Introduction
Testicular germ cell tumors (TGCTs) are relatively
rare, representing ~1-1.5% of all malignancies diagnosed in males
and ~5% of urological tumors in males in general (1). However, TGCTs are the most frequently
observed solid tumor among men aged between 15 and 35 years old
(2). Additionally, it has been
reported that the incidence of TGCTs has increased over the last
30–40 years (3). Despite this, the
5-year disease-free survival of patients with TGCTs is the highest
amongst any other solid malignancy in males due to effective modern
therapy, including platinum-based combination chemotherapy
regimens, and close surveillance following surgery. In addition,
the cure rate for patients with TGCTs is 95–96% (4,5). However,
~5% of patients with TGCTs still develop treatment resistance
(6). Therefore, a better
understanding of the pathogenesis of TGCTs is important in order to
develop novel treatments.
The functional role of axis inhibition protein 1
(AXIN1), a multi-domain scaffold protein, has been associated with
the tumorigenesis and progression of a number of diseases,
including hepatitis B virus-related hepatocellular carcinoma and
gastrointestinal cancer (7–10). AXIN1 acts as a tumor suppressor, and
thus mutations of AXIN1 serve a significant role in
carcinogenesis (8,11). Additionally, in combination with
several different protein complexes AXIN1 has been reported to be
involved in the Wnt, transforming growth factor (TGF)-β, stress
activated protein kinase JNK1 (JNK) and cellular tumor antigen p53
(p53) signaling pathways (12–15).
Furthermore, activation of the Wnt/β-catenin signaling pathway in
human germ cell tumors has been reported (16), a cancer that AXIN1 mutation has
been associated with (17). However,
little information is available regarding the association between
the functional role of AXIN1 and the phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR)
signaling pathway in TGCTs.
The aim of the present study was to determine the
functional role of AXIN1 in TGCTs. Furthermore, whether AXIN1
functions through the PI3K/AKT/mTOR signaling pathway was
investigated. The results of the current study may contribute to
the identification and development of novel drugs for the treatment
of TGCTs.
Materials and methods
Cell lines and culture
The human embryonal carcinoma (EC)-derived cell line
NTera2 was purchased from the Shanghai Institute of Biochemistry
and Cell Biology of the Chinese Academy of Sciences (Shanghai,
China). Cells were maintained in Dulbecco's modified Eagle's medium
(Sigma; Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal
calf serum (BD Biosciences, San Jose, CA, USA), 58.5 mg/ml
L-Glutamine (Gibco), 100 U/ml penicillin and 100 mg/ml streptomycin
(all from Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C
in an incubator with 5% CO2.
Transfection
When the cells reached 70% confluence, they were
subjected to transfection with small interfering RNA (siRNA) or an
expression construct using Lipofectamine® 2000 reagent
(1×106 cells/well) (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. AXIN1 was
knocked down using a siRNA (siAXIN1) and a scrambled siRNA
(siControl) was used as a control. The sequence of siRNAs was
listed as following: siAXIN1, 5′GGG AUA AGC CUG UUC AGG ATT3′, and
siControl, 5′AAA ATC GAC TCG TTT TTG CTC3′. The target sequences
were designed and constructed by Shanghai GenePharma Co., Ltd.
(Shanghai, China). In addition, an AXIN1 expression vector
(pcDNA3.1-AXIN1) was constructed and confirmed by sequencing
(7). As a control, the empty
construct pcDNA3.1 was transfected into NTera2 cells. For the cells
simultaneously transfected with siAXIN1 and pcDNA3.1-AXIN1, the
cells simultaneously transfected with scrambled siRNA and empty
construct were considered as a control.
MTT cell viability assay
The survival of NTera2 cells was determined using
the MTT colorimetric assay. Briefly, cells were collected, washed
with PBS seeded into a 96-well plate (1×106 cells/well)
and incubated at 37°C. After 24, 48, 72 and 96 h incubation, 10 µl
MTT was added to each well, and the cells were cultured for a
further 4 h at 37°C. The absorbance at 595 nm was measured with a
Synergy™ 4 Hybrid Microplate Reader (BioTek Instruments, Inc.,
Winooski, VT, USA).
Apoptosis assay
Cell apoptosis was assessed using an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol. Briefly, the cells were harvested
(2×105 cells/well), washed with PBS and re-suspended
with binding buffer. Subsequently, the mixture was incubated with 5
µl Annexin V-FITC and 5 µl propidium iodide (PI). After incubation
at room temperature in the dark for 15 min, the cells were analyzed
with a flow cytometer (BD LSR Flow Cytometer; BD Biosciences). The
collected data were analyzed using CellQuest™ software (version
3.0; BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
AXIN1 mRNA levels in NTera2 cells transfected with
pcDNA3.1-AXIN1 or siAXIN1 for 48 h was determined using RT-qPCR
analysis. Total RNA was extracted with the TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. First strand 5 µl complementary (c)DNA was
subjected to real-time PCR reaction using an ABI PRISM 7700 System
and TaqMan reagents (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol (18). GAPDH mRNA was used as a reference. The
sequence of mRNA was listed as following: AXIN1; forward,
5′AAC ACA TGG TCA TGC CAA GC3′; reverse, 5′TTC TCA GCG TCC TCT GTG
G3′ (19); and GAPDH; forward,
5′TCC TGC ACC ACC AAC TGC TTAG3′; reverse, 5′AGT GGC AGT GAT GGC
ATG GAC T3′. The PCR thermocycler conditions included: initial
denaturation was performed at 96°C for 15 sec, followed by 30
cycles of denaturation at 97°C for 15 sec, annealing at 62°C for 5
sec, extension at 72°C for 50 sec and the final extension was at
72°C for 10 min. Gene expression was quantified using the
2−ΔΔCq method (20).
