Introduction
Uterine cervical cancer is the second most frequent
cancer in women, causing cancer-associated mortality worldwide
(1). Concurrent chemoradiotherapy
(CCRT) is the standard treatment for locally advanced uterine
cervical cancer, including International Federation of Gynecology
and Obstetrics (FIGO) stage IIIA, IIIB and IVA lesions (2–4) However,
the prognosis of patients with locally advanced uterine cervical
cancer is poor, and the 5-year survival rate is <60% (5,6).
Effective neoadjuvant chemotherapy (NAC) can reduce
tumor size to facilitate hysterectomy and improve the prognosis of
patients with locally advanced cervical cancer (7). However, NAC is ineffective in certain
cases, leaving radiotherapy as the only treatment option, and
thereby delaying the initiation of the core treatment, thus
resulting in worse prognosis (8,9). At
present, no significant predictive markers of NAC efficacy for
locally advanced cervical cancer patient prognosis have been
reported. The identification of such markers would improve
therapeutic decision making and the prognosis of patients with
locally advanced cervical cancer (10–14).
Chemotherapeutic agents generate reactive oxygen
species (ROS) that can overwhelm antioxidant defenses to result in
cell damage and cell death (15–17).
Uncoupling proteins (UCPs) are mitochondrial anion transporters
(18,19). The five known types of UCP (UCP1-5)
have different levels of identity and different tissue distribution
(20). UCP1 is expressed in brown
adipose tissue, which generates heat through uncoupling of
oxidative phosphorylation from the electron transport chain
(20). UCP2 expression has been
identified in the liver, pancreas, adipose tissue, spleen, kidney
and brain, and UCP3 is localized to skeletal muscle (20). UCP2 and UCP3 are activated by
superoxide from the mitochondrial inner membrane, and can reduce
ROS generation (21). UCP4 and UCP5
are specifically localized to the brain, and associated with
reducing the mitochondrial membrane potential (22).
UCP2 is broadly expressed in cancer cells (23,24), and
can suppress mitochondrial ROS production, thus mitigating
oxidative stress (25). Loss of UCP2
function may increase ROS production (25), whereas UCP2 overexpression may promote
cytoprotection by mitigating oxidative stress (26,27).
Furthermore, UCP2 contributes to both carcinogenesis and
chemoresistance (28,29). UCP2 is implicated in human colon
carcinogenesis (24,30), while mitochondrial uncoupling by UCP2
induces pancreatic cancer cell resistance to gemcitabine (28). Furthermore, inhibition of UCP2 by
genipin sensitizes cancer cells to chemotherapeutic agents
(28,29). These findings suggest that UCP2
represents a target for cancer treatment with chemotherapeutic
agents that promote oxidative stress. In the present study, a
correlation between UCP2 expression and the efficacy of NAC for
locally advanced uterine cervical cancer was revealed.
Materials and methods
Patients and samples
The present study included 58 patients with locally
advanced uterine cervical cancer (FIGO stages IIIA and IIIB). All
patients were under 70 years of age, and were treated at Osaka City
University Hospital (Osaka, Japan) from April 1995 to March 2010.
Tumor tissue samples were obtained by punch biopsy prior to NAC.
Patients were divided into two groups based on NAC effectiveness.
For the NAC effective group, surgery was possible, and radiation
therapy was also performed (n=34), while in the NAC ineffective
group, radiation therapy was performed without prior surgery
(n=24). All patients underwent balloon-occluded arterial infusion
chemotherapy for NAC. Cisplatin (Bristol-Myers Squibb, Tokyo,
Japan) was infused intra-arterially through the catheter over 30
min (31).
Written informed consent was obtained from all
patients prior to punch biopsy. The present study was approved by
the institutional review board (IRB) of Osaka City University
Hospital (IRB no. 3524).
Immunohistochemical staining
UCP2 expression was examined in paraffin-embedded
sections using an anti-UCP2 antibody (#ab116263; Abcam, Cambridge,
UK) and a Dako LSAB2 Peroxidase kit (#K0675; Agilent Technologies,
Inc., Santa Clara, CA, USA). Sections (4 µm-thick) were
deparaffinized, rehydrated and immersed in 3% hydrogen peroxide at
room temperature for 10 min to block endogenous peroxidase
activity. An antigen retrieval procedure was performed by immersing
sections in 10 mM citrate buffer (pH 6.0) and heating to 110°C for
20 min in an autoclave. Tissue sections were then washed in PBS and
incubated overnight at 4°C with a 1:100 dilution of the
aforementioned rabbit polyclonal anti-UCP2 antibody. Next, sections
were washed in PBS for 15 min and then incubated for 10 min with
biotinylated goat anti-mouse or anti-rabbit immunoglobulin G (Dako;
Agilent Technologies, Inc.). Sections were then incubated with a
streptavidin-peroxidase complex, and 3,3′-diaminobenzidine was used
as the chromogen. Finally, tissue sections were counterstained with
H&E and the specificity of the immunohistochemical reactions
was verified by omitting the primary antibody.
