24 h
following treatment with 30, 60 or 90 µg/ml pPeOp, and 100
µg/ml 5-FU (positive control) compared with the untreated control
group and 90 µg/ml PVP-treated cells (negative control). This
decrease was significant for all concentrations of pPeOp
compared with the untreated control group (P<0.01; Fig. 1B). As presented in Fig. 1B, the relative cell viability of each
group was as follows: 30 µg/ml pPeOp, 64.97±0.172%; 60
µg/ml, 39.42±0.021%; 90 µg/ml, 35.23±0.074%; PVP-treated negative
control, 91.00±0.017%; and 5-FU-treated positive control,
73.81±0.118%. Compared with the 5-FU positive control group, an
increase in pPeOp concentration led to increased inhibition
of proliferation in a dose-dependent manner.
pPeOp arrests the MC-4 cell cycle in S
phase
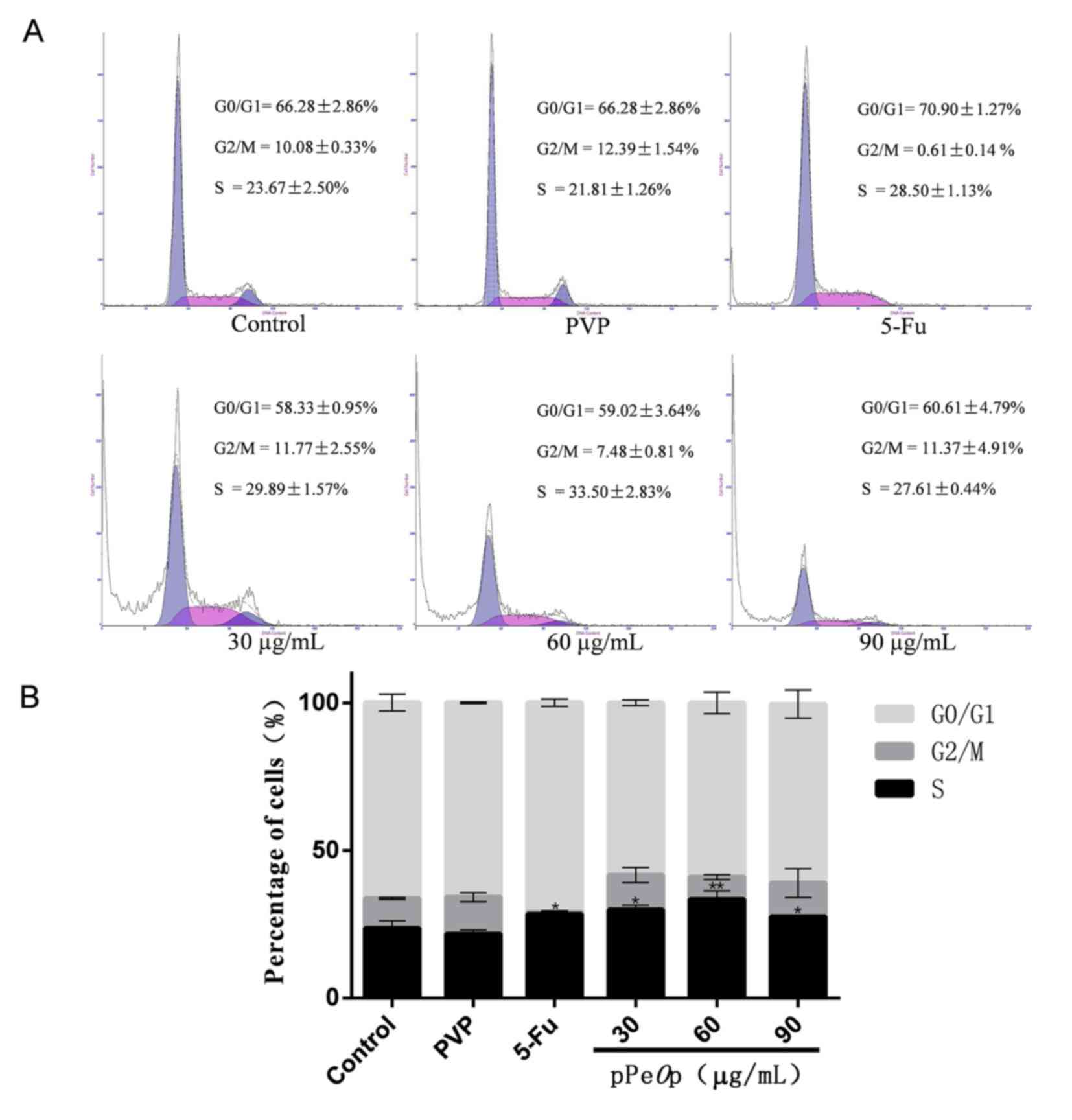
Treatment of MC-4 cells with various concentrations
of pPeOp led to alterations in cell cycle distribution
following 24 h, as presented in Fig.