Western blotting
A total of 24 h after transfection, protein was
extracted from the cells by using a radioimmunoprecipitation lysis
extraction kit (Thermo Fisher Scientific, Inc.). The protein
concentration was measured using a BCA Protein Assay kit (Pierce;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. A total of 20 µg per lane of protein samples were
resolved on a 12% gel using SDS-PAGE and blotted onto
polyvinylidene difluoride membranes. The resulting membranes were
blocked in 5% milk in 0.1% Tris-buffered saline-Tween for 2 h.
Subsequently, the membranes were treated with the following primary
monoclonal antibodies overnight at 4°C: Anti-AXIN1 (cat. no.
ab131372; dilution, 1:1,000; Abcam, Cambridge, UK), anti-apoptosis
regulator Bax (Bax) (cat. no. sc-65,532; dilution, 1:1,000),
anti-B-cell lymphoma (Bcl)-2 (cat. no. sc-509; dilution, 1:1,000),
anti-phosphorylated (p)-mTOR (cat. no. sc-293132; dilution,
1:1,000), anti-mTOR (cat. no. sc-400140; dilution, 1:1,000),
anti-p-AKT (cat. no. sc-7985-R; dilution, 1:1,000), anti-AKT (cat.
no. sc-24500; dilution, 1:1,000), anti-P-70S ribosomal protein S6
(S6) (cat. no. sc-8416; dilution, 1:1,000), or anti-S6 (cat. no.
sc-9027; dilution, 1:1,000). GAPDH (cat. no. sc-293335; dilution,
1:1,000) was used as the internal control. Then the appropriate
goat anti-rabbit IgG (cat. no. sc-2004; dilution, 1:10,000) or goat
anti-mouse IgG (cat. no. sc-2005; dilution, 1:10,000) were used for
2 h at room temperature. All antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA) except anti-AXIN1.
Immunoreactive protein bands were detected by enhanced
chemiluminescence western blotting substrate (Pierce; Thermo
Fischer Scientific, Inc.).
Statistical analysis
All experiments were performed ≥2 times. The data