UCP2 expression levels were assessed quantitatively
using the weighted score method of Sinicrope et al (32). The mean percentage of stained tumor
cells was scored as follows: 0, ≤5%; 1, 5< and ≤25%; 2, 25<
and ≤50%; 3, 50< and ≤75%; 4, >75%. Staining intensity was
classified into three categories: 1+, weak; 2+, moderate; and 3+,
intense. The weighted score was determined by multiplying the score
of percentage of stained tumor cells by that of staining intensity
for each tissue specimen.
Cell culture
The human uterine cervical cancer cell line Ca Ski
(no. IFO50007; Japanese Collection of Research Biosources Cell
Bank, Osaka, Japan) was maintained in RPMI medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.). Cells were cultured
in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
Immunofluorescence staining
Cells were seeded at a density of 4×103
cells/well in 4-well chamber slides. After 48 h, the culture medium
was removed, and the cells were washed three times in PBS. Cells
were fixed with cold ethanol at 4°C for 10 min and then washed
three times in PBS. Samples were blocked with 1% bovine serum
albumin (Gibco; Thermo Fisher Scientific, Inc.) in PBS for 30 min
at room temperature. Upon washing with PBS, samples were incubated
with an anti-UCP2 antibody (1:250 dilution; no. scb-sc6525; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Upon
washing with PBS, cells were exposed to a fluorescein
isothiocyanate-conjugated anti-goat secondary antibody (1:200
dilution; no. F-2761; Dako, Agilent Technologies, Inc.) for 1 h at
room temperature. Immunofluorescent images were captured using a
fluorescence microscope (BX50; Olympus Corporation, Tokyo,
Japan).
Chemosensitivity assay
The sensitivity of cells to cisplatin was examined
using Cell Counting kit-8 (CCK-8; Dojindo Mole-cular Technologies,
Inc., Kumamoto, Japan). Approximately 1×103 cells were
seeded into each well of a 96-well tissue culture plate, and 24 h
later, the culture medium was removed from each well and replaced
with 100 µl fresh medium. Next, 100 µl dimethyl sulfoxide (DMSO) or
DMSO containing 1 µM genipin (#G-4796; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added to each well. Cells were then treated
with cisplatin (0–10 µg/ml) for 24 h. Subsequently, 10 µl CCK-8 was
added, followed by 2 h of incubation. The absorbance at 450 nm was
then measured with a microplate reader (Corona Electric Co., Ltd.,
Ibaraki, Japan). Dose-response graphs were constructed based on the
percentage of viable cells compared with that of control untreated
cells.
Statistical analysis
Data are presented as the mean ± standard deviation
in tables and as the mean + standard error in figures. Kaplan-Meier
and log-rank analyses were performed to evaluate prognosis.
Weighted scores were compared using the Mann-Whitney U test.