2. Compared with the untreated control, following treatment
with pPeOp there was a significantly increased proportion of
cells in S phase (P<0.05), but a decreased proportion of cells
in G0/G1 phase (Fig. 2B).
pPeOp at a concentration of 30 and 90 µg/ml also increased
the proportion of cells in G2/M phase compared with the untreated
control, whereas 60 µg/ml pPeOp decreased the proportion of
cells in G2/M phase (Fig. 2B). In
addition, compared with the 5-FU-treated group, the proportion of
cells in S phase in the 30 and 60 µg/ml pPeOp-treated groups
increased; however, cells treated with 90 µg/ml pPeOp
exhibited a decreased proportion of cells in S phase. These results
indicate that in S phase cell cycle arrest is induced by
pPeOp.
pPeOp decreases cell migration
A wound healing assay was used to assess the role of
pPeOp in the migration of MC-4 cells. Microscopic analysis
(Fig. 3) demonstrated that
pPeOp decreased the migratory rate of MC-4 cells in a
concentration-dependent manner, compared with the untreated control
cells. These results indicate that pPeOp has a negative
effect on MC-4 cell migration.
pPeOp affects the mRNA and protein
expression of cell cycle- and migration-associated proteins in MC-4
cells
As presented in Fig.
4A, the mRNA expression levels of the cyclin B, cyclin D1, CDK1
and CDK2 were decreased in MC-4 cells treated with pPeOp in
a concentration-dependent manner compared with the untreated
control group. However, the mRNA expression levels of cyclin A and
CDK4 mRNA were increased compared with the untreated control in a
concentration-dependent manner (Fig.
4A). In addition, the mRNA expression levels of cell
migration-associated MMP2 and MMP9 in MC-4 cells were decreased
significantly at all concentrations of pPeOp used
(P<0.001; Fig. 4B). Furthermore,
the expression of the cycle arrest-associated proteins cyclin D1,
CDK4, cyclin B, CDK1, cyclin A and CDK2, and migration-associated
proteins MMP-2 and MMP-9, were analyzed by western blotting, with
similar results to their corresponding mRNA expression (Fig. 4C). Quantification of band density
relative to that of β-actin demonstrated statistically significant
differences in protein expression between the untreated control