are expressed as the mean ± standard deviation. Data analysis was
carried out SPSS software (version 16.0; SPSS, Inc., Chicago, IL,
USA). One-way analysis of variance followed by Tukey post hoc test
was performed to calculate the P-values. P<0.05 was considered
to indicate a statistically significant difference.
Results
Confirming the results of siRNA and
construct transfection on AXIN1 expression
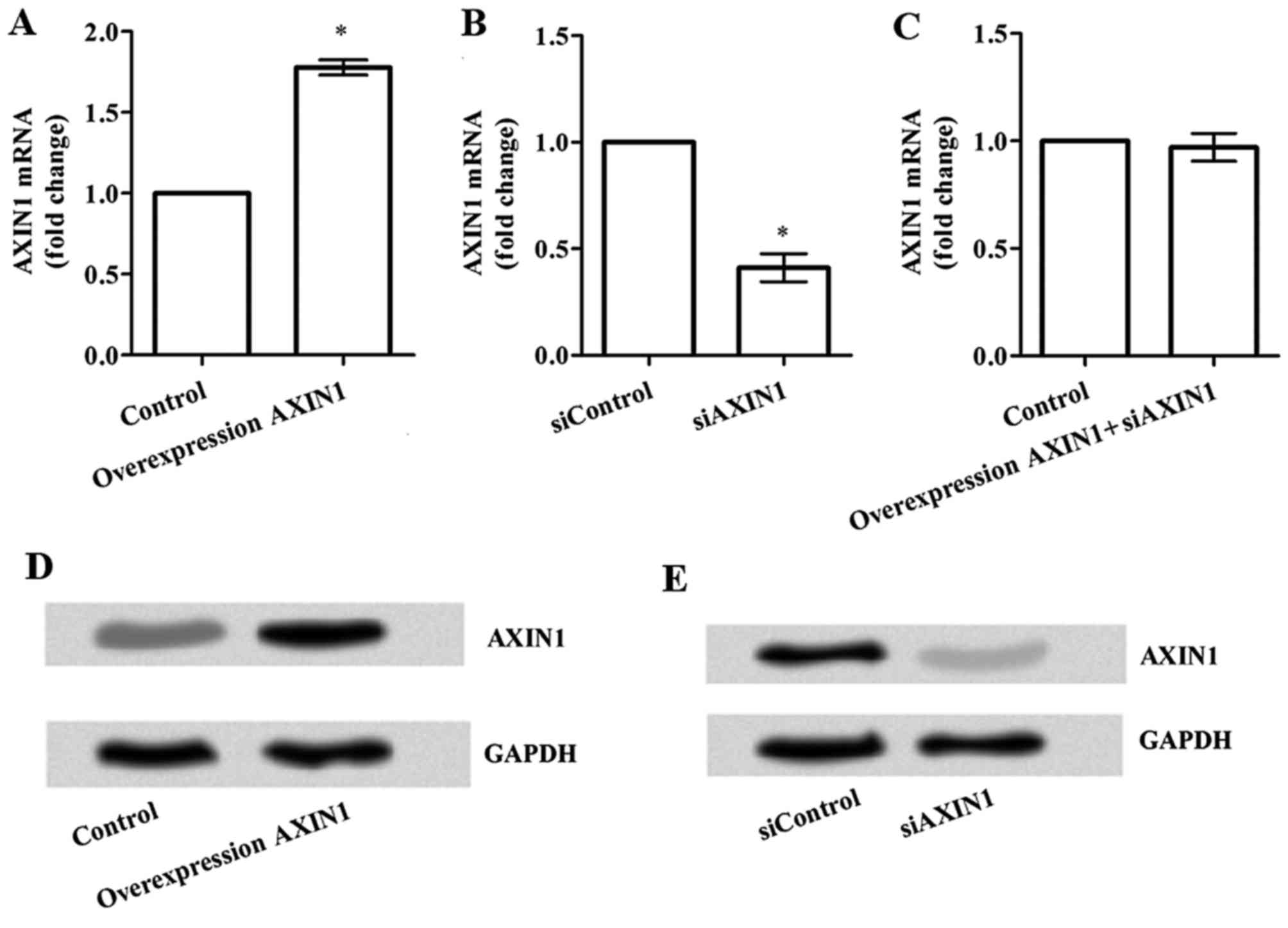
To explore the effects of transfection on AXIN1
expression, an AXIN1 expression vector (pcDNA3.1-AXIN1) and
AXIN1-specific siRNA were constructed. As illustrated in Fig. 1A, the mRNA expression of AXIN1
was significantly increased following transfection with
pcDNA3.1-AXIN1 compared with the control group (P<0.05). By
contrast, the mRNA expression levels of AXIN1 were
significantly lower in the siAXIN1 group compared with the
siControl group (P<0.05; Fig. 1B).
However, no significant difference was observed in AXIN1
mRNA expression following simultaneous transfection with
pcDNA3.1-AXIN1+siAXIN1 compared with the control group (Fig. 1C). Western blots for the protein level
of AXIN1 revealed similar results (Fig.
1D and E).
Effect of AXIN1 overexpression and
silencing on TGCT cell viability
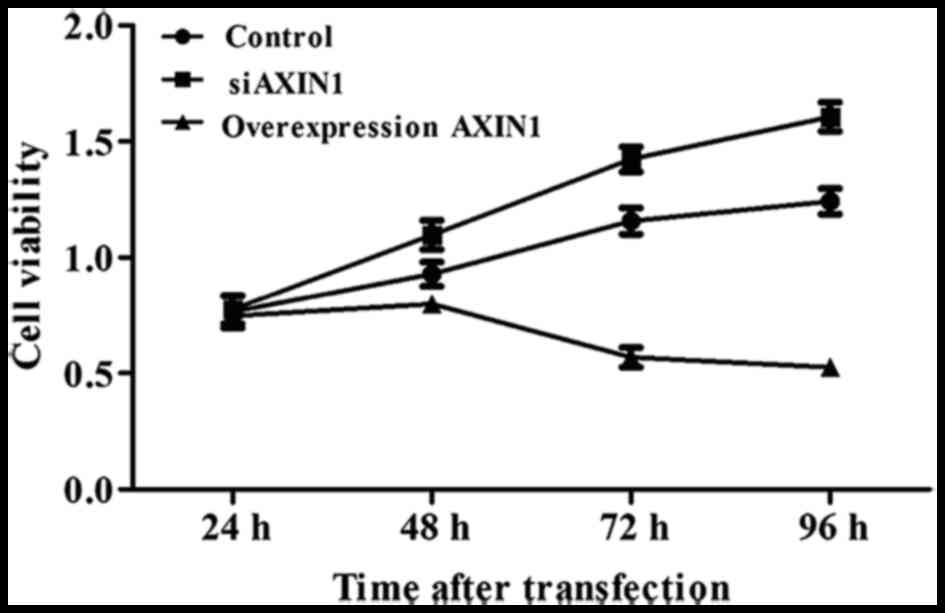
To explore the effects of AXIN1 overexpression and
silencing on TGCT cell viability, an MTT assay was performed. A
total of 24 h after transfection, cell viability was significantly
increased by in the group transfected with siAXIN1 and markedly
decreased in the group transfected with pcDNA3.1-AXIN1 compared
with the control group of cells simultaneously transfected with
scrambled siRNA and empty construct (both P<0.05; Fig. 2), indicating that AXIN1 acts as a
tumor suppressor in TGCTs.