Student's t was performed to identify significant differences
between the means of two groups, and χ2 tests was
performed identify the association between the categorical
variables of two groups. SPSS software version 21.0 (IBM SPSS,
Armonk, NY, USA) was used for all statistical analyses. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patients' characteristics
A total of 58 patients with locally advanced uterine
cervical cancer were divided into two groups: The NAC effective
group (n=34) and the NAC ineffective group (n=24). Table I contains patients' age, FIGO stage,
histology and tumor size details. There were no significant
differences in these parameters between the two groups.
 | Table I.Characteristics of patients in the NAC
effective and ineffective groups. |
Table I.
Characteristics of patients in the NAC
effective and ineffective groups.
| Characteristics | NAC effective
(no.) | NAC ineffective
(no.) | P-value |
|---|
| No. of patients | 34 | 24 |
|
| Age (years) |
|
| 0.394a |
| Mean ±
SD | 49.3±12.9 | 52.2±11.7 |
|
|
Range | 24–69 | 36–68 |
|
| FIGO stage |
|
| 0.397b |
| IIIA | 1 | 0 |
|
| IIIB | 33 | 24 |
|
| Histology |
|
| 0.400b |
| SCC | 29 | 19 |
|
| A | 5 | 3 |
|
| AS | 0 | 1 |
|
|
Others | 0 | 1 |
|
| Tumor size
(mm) |
|
| 0.144a |
| Mean ±
SD | 46.9±17.2 | 53.7±14.9 |
|
UCP2 expression in uterine cervical
cancer tissue
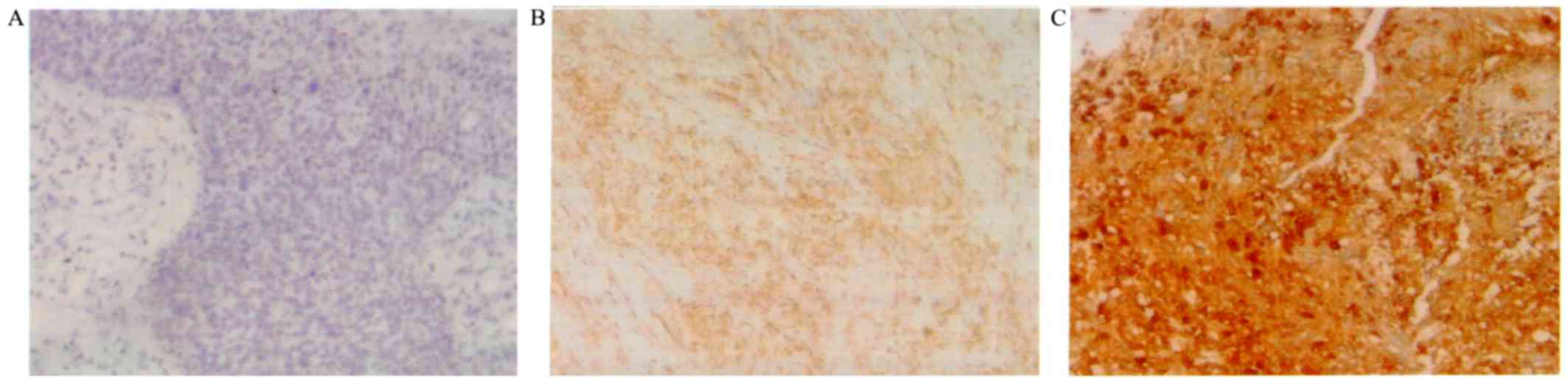
Cytop-lasmic expression of UCP2 was observed in
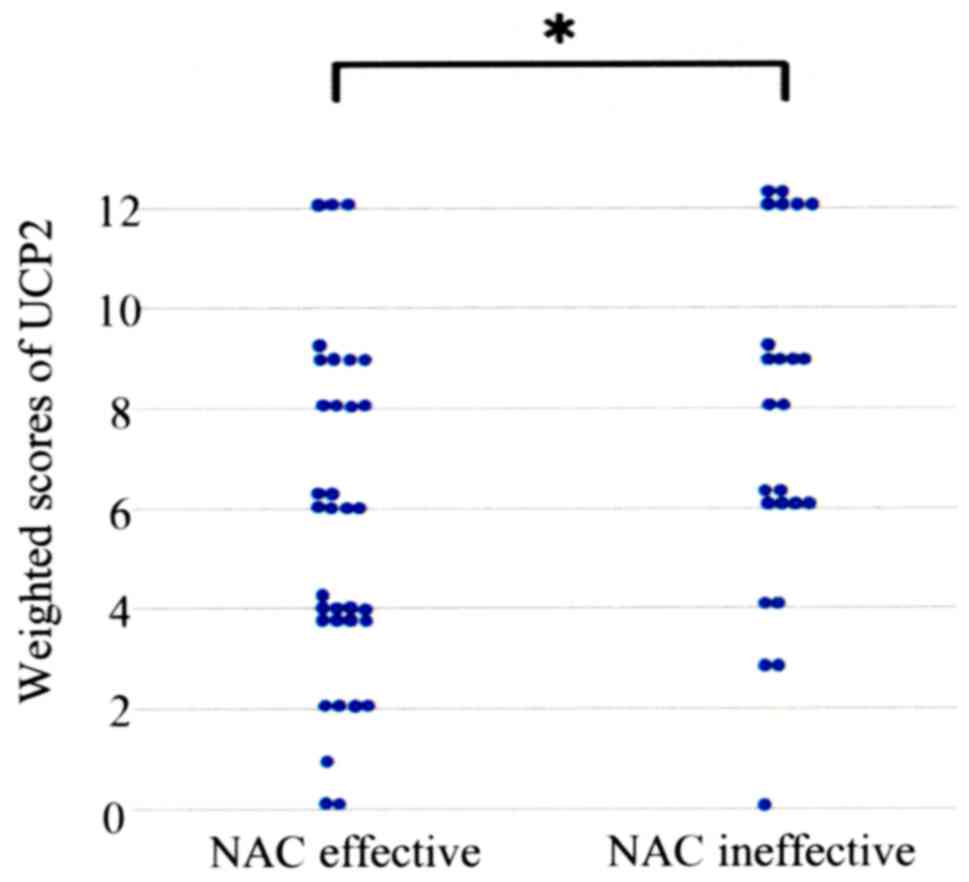
tumor cells (Fig. 1). Table II shows the UCP2 weighted scores in
the tissues of the two patient groups. The means of weighted scores
in the NAC effective and ineffective groups were 5.71 and 7.63,
respectively. The UCP2 weighted score was significantly higher in
the NAC ineffective group compared with that in the NAC effective
group (P=0.038; Table II and
Fig. 2).
 | Table II.Weighted scores for uncoupling
protein 2 expression in the NAC effective and ineffective
groups. |
Table II.
Weighted scores for uncoupling
protein 2 expression in the NAC effective and ineffective
groups.
|
| No. of
patients |
|---|
|
|
|
|---|
| Weighted score | NAC
effectivea | NAC
ineffectiveb |
|---|
| 0 | 2 | 1 |
| 1 | 1 | 0 |
| 2 | 4 | 0 |
| 3 | 0 | 2 |
| 4 | 9 | 2 |
| 6 | 6 | 6 |
| 8 | 4 | 2 |
| 9 | 5 | 5 |
| 12 | 3 | 6 |
| Total | 34 | 24 |
| Mean | 5.71 | 7.63 |
Next, cases were divided into two groups based on
their UCP2 expression levels: The low UCP2 expression group
(weighted score, 0–4) and the high UCP2 expression group (weighted
score, 6–12). Table III lists the
characteristics of the high and low expression groups, with
analyses revealing no significant differences between the two
groups.
 | Table III.Characteristics of patients in the
low and high UCP2 expression groups. |
Table III.
