Introduction
Bendamustine is a bifunctional alkylating agent that
combines the alkylating properties of 2-chloroethylamine and the
antimetabolite properties of a benzimidazole ring (1). Bendamustine acts primarily as an
alkylating agent that induces interstrand DNA cross-linking and
subsequent strand breaks (2), but
partial cross-resistance suggests that bendamustine has an
alternative underlying mechanism of action from that of other
alkylating agents (3,4). Results of previous clinical trials have
demonstrated that bendamustine is safe and effective as a single
agent for the treatment of chronic lymphocytic leukemia (CLL)
(5) and rituximab-resistant low-grade
lymphomas (6). The clinical
application of bendamustine has been extended to diffuse large B
cell lymphoma (7) and aggressive
lymphomas (8). Although bendamustine
as a monotherapy and in combination with rituximab appears to be
useful in treating CLL and untreated indolent lymphomas (5,9), combined
chemotherapy with other therapeutic agents is required for the
treatment of relapsed cases and refractory malignancies including
aggressive lymphomas.
Combined chemotherapy remains the primary approach
for patients with hematological malignancies. Previous preclinical
studies have demonstrated the combined effects of bendamustine with
other anticancer agents (10).
Certain combinations have been applied clinically (11), but a precise investigation of their
effects is required for validation. To establish safer and more
effective regimens, in the present study, a systematic screening
for suitable drugs to be used in combination with bendamustine for
use against intractable lymphoid malignancies was conducted and the
underlying molecular mechanisms for the effects of favorable
combinations were investigated. In total, >50 compounds and
extracts were examined, including anticancer agents,
differentiation inducers and inhibitors of oncogenic signal
transduction. Potentiation of the growth-inhibitory activities of
various agents in human lymphoma BALM3 cells in the presence of
bendamustine was evaluated by isobogram analysis, as described
previously (12). As a result, it was
identified that combinations of bendamustine and MK615, an extract
of Japanese apricot, were favorable. Japanese apricot has been used
for centuries as a traditional medicine and food in Japanese
culture. Japanese apricot contains a number of chemicals, including
citric acid, malic acid, cyanogenic glycosides and triterpenoids.
MK615 is a sticky extract from Japanese apricot, called Ume
in Japanese, and has been used for a number of years as an
anti-inflammatory agent, for the treatment of intestinal disorders
and as an antipyretic (13). A number
of triterpenoids in MK615 are considered to exhibit antineoplastic
effects. We and other investigators have reported previously that
MK615 inhibits the proliferation of various cancer cells, including
gastric, breast, hepatocellular, colon and pancreatic cancer cells
(12,14,15). MK615
markedly suppressed cutaneous metastases in a patient with advanced
malignant melanoma (16). These
results suggest that MK615 may be useful for treating human
malignant tumors. In the present study, the underlying molecular
mechanisms for the synergism of MK615 and bendamustine were
examined. The results of the present study may provide important
information for the establishment of effective bendamustine-based
regimens.
Materials and methods
Materials
MK615 (Misatol®GL) was prepared as
described previously (12) and
obtained from AdaBio Co., Ltd. (Takasaki, Japan). As
Misatol®GL is a sticky extract, an equal volume of PBS
was added to Misatol®GL. The 50% diluted
Misatol®GL was used as MK615 solution. Ursolic acid and
MTT were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). Bendamustine, VE-821 and KU-60019 were obtained from
Selleck Chemicals (Houston, TX, USA). The general caspase inhibitor
benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (Z-VAD-FMK) was
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Propidium iodide (PI) was purchased from BioVision Inc. (Milpitas,
CA, USA).
Cells and cell culture
Human B cell lymphoma (BALM3, SU-DHL-4, U698 M and
SKW4), lymphoblastoid (BALM1) and myeloma (RPMI8226) cells were
cultured in suspension in RPMI-1640 medium (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum
(BioWest, Nuaille, France) and 80 µg/ml gentamicin at 37°C in a
humidified atmosphere containing 5% CO2. The characteristics of the
lymphoid cell lines used in the present study have been described
previously (17).