Effect of AXIN1 overexpression and
silencing on TGCT cell apoptosis
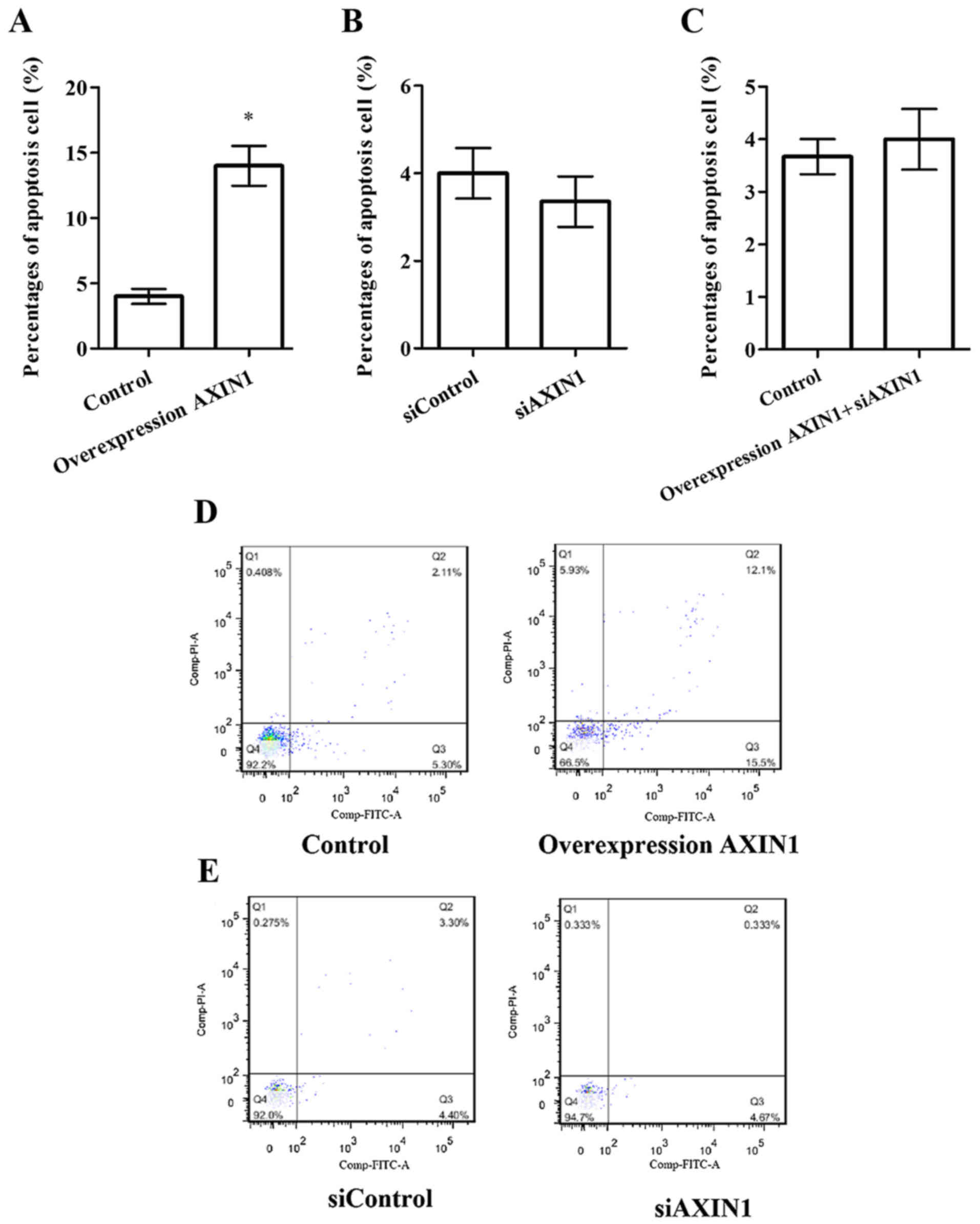
Flow cytometry was performed to explore the effects
of AXIN1 overexpression and silencing on TGCT cell apoptosis
(Fig. 3). There was no significant
difference in the percentage of apoptosis between the siAXIN1
(Fig. 3B) or pcDNA3.1-AXIN1+siAXIN1
(Fig. 3C) groups compared with their
respective controls. However, the percentage of apoptosis was
significantly increased in the pcDNA3.1-AXIN1 group compared with
the control group (Fig. 3A;
P<0.05), suggesting that overexpression of AXIN1 induces TGCT
cell apoptosis.