Characteristics of patients in the
low and high UCP2 expression groups.
|
| No. of
patients |
|
|---|
|
|
|
|
|---|
|
Characteristics | UCP2 expression
(score ≤4) | UCP2 expression
(score ≥6) | P-value |
|---|
| No. of
patients | 21 | 37 |
|
| Age (years) |
|
| 0.594a |
| Mean ±
SD | 51.7±12.4 | 49.8±12.5 |
|
|
Range | 24–68 | 24–69 |
|
| FIGO stage |
|
| 0.447b |
|
IIIA | 0 | 1 |
|
|
IIIB | 21 | 36 |
|
| Histology |
|
| 0.759b |
|
SCC | 17 | 31 |
|
| A | 3 | 5 |
|
| AS | 0 | 1 |
|
|
Others | 1 | 0 |
|
| Tumor size
(mm) |
|
| 0.144a |
| Mean ±
SD | 47.0±15.9 | 54.0±17.3 |
|
NAC effectiveness correlates with UCP2
expression
Within the low UCP2 expression group, 16 cases (76%)
belonged to the NAC effective group, while 5 (24%) belonged to the
NAC ineffective group. In the high UCP2 expression group, 18 cases
(49%) belonged to the NAC effective group and 19 (51%) to the NAC
ineffective group. The low UCP2 expression group was more sensitive
to NAC than the high UCP2 expression group (P=0.041; Table IV).
 | Table IV.Number of patients with low and high
uncoupling protein 2 expression in the NAC effective and
ineffective groups. |
Table IV.
Number of patients with low and high
uncoupling protein 2 expression in the NAC effective and
ineffective groups.
| UCP2
expression | NAC+OP+R, no.
(%) | NAC+R, no. (%) | P-value |
|---|
| Low expression
(score ≤6) | 16 (76) | 5 (24) | 0.041a |
| High expression
(score ≥8) | 18 (49) | 19 (51) |
|
Survival
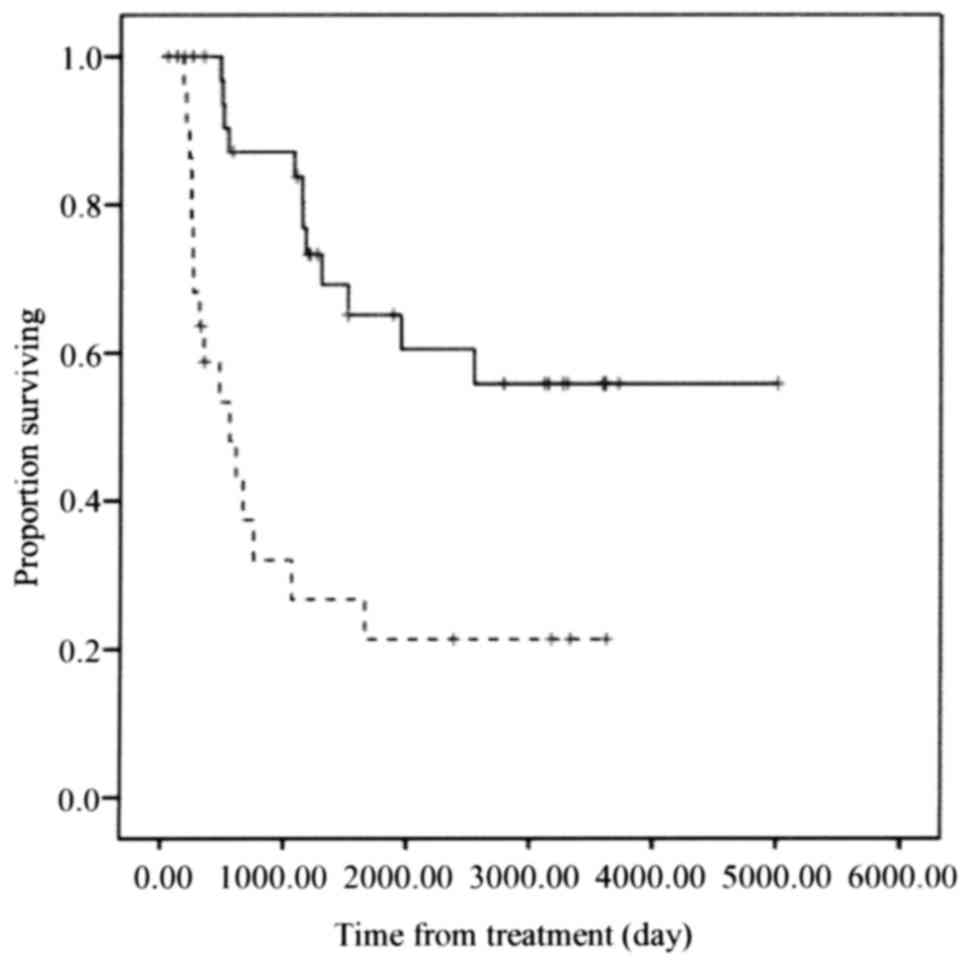
The NAC effective group exhibited significantly
improved overall survival compared with that of the NAC ineffective
group (P<0.001; Fig. 3).