cells and cells treated with pPeOp, as presented in Fig. 4D.
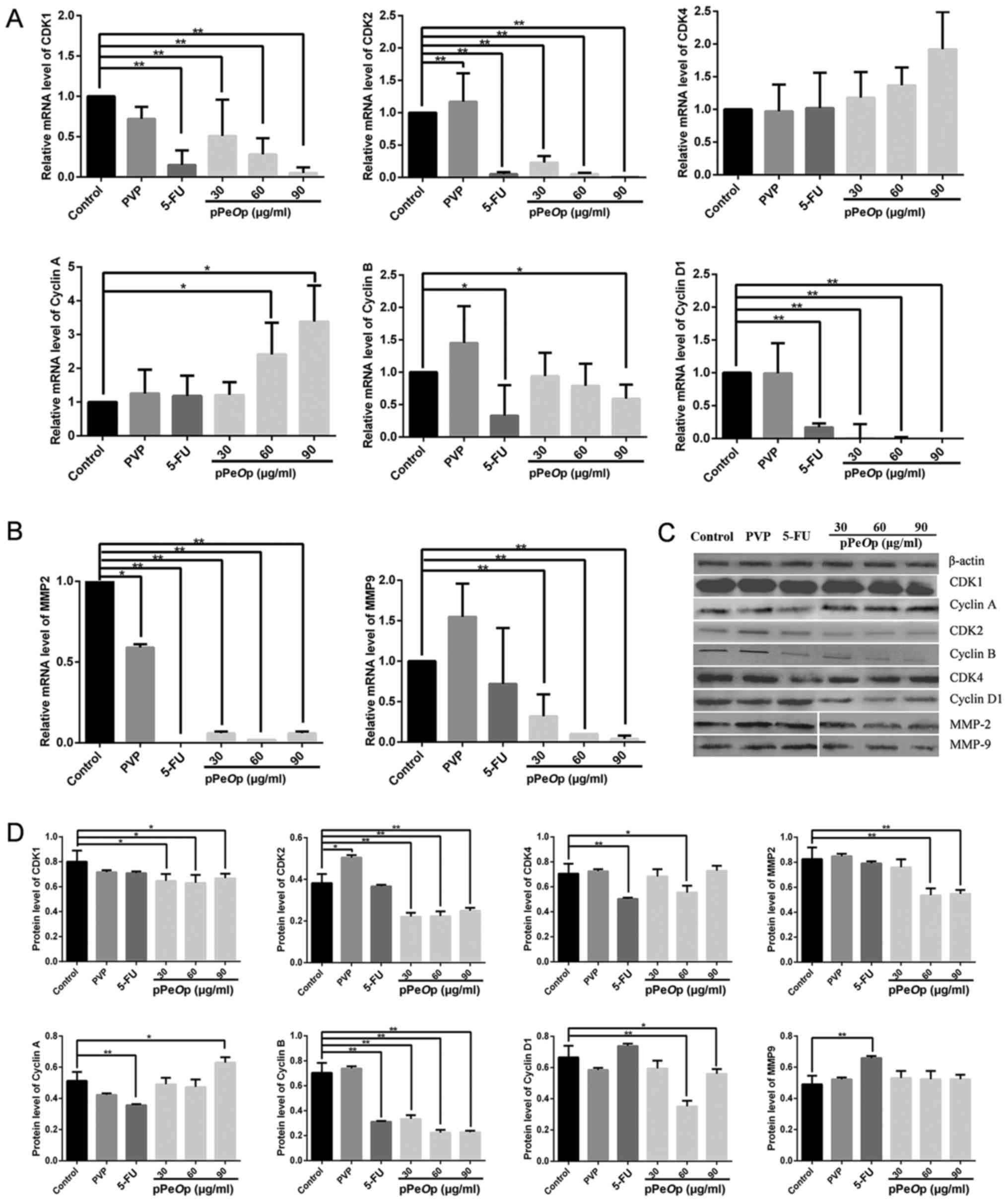 | Figure 4.pPeOp affects the expression
of cell cycle- and migration-associated genes/proteins in MC-4
cells. Cells were treated with pPeOp (30, 60 or 90 µg/ml), 90 µg/ml
PVP (negative control) or 100 µg/ml 5-FU (positive control) for 24
h. In addition, untreated cells were used as the control group.
Reverse transcription-quantitative polymerase chain reaction
analysis was used to assess cell cycle-associated gene expression
levels. All data were normalized to the untreated control. (A)
Effects of pPeOp on the mRNA expression levels of the cell
cycle-associated genes CDK1, CDK2, CDK4, cyclin A, cyclin B and
cyclin D1. (B) Effects of pPeOp on the mRNA expression
levels of the migration-associated genes MMP-2 and MMP-9. (C)
Western blotting was conducted to examine the effects of
pPeOp on the expression of cell cycle- and
migration-associated proteins, with β-actin used as a loading
control. (D) Quantification of protein expression levels of cell
cycle- and migration-associated proteins normalized to β-actin.
*P<0.05, **P<0.01. pPeOp, purified Omphalia
lapidescens protein; PVP, polyvinylpyrrolidone; 5-FU,
5-fluorouracil; CDK, cyclin-dependent kinase; MMP, matrix
metalloproteinase. |
Discussion
Migration, an important biological characteristic of
cancer cells, is observed in gastric cancer (16,17). The
decreased survival rate of patients with gastric cancer may be
attributed to the migration of gastric cancer cells into the lymph
nodes and peritoneum (18,19). MMP-2 and MMP-9 are the matrix
metalloproteinases primarily involved in degradation of type IV
collagen in the basement membrane, and are associated with
malignant tumor infiltration and migration (20–22). MMPs
are important regulatory molecules in tumor migration, and exhibit
extensive and increased expression in various types of human
malignant tumor (23). In
tumorigenesis MMPs regulate the degradation of the ECM, regulate
tumor angiogenesis, alter the function of cell adhesion molecules
and mediate the proliferation of tumor cells (24). MMP-2 and MMP-9 may be biomarkers of
the migratory ability of cancer cells (25–27).