Effect of AXIN1 overexpression and
silencing on the expression of Bax and Bcl-2 protein in TGCT
cells
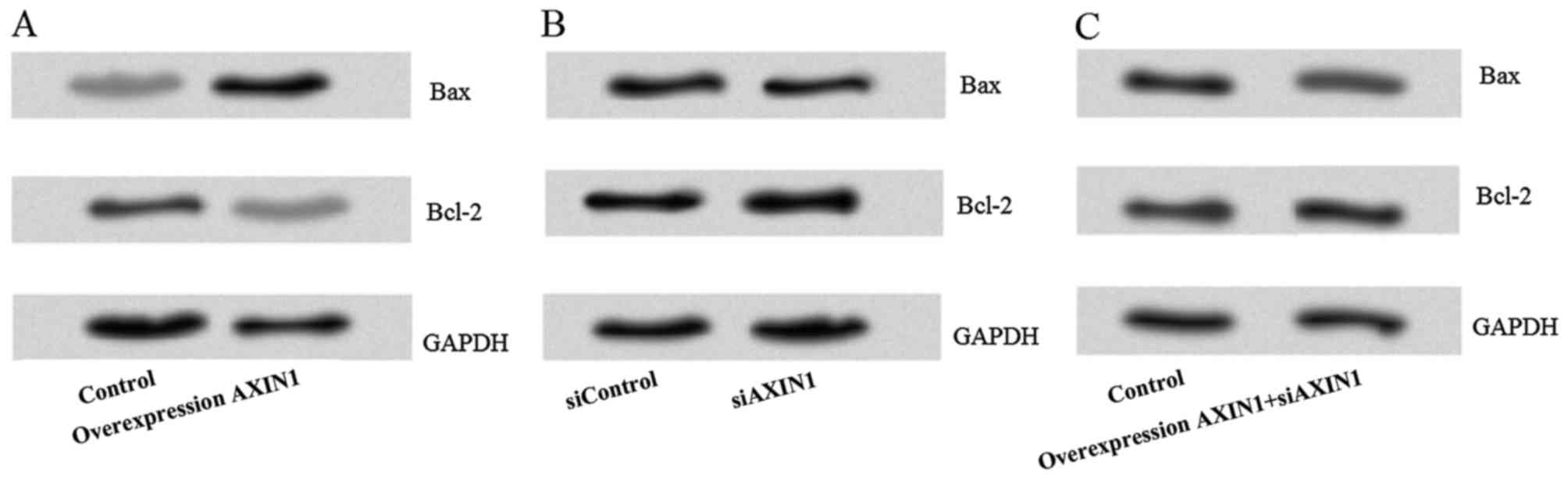
To explore the potential of AXIN1
overexpression-induced TGCT cell apoptosis, the levels of the
apoptosis-associated proteins Bax and Bcl-2 in NTera2 cells
transfected with siAXIN1 and/or pcDNA3.1-AXIN1 were measured. This
revealed that the expression of Bax was markedly increased and the
expression of Bcl-2 was markedly decreased after transfection with
pcDNA3.1-AXIN1 compared with the control group (Fig. 4A). However, there was no notable
difference in the protein levels of Bax or Bcl-2 in the siAXIN1
(Fig. 4B) or pcDNA3.1-AXIN1+siAXIN1
(Fig. 4C) groups compared with their
respective controls.
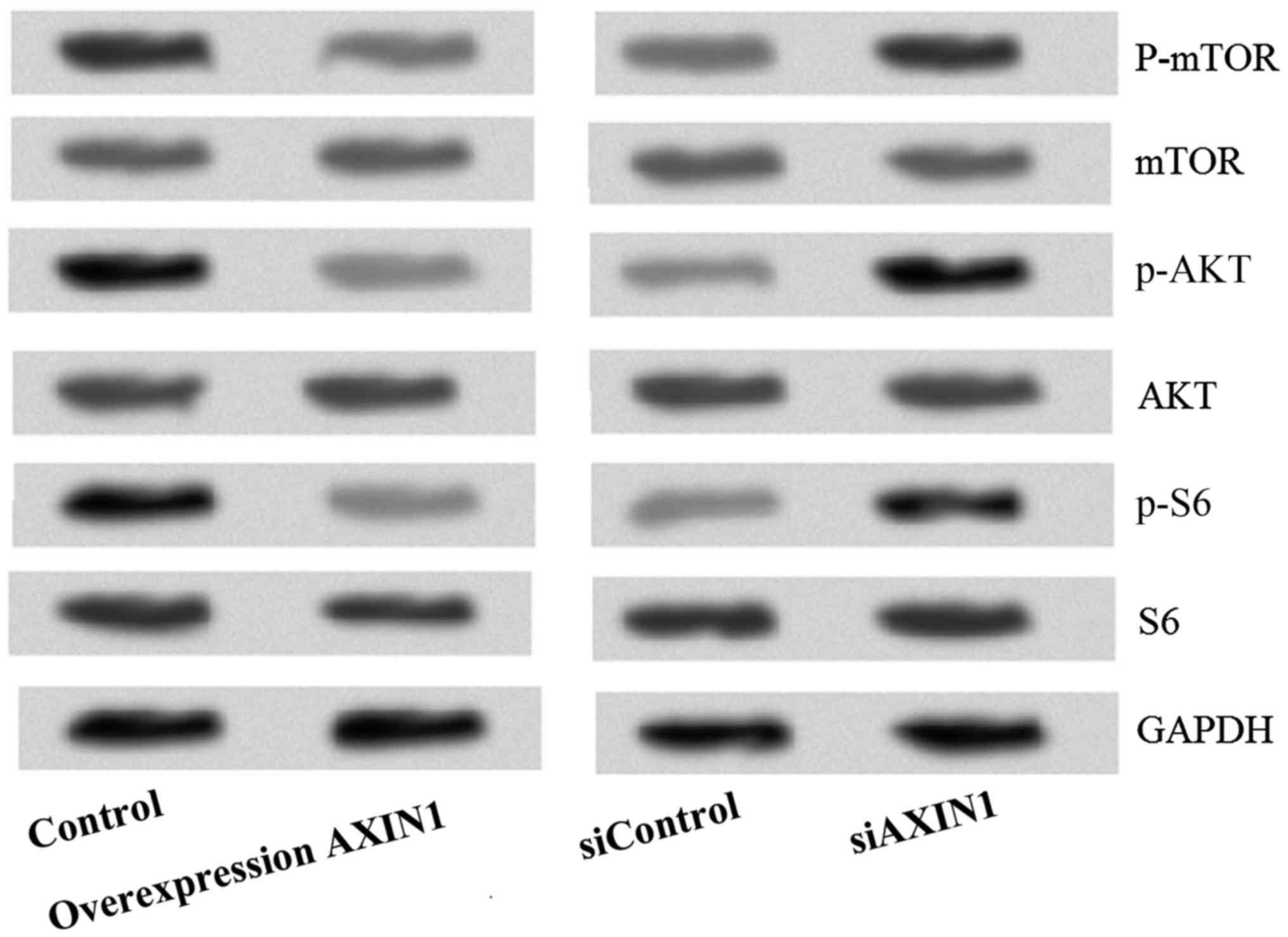
Effect of AXIN1 overexpression and
silencing on the PI3K/AKT/mTOR signaling pathway in TGCT cells
To investigate the potential signaling pathway
through which AXIN1 overexpression was inducing TGCT cell
apoptosis, the levels of PI3K/AKT/mTOR signaling pathway proteins
(p-mTOR, mTOR, p-AKT, AKT, p-S6 and S6) was investigated (Fig. 5). The results demonstrated that the
protein levels of p-AKT, P-mTOR and p-S6 were markedly reduced
following transfection with pcDNA3.1-AXIN1, indicating that the
PI3K/AKT/mTOR signaling pathway is inhibited by AXIN1
overexpression.
Discussion
In the present study, AXIN1 was confirmed to be a
candidate tumor suppressor gene in TGCTs. Overexpression of AXIN1
could inhibit TGCT cell viability and induce human EC-derived
NTera2 cell apoptosis through increasing the expression of
proapoptotic Bax protein, while decreasing the expression of
antiapoptotic Bcl-2 protein. The PI3K/Akt/mTOR signaling pathway
was also demonstrated to serve an important role in AXIN1
overexpression-induced TGCT cell apoptosis. These results suggest
that AXIN1 is a potential target for gene therapy in
TGCTs.
AXIN1 was previously identified to be a product of
the mouse Fused locus, and is mapped to human chromosome