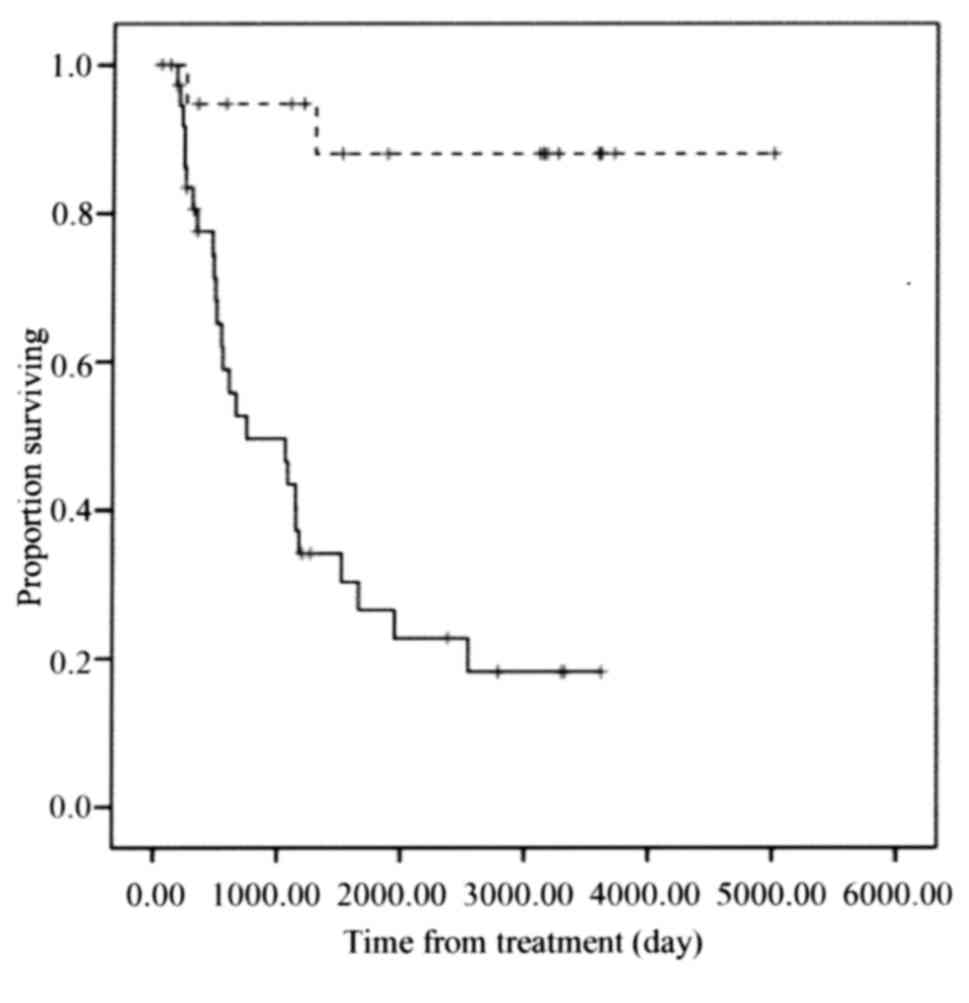
Furthermore, the low UCP2 expression group exhibited significantly
improved overall survival compared with that of the high UCP2
expression group (P<0.001; Fig.
4).
Inhibition of UCP2 by genipin enhances
the sensitivity of cervical cancer cells to cisplatin
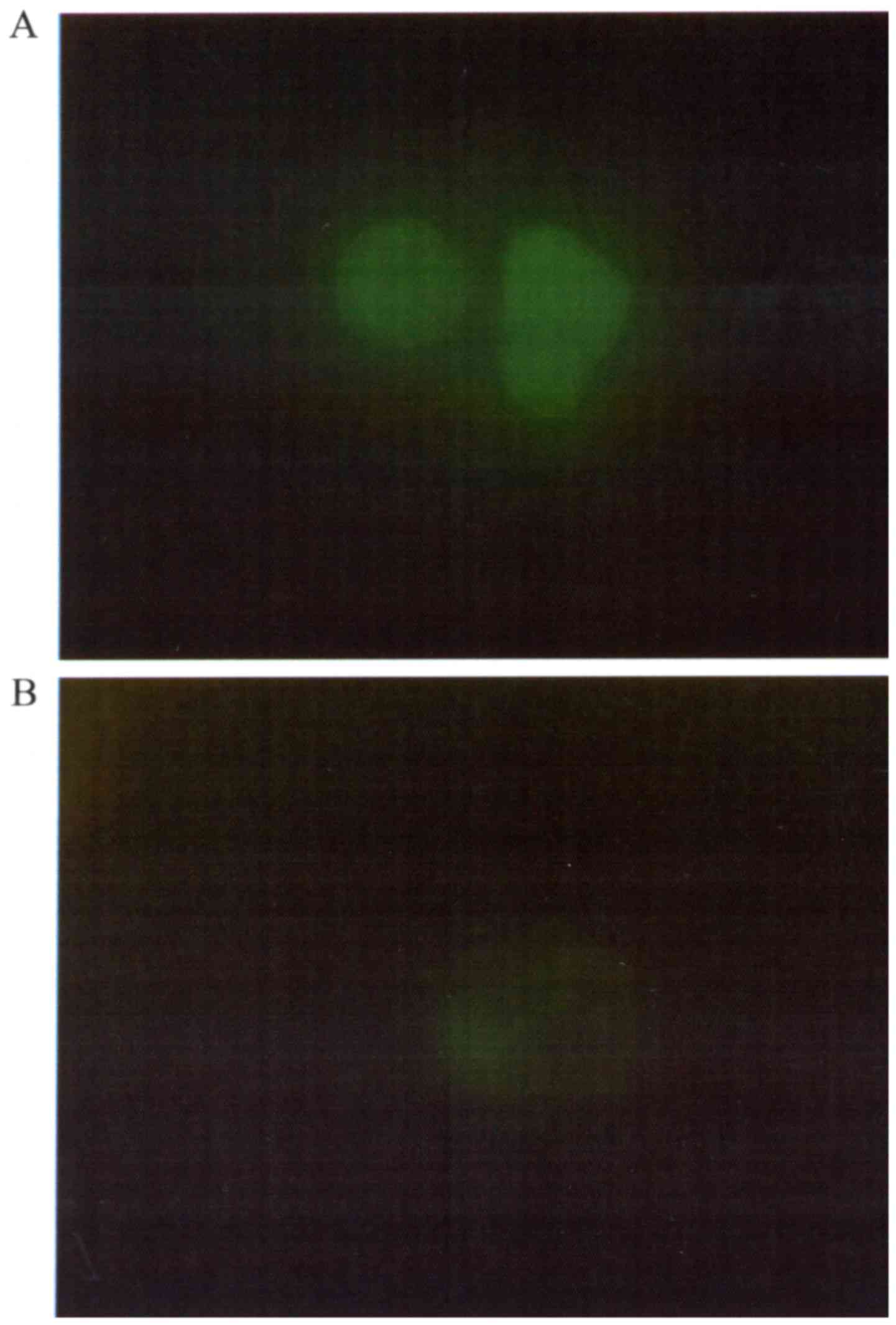
The expression of UCP2 protein in the uterine
cervical cancer cell line Ca Ski was confirmed by
immunofluorescence analysis. UCP2 protein expression in Ca Ski
cells was almost completely eliminated following 24 h of incubation