An uncontrolled cell cycle is closely associated
with tumor occurrence, development and malignancy (28,29). Once
cell proliferation or inappropriate cell death occurs, it typically
results in a tumor, and it has been demonstrated that the
regulation of the cell cycle is an important mechanism in
tumorigenesis. Arrest of the various phases of the cell cycle may
be used to inhibit cell proliferation and induce differentiation or
apoptosis (30,31). Cancer is a disease that causes
disorders in the cell cycle and uncontrolled proliferation due to
the combined effects of hereditary and environmental factors
(32). Therefore, the exploration of
the fundamental mode of tumor cell cycle regulation, examining the
important issue of targeted drug therapy, has become central to
research in the field of tumor-associated diseases.
The aim of studying the effects of the protein
pPeOp from O. lapidescens Schroet on the migration
and cell cycle distribution of the human gastric cancer cell line
MC-4 was to elucidate the underlying molecular mechanisms through
which pPeOp functions. pPeOp was demonstrated to
significantly inhibit the proliferation of MC-4 cells; the
proliferation of MC-4 cells was arrested in the S phase and led to
abnormal distribution of G1 and G2 phase
cells compared with the conventional chemotherapy drug 5-FU.
Results from RT-qPCR analysis of the mRNA expression levels of cell
cycle-associated genes and from the cell cycle analysis identified
that pPeOp induced upregulation of cyclin A and CDK4, which
arrested cells in S phase. Previous studies have suggested that
cyclins and CDKs are essential regulatory proteins in the cell
cycle, and arrest of the cell cycle at the
G0/G1 or G2/M phases is one of the
mechanisms caused by anticancer therapies (33–35). The
results of the present study demonstrated that pPeOp is able
to cause an abnormal G0/G1 and
G2/M phase distribution of cells via downregulating the
expression of cyclin D1, cyclin B, CDK1, and CDK2 genes in a
concentration-dependent manner. In order to further clarify the
role of pPeOp, the alteration in cell cycle-associated
protein expression was examined, which revealed similar results to
RT-qPCR analysis. Expression of cyclin B protein was notably
downregulated and no marked expression was identified in the
pPeOp-treated cells, indicating that pPeOp induces
MC-4 cell apoptosis, which makes detection of the already low level
of the corresponding expression of cyclin D1 difficult.
Furthermore, the results of the wound healing assay demonstrated
that pPeOp decreased the migratory rate of MC-4 cells in a
concentration-dependent manner. In addition, pPeOp had a
marked lethal effect on MC-4 cells, which is consistent with Chen
et al (14). However, the
effect of pPeOp on cell migration was not investigated by
Chen et al. In the present study, pPeOp induced
apoptosis in the majority of the cells and induced cell migration.
Additionally, the secretion of MMP-2 and MMP-9 decreased as shown
by western blotting results. Cell migration was inhibited by the
expression of MMP-2 and MMP-9. Concomitant with an increase in the
concentration of pPeOp, the expression levels of MMP-2 and
MMP-9 protein were decreased.
The downregulation of cyclin D1, cyclin B, CDK1 and
CDK2, and upregulation of cyclin A and CDK4 by pPeOp
arrested MC-4 cells in the S phase of the cell cycle and led to an
abnormal distribution of G0/G1 and
G2/M phase cells. Furthermore, by downregulating MMP-2
and MMP-9 expression, pPeOp inhibited the migration of MC-4
cells. These results indicate that pPeOp serves a role in
cell cycle arrest and the inhibition of migration of MC-4 gastric
tumor cells. The identification and determination of the expression
of other proteins that may be involved in the underlying molecular
mechanism of action of pPeOp is warranted by further
study.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation Project (grant no. 81374023),
Zhejiang Provincial Natural Science Foundation (grant no. Y207765)
and the Zhejiang Provincial Medical and Health Science and
Technology Project (grant no. 2015106212).
References
|
1
|
Song W, Liu YY, Peng JJ, Liang HH, Chen
HY, Chen JH, He WL, Xu JB, Cai SR and He YL: Identification of
differentially expressed signatures of long non-coding RNAs
associated with different metastatic potentials in gastric cancer.
J Gastroenterol. 51:119–129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi HJ, Ki CS, Suh SP and Kim JW:
Presymptomatic identification of CDH1 germline mutation in a
healthy Korean individual with family history of gastric cancer.