16p13.3 with 87% similarity to the mouse protein (21). AXIN1 is associated with regulating
axis formation during embryonic development (21). Two naturally occurring splicing
variants of AXIN1, variant 1 (AXIN1V1) and variant 2 (AXIN1V2) have
been reported. AXIN1V1 encodes an 862-amino acid (AA)-long
polypeptide, whereas AXIN1V2 is a shorter form of AXIN that lacks
36 AAs from exon 8 (8). AXIN1 serves
as a scaffold protein through interacting with numerous proteins,
enabling it to facilitate the degradation β-catenin, which suggests
that it has a tumor suppressor function (22). Several signaling pathways, including
that of Wnt, TGF-β, JNK1 and p53, have been reported to be
associated with AXIN1 (23–25). A previous study suggested that AXIN1
mutation may be associated with germ cell tumors (17). However, there is little information
with respect to the effect of AXIN1 overexpression, rather than
mutation, on TGCTs.
One of the mechanisms underlying tumorigenesis is
the activation of essential cellular signaling pathways. The
essential and well-studied PI3K/AKT/mTOR signaling pathway serves
important roles in tumorigenesis (26,27). This
signaling pathway contributes to cell proliferation,
differentiation, metabolism, cytoskeletal reorganization and
apoptosis (28). Activation of the
PI3K/AKT/mTOR signaling pathway promotes cancer cell survival and
therapy resistance (29–31). Following activation of the
PI3K/AKT/mTOR signaling pathway by membrane tyrosine kinase growth
factor receptors or G protein-coupled receptors (32,33),
functional PI3K is translocated to the plasma membrane where it
causes the phosphorylation of phosphatidylinositol
3,4,5-triphosphate (PIP3) (33).
Thereafter, p-PIP3 recruits 3′-phosphoinositide-dependent kinase 1
(PDK1) and AKT (34), leading to the
phosphorylation and activation of AKT. Phosphorylation of AKT
increases cell survival by inactivating proapoptotic factors,
including Bax (34,35). Subsequently, p-AKT activates several
downstream targets, including those in the mTOR signaling pathway.