with 1 µM genipin (Fig. 5A and B).
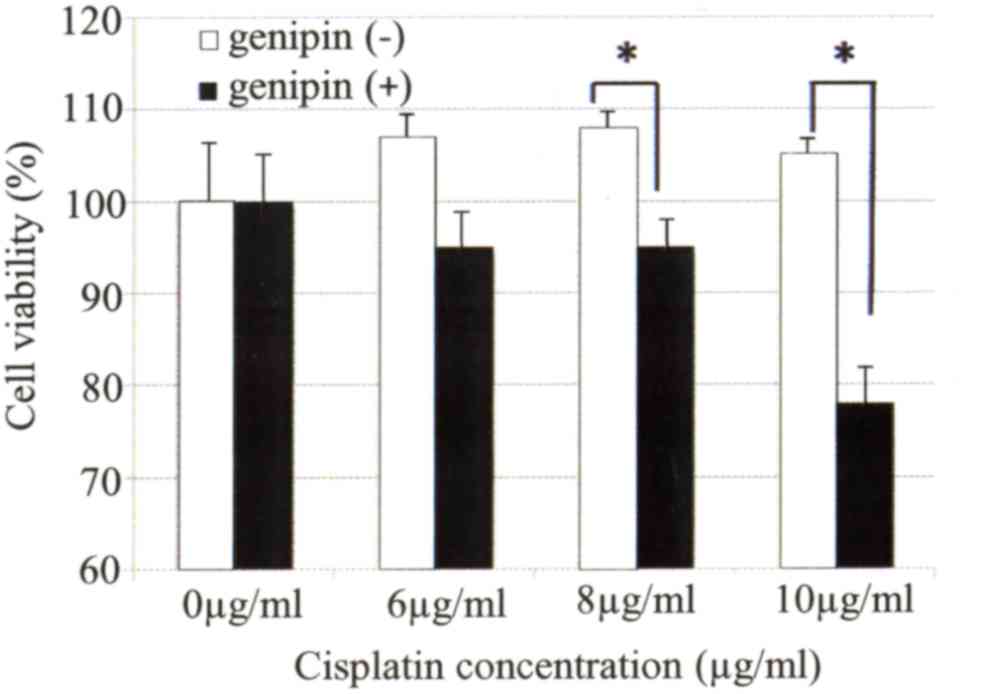
Next, it was examined whether the sensitivity of uterine cervical
cancer cells to cisplatin was affected by treatment with genipin.
Genipin-mediated inhibition of UCP2 expression in Ca Ski cells
significantly enhanced their sensitivity to cisplatin (Fig. 6).
Discussion
CCRT is regularly recommended for patients with
locally advanced uterine cervical cancer. However, effective NAC
can reduce tumor size, thus facilitating hysterectomy for locally
advanced uterine cervical cancer (7).