Ann Lab Med. 34:386–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang C, Chen X, Alattar M, Wei J and Liu
H: MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis
of gastric cancer. Cancer Gene Ther. 22:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao J, Liu Y, Huang G, Cui P, Zhang W and
Zhang Y: Long non-coding RNAs in gastric cancer: Versatile
mechanisms and potential for clinical translation. Am J Cancer Res.
5:907–927. 2015.PubMed/NCBI
|
|
5
|
Robertson-Tessi M, Gillies RJ, Gatenby RA
and Anderson AR: Impact of metabolic heterogeneity on tumor growth,
invasion, and treatment outcomes. Cancer Res. 75:1567–1579. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Li L, Liu R and Lin HS: Establishing
Chinese medicine characteristic tumor response evaluation system is
the key to promote internationalization of Chinese medicine
oncology. Chin J Integr Med. 18:730–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo
N, Li XK and Tang W: Chinese herbal medicines as adjuvant treatment
during chemo- or radio-therapy for cancer. Biosci Trends.
4:297–307. 2010.PubMed/NCBI
|
|
8
|
Ling CQ, Yue XQ and Ling C: Three
advantages of using traditional Chinese medicine to prevent and
treat tumor. J Integr Med. 12:331–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YT, Lin MA, Cheng DQ, Shi ZJ, Zhu JL
and Wu J: Effect of proteins extracted from mycelia of Omphalia
lapidescens on inhibiting H, liver cancer in mice and regulating
immune function. Zhong Yao Cai. 32:1870–1874. 2009.(In Chinese).
PubMed/NCBI
|
|
10
|
Yan H, Rong X, Chen PT, Zhang X and Ma ZQ:
Two new steroids from sclerotia of the fungus Omphalia lapidescens.
J Asian Nat Prod Res. 16:265–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohno N, Saito K, Nemoto J, Kaneko S,
Adachi Y, Nishijima M, Miyazaki T and Yadomae T:
Immunopharmacological characterization of a highly branched fungal
(1->3)-beta-D-glucan, OL-2, isolated from Omphalia lapidescens.
Biol Pharm Bull. 16:414–419. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saito K, Nishijima M, Ohno N, Yadomae T
and Miyazaki T: Structure and antitumor activity of the
less-branched derivatives of an alkali-soluble glucan isolated from
Omphalia lapidescens. (Studies on fungal polysaccharide. XXXVIII).
Chem Pharm Bull (Tokyo). 40:261–263. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang G, Huang Y, Bian Y, Wong JH, Ng TB
and Wang H: Hypoglycemic activity of the fungi Cordyceps
militarisCordyceps sinensisTricholoma mongolicum, and Omphalia
lapidescens in streptozotocin-induced diabetic rats. Appl Microbiol
Biotechnol. 72:1152–1156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YT, Lu QY, Lin MA, Cheng DQ, Ding ZS
and Shan LT: A PVP-extract fungal protein of Omphalia lapidescens
and its antitumor activity on human gastric tumors and normal
cells. Oncol Rep. 26:1519–1526. 2011.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Pan T, Zhong X and Cheng C:
Androgen receptor promotes esophageal cancer cell migration and
proliferation via matrix metalloproteinase 2. Tumour Biol.
36:5859–5864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin Y, Peng N, Zhuang H, Zhang D, Wang Y
and Hua ZC: Heat shock proteins HSP70 and MRJ cooperatively
regulate cell adhesion and migration through urokinase receptor.
BMC Cancer. 14:6392014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun A, Yu G, Dou X, Yan X, Yang W and Lin
Q: Nedd4-1 is an exceptional prognostic biomarker for gastric
cardia adenocarcinoma and functionally associated with metastasis.
Mol Cancer. 13:2482014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao K, Xie D, Cao P, Zou Q, Lu C, Xiao S,
Zhou J and Peng X: SiRNA-mediated flotillin-2 (Flot2)
downregulation inhibits cell proliferation, migration, and invasion
in gastric carcinoma cells. Oncol Res. 21:271–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu S, Zhu Q, Zhang Y, Song W, Wilson MJ
and Liu P: Dual-functions of miR-373 and miR-520c by differently
regulating the activities of MMP2 and MMP9. J Cell Physiol.
230:1862–1870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tong WW, Tong GH, Chen XX, Zheng HC and
Wang YZ: HIF2α is associated with poor prognosis and affects the
expression levels of survivin and cyclin D1 in gastric carcinoma.