Activation of mTOR leads to an increase in protein synthesis,
including that of certain proteins that are associated with the
pathogenesis of a number of tumors, such as cyclin D1 (36). Additionally, activated mTOR can
directly phosphorylate PDK1 and activate ribosomal protein S6
kinase b-1 (P70S6K). P70S6K initiates the
ribosomal translation of mRNA into proteins that is essential for
cell growth, progression and metabolism by phosphorylating S6.
Thus, activation of PI3K/AKT/mTOR signaling pathway is a key event
in tumorigenesis.
Since AXIN1 may act as a tumor suppressor in TGCTs,
the theory that this this protective effect may be through
inhibition of the PI3K/AKT/mTOR signaling pathway was investigated.
To confirm this hypothesis, transfection techniques were utilized
to dysregulate the expression of AXIN1 in NTera2 cells. The effects
of transfection on AXIN1 expression were confirmed by RT-qPCR and
western blotting. The effects of AXIN1 dysregulation on NTera2 cell
viability and apoptosis were then examined. The results showed that
NTera2 cell viability was significantly increased by knockdown of
AXIN1 and markedly decreased by overexpression of AXIN1, further
suggesting that AXIN1 functions as a tumor suppressor in TGCTs. In
addition, the percentage of apoptotic cells was significantly
increased by overexpression of AXIN1. This result was in line with
those of previous studies, which also suggested that AXIN1 could
significantly induce cancer cell apoptosis (11,37). Next,
the potential mechanism of AXIN1 overexpression-induced apoptosis
was explored. Overexpression of AXIN1 markedly increased Bax
protein expression and markedly decreased Bcl-2 protein expression.
One explanation for this effect is that AXIN1 acts of AKT to
inhibit the PI3K/AKT/mTOR signaling pathway, decreasing
apoptosis-associated protein expression and apoptosis.
The levels of PI3K/AKT/mTOR signaling pathway
proteins were assessed in the present study. This demonstrated that
the protein levels of p-AKT, p-mTOR and p-S6 were markedly reduced
by overexpression of AXIN1, indicating that the PI3K/AKT/mTOR
signaling pathway is inhibited by overexpression of AXIN1. Arnold
et al (38) suggested that
AXIN1 could negatively regulate proto-oncogene c-Myc protein
expression at a post-translational level, thus acting a tumor
suppressor (38). However, in the
present study it remains unclear whether AXIN1 regulates or
coordinates with oncoproteins to exert antitumor activity.
In conclusion, the results of the present study
confirm that AXIN1 is a candidate tumor suppressor gene in TGCTs
and indicate that it exerts this effect through inhibiting the
PI3K/AKT/mTOR signaling pathway. This suggests that AXIN1 is a
potential target for gene therapy in TGCTs. However, further
research is required to determine the mechanism through which AXIN1
regulates the PI3K/AKT/mTOR signaling pathway in cancer.
References
|
1
|
Chia VM, Quraishi SM, Devesa SS, Purdue
MP, Cook MB and McGlynn KA: International trends in the incidence
of testicular cancer, 1973–2002. Cancer Epidemiol Biomarkers Prev.
19:1151–1159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vasdev N, Moon A and Thorpe AC:
Classification, epidemiology and therapies for testicular germ cell
tumours. Int J Dev Biol. 57:133–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Purdue MP, Devesa SS, Sigurdson AJ and
McGlynn KA: International patterns and trends in testis cancer
incidence. Int J Cancer. 115:822–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ries LAG, Young JL Jr, Keel GE, Eisner MP,
Lin YD and Horner M-JD: SEER Survival Monograph: Cancer Survival
Among Adults: U.S. SEER Program, 1988–2001. Patient and Tumor
CharacteristicsNational Cancer Institute, SEER Program, NIH Pub.
No. 07-6215. Bethesda, MD: 2007
|
|
5
|
Hayes-Lattin B and Nichols CR: Testicular
cancer: A prototypic tumor of young adults. Semin Oncol.
36:432–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raghavan D: Testicular cancer: Maintaining
the high cure rate. Oncology (Williston Park). 17:218–229, 234-235.
2003.PubMed/NCBI
|
|
7
|
Li J, Quan H, Liu Q, Si Z, He Z and Qi H:
Alterations of axis inhibition protein 1 (AXIN1) in hepatitis B
virus-related hepatocellular carcinoma and overexpression of AXIN1
induces apoptosis in hepatocellular cancer cells. Oncol Res.
20:281–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salahshor S and Woodgett JR: The links
between axin and carcinogenesis. J Clin Pathol. 58:225–236. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JY, Park WS, Nam SW, Kim SY, Lee SH,
Yoo NJ, Lee JY and Park CK: Mutations of beta-catenin and AXIN I
genes are a late event in human hepatocellular carcinogenesis.
Liver Int. 25:70–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mazzoni SM and Fearon ER: AXIN1 and AXIN2
variants in gastrointestinal cancers. Cancer Lett. 355:1–8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa
N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al:
AXIN1 mutations in hepatocellular carcinomas and growth suppression
in cancer cells by virus-mediated transfer of AXIN1. Nat Genet.