Such treatment can improve the prognosis of patients with locally
advanced cervical cancer (7).
However, NAC is ineffective in certain cases, leaving radiotherapy
as the only treatment option, and thereby delaying the initiation
of the core treatment and resulting in worse prognosis (10,11).
Therefore, the identification of prognostic factors that indicate
the likely efficacy of NAC for patients with locally advanced
uterine cervical cancer should improve patient prognosis.
UCPs are mitochondrial anion transporters, and
mitochondrial uncoupling reduces the production of ROS (18,19). UCP2
is broadly expressed in cancer cells, and the expression of UCP2 is
associated with ROS levels in various tissues (23,24).
Cancer cells reduce ROS production through the expression of UCP2;
thus, high expression of UCP2 may protect cells from oxidative
stresses and cell damage (30,33). UCP2
contributes to both carcinogenesis and chemoresistance (28,29).
Additionally, overexpression of UCP2 in cancer cells facilitates
resistance to gemcitabine, while downregulation of UCP2 results in
significantly increased cell death following chemotherapy (34).
The current study demonstrates a significant
correlation between UCP2 expression and NAC effectiveness in
patients with locally advanced uterine cervical cancer. Patients
with low UCP2 expression tended to be sensitive to NAC, and were
able to undergo surgery following NAC. The overall survival time
was significantly longer in the NAC effective group compared with
that in the NAC ineffective group. Similarly, the overall survival
time was significantly longer in the low UCP2 expression group
compared with that in the high UCP2 expression group.
These results suggest that UCP2 expression levels in
patients with locally advanced uterine cervical cancer are
associated with the likely effectiveness of NAC. Therefore, UCP2
represents a potential predictive marker of whether NAC is likely
to be effective in patients with locally advanced uterine cervical
cancer.
The present study revealed that the proliferation of
Ca Ski cells was suppressed by the addition of genipin following
chemotherapy. This is consistent with previous reports using other
cancer cells (24,28,29). The
present study is the first to report a correlation between UCP2
expression and NAC efficacy for locally advanced uterine cervical
cancer.
In summary, UCP2 expression may become a predictive
marker of whether NAC is effective for patients with locally
advanced uterine cervical cancer. Such knowledge could be helpful
for improving the prognosis of patients with locally advanced
uterine cervical cancer.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ebina Y, Yaegashi N, Katabuchi H, Nagase
S, Udagawa Y, Hachisuga T, Saito T, Mikami M, Aoki Y and Yoshikawa
H: Japan Society of Gynecologic Oncology guidelines 2011 for the
treatment of uterine cervical cancer. Int J Clin Oncol. 20:240–248.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National comprehensive cancer network, .
NCCN clinical practice guidelines in oncology-cervical
cancer-version II. 2013.
|
|
5
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eifel PJ, Winter K, Morris M, Levenback C,
Grigsby PW, Cooper J, Rotman M, Gershenson D and Mutch DG: Pelvic
irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: An update of
radiation therapy oncology group trial (RTOG) 90–01. J Clin Oncol.
22:872–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishiko O, Sumi T, Yasui T, Matsumoto Y,
Kawamura N, Ogita S, Kamino T, Nakamura K and Yamada R:
Balloon-occluded arterial infusion chemotherapy, simple total
hysterectomy, and radiotherapy as a useful combination-therapy for
advanced cancer of the uterine cervix. Oncol Rep. 7:141–144.
2000.PubMed/NCBI
|
|
8
|
Souhami L, Gil RA, Allan SE, Canary PC,
Araújo CM, Pinto LH and Silveira TR: A randomized trial of
chemotherapy followed by pelvic radiation therapy in stage IIIB
carcinoma of the cervix. J Clin Oncol. 9:970–977. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tattersall MH, Lorvidhaya V, Vootiprux V,
Cheirsilpa A, Wong F, Azhar T, Lee HP, Kang SB, Manalo A, Yen MS,
et al: Randomized trial of epirubicin and cisplatin chemotherapy
followed by pelvic radiation in locally advanced cervical cancer.
Cervical cancer study group of the Asian Oceanian clinical oncology
association. J Clin Oncol. 13:444–451. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishiko O, Sumi T, Yasui T, Matsumoto Y,
Ogita S, Kaminou T, Nakamura K and Yamada R: Tumor marker and MR
imaging criteria for evaluating the efficacy of cyclic
balloon-occluded arterial infusion for advanced cancer of the
uterine cervix. Oncol Rep. 7:827–830. 2000.PubMed/NCBI
|
|
11
|
Ishiko O, Sumi T, Yoshida H, Ogita S and
Yamada R: Expression of apoptosis regulatory proteins in advanced
cancer of the uterine cervix after cyclic balloon-occluded arterial
infusion chemotherapy. Int J Oncol. 18:1151–1155. 2001.PubMed/NCBI
|
|
12
|
Okamoto E, Sumi T, Misugi F, Nobeyama H,
Hattori K, Yoshida H, Matsumoto Y, Yasui T, Honda K and Ishiko O:
Expression of apoptosis-related proteins in advanced uterine
cervical cancer after balloon-occluded arterial infusion
chemotherapy as an indicator of the efficiency of this therapy. Int
J Mol Med. 15:41–47. 2005.PubMed/NCBI
|
|
13
|
Nobeyama H, Sumi T, Misugi F, Okamoto E,
Hattori K, Matsumoto Y, Yasui T, Honda K, Iwai K and Ishiko O:
Association of HPV infection with prognosis after neoadjuvant
chemotherapy in advanced uterine cervical cancer. Int J Mol Med.