Int J Oncol. 46:233–242. 2015.PubMed/NCBI
|
|
22
|
Płuciennik E, Nowakowska M, Pospiech K,
Stępień A, Wołkowicz M, Gałdyszyńska M, Popęda M, Wójcik-Krowiranda
K, Bieńkiewicz A and Bednarek AK: The role of WWOX tumor suppressor
gene in the regulation of EMT process via regulation of
CDH1-ZEB1-VIM expression in endometrial cancer. Int J Oncol.
46:2639–2648. 2015.PubMed/NCBI
|
|
23
|
Leight JL, Tokuda EY, Jones CE, Lin AJ and
Anseth KS: Multifunctional bioscaffolds for 3D culture of melanoma
cells reveal increased MMP activity and migration with BRAF kinase
inhibition. Proc Natl Acad Sci USA. 112:pp. 5366–5371. 2015;
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng L, Zhang YM, Zhan YZ and Liu CX:
Momordica cochinchinensis seed extracts suppress migration and
invasion of human breast cancer ZR-75-30 cells via down-regulating
MMP-2 and MMP-9. Asian Pac J Cancer Prev. 15:1105–1110. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
da Rosa MR, Falcão AS, Fuzii HT, da Silva
Kataoka MS, Ribeiro AL, Boccardo E, De Siqueira AS, Jaeger RG, de
Jesus Viana Pinheiro J and de Melo Alves S Júnior: EGFR signaling
downstream of EGF regulates migration, invasion and MMP secretion
of immortalized cells derived from human ameloblastoma. Tumour
Biol. 35:11107–11120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu F, Zhou J, Zhou P, Chen W and Guo F:
The ubiquitin ligase CHIP inactivates NF-κB signaling and impairs
the ability of migration and invasion in gastric cancer cells. Int
J Oncol. 46:2096–2106. 2015.PubMed/NCBI
|
|
27
|
Kim D, Lee D, Jang YL, Chae SY, Choi D,
Jeong JH and Kim SH: Facial amphipathic deoxycholic acid-modified
polyethyleneimine for efficient MMP-2 siRNA delivery in vascular
smooth muscle cells. Eur J Pharm Biopharm. 81:14–23. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matusewicz L, Meissner J, Toporkiewicz M
and Sikorski AF: The effect of statins on cancer cells-review.
Tumour Biol. 36:4889–4904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parajuli KR, Zhang Q, Liu S and You Z:
Aminomethylphosphonic acid and methoxyacetic acid induce apoptosis
in prostate cancer cells. Int J Mol Sci. 16:11750–11765. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saiko P, Steinmann MT, Schuster H, Graser
G, Bressler S, Giessrigl B, Lackner A, Grusch M, Krupitza G,
Bago-Horvath Z, et al: Epigallocatechin gallate, ellagic acid, and
rosmarinic acid perturb dNTP pools and inhibit de novo DNA
synthesis and proliferation of human HL-60 promyelocytic leukemia
cells: Synergism with arabinofuranosylcytosine. Phytomedicine.
22:213–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hong S, Gu Y, Gao Z, Guo L, Guo W, Wu X,
Shen Y, Sun Y, Wu X and Xu Q: EGFR inhibitor-driven endoplasmic
reticulum stress-mediated injury on intestinal epithelial cells.
Life Sci. 119:28–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Evers B, Helleday T and Jonkers J:
Targeting homologous recombination repair defects in cancer. Trends
Pharmacol Sci. 31:372–380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Rader J, He S, Phung T and Thiele
CJ: CASZ1 inhibits cell cycle progression in neuroblastoma by
restoring pRb activity. Cell Cycle. 12:2210–2218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao Z, Lin W, Huang Z, Chen X, Zhao J,
Zheng L, Ye H, Liu Z, Liao L and Du J: Ethyl acetate extraction
from a Chinese herbal formula, Jiedu Xiaozheng Yin, inhibits the
proliferation of hepatocellular carcinoma cells via induction of
G0/G1 phase arrest in vivo and in vitro. Int J Oncol. 42:202–210.
2013.PubMed/NCBI
|
|
35
|
Marconett CN, Morgenstern TJ, San Roman
AK, Sundar SN, Singhal AK and Firestone GL: BZL101, a phytochemical
extract from the Scutellaria barbata plant, disrupts proliferation
of human breast and prostate cancer cells through distinct
mechanisms dependent on the cancer cell phenotype. Cancer Biol
Ther. 10:397–405. 2010. View Article : Google Scholar : PubMed/NCBI
|