24:245–250. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kishida S, Yamamoto H, Ikeda S, Kishida M,
Sakamoto I, Koyama S and Kikuchi A: Axin, a negative regulator of
the wnt signaling pathway, directly interacts with adenomatous
polyposis coli and regulates the stabilization of beta-catenin. J
Biol Chem. 273:10823–10826. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng F, Li S, Xu W, Zou Z, Ke Z and Zeng
F: AXIN1-related CSRNP1 mRNA expression and its transcriptional
regulation in TGF-beta1-induced tumor cells. Nan Fang Yi Ke Da Xue
Xue Bao. 33:1122–1126. 2013.(In Chinese). PubMed/NCBI
|
|
14
|
Zhang Y, Neo SY, Wang X, Han J and Lin SC:
Axin forms a complex with MEKK1 and activates c-Jun NH(2)-terminal
kinase/stress-activated protein kinase through domains distinct
from Wnt signaling. J Biol Chem. 274:35247–35254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rui Y, Xu Z, Lin S, Li Q, Rui H, Luo W,
Zhou HM, Cheung PY, Wu Z, Ye Z, et al: Axin stimulates p53
functions by activation of HIPK2 kinase through multimeric complex
formation. EMBO J. 23:4583–4594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fritsch MK, Schneider DT, Schuster AE,
Murdoch FE and Perlman EJ: Activation of Wnt/beta-catenin signaling
in distinct histologic subtypes of human germ cell tumors. Pediatr
Dev Pathol. 9:115–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dahmen RP, Koch A, Denkhaus D, Tonn JC,
Sörensen N, Berthold F, Behrens J, Birchmeier W, Wiestler OD and
Pietsch T: Deletions of AXIN1, a component of the WNT/wingless
pathway, in sporadic medulloblastomas. Cancer Res. 61:7039–7043.
2001.PubMed/NCBI
|
|
18
|
Ngalame NN, Tokar EJ, Person RJ and
Waalkes MP: Silencing KRAS overexpression in arsenic-transformed
prostate epithelial and stem cells partially mitigates malignant
phenotype. Toxicol Sci. 142:489–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Figeac N and Zammit PS: Coordinated action
of Axin1 and Axin2 suppresses β-catenin to regulate muscle stem
cell function. Cell Signal. 27:1652–1665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek
TJ, Perry WL III, Lee JJ, Tilghman SM, Gumbiner BM and Costantini
F: The mouse Fused locus encodes Axin, an inhibitor of the Wnt
signaling pathway that regulates embryonic axis formation. Cell.
90:181–192. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de la Roche M, Ibrahim AE, Mieszczanek J
and Bienz M: LEF1 and B9L shield β-catenin from inactivation by
Axin, desensitizing colorectal cancer cells to tankyrase
inhibitors. Cancer Res. 74:1495–1505. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of beta-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
L'Esperance S, Popa I, Bachvarova M,
Plante M, Patten N, Wu L, Têtu B and Bachvarov D: Gene expression
profiling of paired ovarian tumors obtained prior to and following
adjuvant chemotherapy: Molecular signatures of chemoresistant
tumors. Int J Oncol. 29:5–24. 2006.PubMed/NCBI
|
|
25
|
Rada P, Rojo AI, Offergeld A, Feng GJ,
Velasco-Martín JP, González-Sancho JM, Valverde ÁM, Dale T,
Regadera J and Cuadrado A: WNT-3A regulates an Axin1/NRF2 complex
that regulates antioxidant metabolism in hepatocytes. Antioxid
Redox Signal. 22:555–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Porta C, Paglino C and Mosca A: Targeting
PI3K/Akt/mTOR signaling in cancer. Front Oncol. 4:642014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: Focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cavazzoni A, Bonelli MA, Fumarola C, La
Monica S, Airoud K, Bertoni R, Alfieri RR, Galetti M, Tramonti S,
Galvani E, et al: Overcoming acquired resistance to letrozole by
targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones.
Cancer Lett. 323:77–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stern DF: ERBB3/HER3 and ERBB2/HER2 duet
in mammary development and breast cancer. J Mammary Gland Biol
Neoplasia. 13:215–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao L and Vogt PK: Class I PI3K in
oncogenic cellular transformation. Oncogene. 27:5486–5496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grewe M, Gansauge F, Schmid RM, Adler G
and Seufferlein T: Regulation of cell growth and cyclin D1
expression by the constitutively active FRAP-p70s6K pathway in
human pancreatic cancer cells. Cancer Res. 59:3581–3587.
1999.PubMed/NCBI
|
|
37
|
Biechele TL, Kulikauskas RM, Toroni RA,
Lucero OM, Swift RD, James RG, Robin NC, Dawson DW, Moon RT and
Chien AJ: Wnt/β-catenin signaling and AXIN1 regulate apoptosis
triggered by inhibition of the mutant kinase BRAFV600E in human
melanoma. Sci Signal. 5:ra32012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arnold HK, Zhang X, Daniel CJ, Tibbitts D,
Escamilla-Powers J, Farrell A, Tokarz S, Morgan C and Sears RC: The
Axin1 scaffold protein promotes formation of a degradation complex
for c-Myc. EMBO J. 28:500–512. 2009. View Article : Google Scholar : PubMed/NCBI
|