14:101–105. 2004.PubMed/NCBI
|
|
14
|
Panici P Benedetti, Bellati F, Manci N,
Pernice M, Plotti F, Di Donato V, Calcagno M, Zullo MA, Muzii L and
Angioli R: Neoadjuvant chemotherapy followed by radical surgery in
patients affected by FIGO stage IVA cervical cancer. Ann Surg
Oncol. 14:2643–2648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pelicano H, Carney D and Huang P: ROS
stress in cancer cells and therapeutic implications. Drug Resist
Updat. 7:97–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alexandre J, Batteux F, Nicco C, Chéreau
C, Laurent A, Guillevin L, Weill B and Goldwasser F: Accumulation
of hydrogen peroxide is an early and crucial step for
paclitaxel-induced cancer cell death both in vitro and in vivo. Int
J Cancer. 119:41–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fruehauf JP and Meyskens FL Jr: Reactive
oxygen species: A breath of life or death? Clin Cancer Res.
13:789–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boss O, Muzzin P and Giacobino JP: The
uncoupling proteins, a review. Eur J Endocrinol. 139:1–9. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fleury C and Sanchis D: The mitochondrial
uncoupling protein-2: Current status. Int J Biochem Cell Biol.
31:1261–1278. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baffy G: Uncoupling protein-2 and cancer.
Mitochondrion. 10:243–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Echtay KS, Murphy MP, Smith RA, Talbot DA
and Brand MD: Superoxide activates mitochondrial uncoupling protein
2 from the matrix side. Studies using targeted antioxidants. J Biol
Chem. 277:47129–47135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoang T, Smith MD and Jelokhani-Niaraki M:
Toward understanding the mechanism of ion transport activity of
neuronal uncoupling proteins UCP2, UCP4, and UCP5. Biochemistry.
51:4004–4014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carretero MV, Torres L, Latasa U,
Garcia-Trevijano ER, Prieto J, Mato JM and Avila MA: Transformed
but not normal hepatocytes express UCP2. FEBS Lett. 439:55–58.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horimoto M, Resnick MB, Konkin TA,
Routhier J, Wands JR and Baffy G: Expression of uncoupling
protein-2 in human colon cancer. Clin Cancer Res. 10:6203–6207.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duval C, Nègre-Salvayre A, Dogilo A,
Salvayre R, Pénicaud L and Casteilla L: Increased reactive oxygen
species production with antisense oligonucleotides directed against
uncoupling protein 2 in murine endothelial cells. Biochem Cell
Biol. 80:757–764. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mattiasson G, Shamloo M, Gido G, Mathi K,
Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T,
Gonzalez-Zulueta M, et al: Uncoupling protein-2 prevents neuronal
death and diminishes brain dysfunction after stroke and brain
trauma. Nat Med. 9:1062–1068. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teshima Y, Akao M, Jones SP and Marbán E:
Uncoupling protein-2 overexpression inhibits mitochondrial death
pathway in cardiomyocytes. Circ Res. 93:192–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pozza E Dalla, Fiorini C, Dando I,
Menegazzi M, Sgarbossa A, Costanzo C, Palmieri M and Donadelli M:
Role of mitochondrial uncoupling protein 2 in cancer cell
resistance to gemcitabine. Biochim Biophys Acta. 1823:1856–1863.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mailloux RJ, Adjeitey CN and Harper ME:
Genipin-induced inhibition of uncoupling protein-2 sensitizes
drug-resistant cancer cells to cytotoxic agents. PLoS One.
5:e132892010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Derdák Z, Fülöp P, Sabo E, Tavares R,
Berthiaume EP, Resnick MB, Paragh G, Wands JR and Baffy G: Enhanced
colon tumor induction in uncoupling protein-2 deficient mice is
associated with NF-kappaB activation and oxidative stress.
Carcinogenesis. 27:956–961. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsuji K, Yamada R, Kawabata M, Mitsuzane
K, Sato M, Iwahashi M, Kitayama S and Nakano R: Effect of balloon
occluded arterial infusion of anticancer drugs on the prognosis of
cervical cancer treated with radiation therapy. Int J Radiat Oncol
Biol Phys. 32:1337–1345. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: Bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
33
|
Collins P, Jones C, Choudhury S, Damelin L
and Hodgson H: Increased expression of uncoupling protein 2 in
HepG2 cells attenuates oxidative damage and apoptosis. Liver Int.
25:880–887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pozza E Dalla, Fiorini C, Dando I,
Menegazzi M, Sgarbossa A, Costanzo C, Palmieri M and Donadelli M:
Role of mitochondrial uncoupling protein 2 in cancer cell
resistance to gemcitabine. Biochim Biophy Acta. 1823:1856–1863.
2012. View Article : Google Scholar
|