Assay of cell proliferation and
viability
Cells were seeded at 1×105 cells/ml in a
24-well plate. Following culture with or without the test compounds
for 2, 3, 4, 5, or 6 days, cell numbers were counted using a model
Z1 Coulter Counter (Beckman Coulter, Inc., Brea, CA, USA). Cell
viability was determined using either a modified MTT assay
(12) or a trypan blue dye exclusion
test using an automated cell counter (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Colony-forming assay
Cells (1×104 cells/dish) were plated into
1.1 ml semisolid methylcellulose medium containing 0.8%
methylcellulose and 20% fetal bovine serum in triplicate for 14
days. A 0.1 ml volume of PBS containing various concentrations of
MK615 and/or bendamustine was added to the semisolid medium. Images
of colonies were captured using an inverted microscope.
Apoptosis assay
For examination of morphology, Cytospin slide
preparations of >300 cells were stained with
May-Grünwald-Giemsa. DNA fragmentation was analyzed as follows:
Cells were collected following exposure to bendamustine and/or
MK615, and DNA was extracted using an Apoptotic DNA Ladder
Detection kit (Abcam Japan, Tokyo), according to the manufacturer's
protocol. Equal amounts of DNA (1 µg) were analyzed by
electrophoresis on 1.5% agarose gels stained with ethidium
bromide.
For the Annexin V-binding assay, cells were labeled
with fluorescein isothiocyanate-labeled Annexin V using an Annexin
V-FITC kit (BioVision, Inc.). Following staining, cells were washed
and analyzed by flow cytometry using a BD FACSCalibur™ instrument
and BD CellQuest Pro (version 6.0) software (both BD Biosciences,
San Jose, CA, USA).
Western blot analysis
Cells were packed following washing with ice-cold
PBS and then lysed at a concentration of 1×107 cells/ml
in lysis buffer (Sample Buffer; Wako Pure Chemical Industries,
Ltd., Osaka, Japan). Protein concentration was quantified using
Protein Quantification Kit-Rapid (Wako Pure Chemical Industries,
Ltd.). Equal amounts of protein (10 µg) were separated by SDS/PAGE
(10% gels) prior to transfer to a polyvinylidene fluoride membrane
(Bio-Rad Laboratories), and then blocked with Block Ace (DS Pharma
Biomedical Co., Ltd, (Osaka, Japan) for 60 min at room temperature.
The membranes were incubated with anti-phospho (p) checkpoint
kinase (Chk) 1, Chk1, pChk2, Chk2 and β-actin antibodies (nos.
2349, 2360, 2197, 6334, and 4970; Cell Signaling Technology, Inc.;
dilution, 1:500) at 4°C overnight. Active caspase-3 was examined by
western blot analysis using anti-cleaved caspase-3 antibody (no.
9664; Cell Signaling Technology, Inc., Danvers, MA, USA; dilution,
1:500). All western blots presented are representative of ≥3
independent experiments.
Immunofluorescence
All procedures were performed at room temperature.
Cells were fixed with 4% paraformaldehyde in PBS for 10 min, and
then permeabilized with 0.3% Tween-20 for 15 min. Following
fixation, cells were washed three times with PBS and then blocked
with blocking buffer (1% bovine serum albumin in PBS) for 60 min.
Cells were incubated with an anti-Rad51 (ab213; Abcam, Cambridge,
UK) and anti-phosphorylated histone H2AX (γH2AX) (no. 9718; Cell
Signaling Technology, Inc.) antibodies (both dilution, 1:100) for
60 min, washed with blocking buffer and incubated for 60 min with
Alexa Fluor® 488-conjugated anti-mouse and Alexa
Fluor® 594-conjugated anti-rabbit secondary antibodies
(nos. 4408 and 8889; Cell Signaling Technology, Inc.; dilution,
1:100). Confocal images were captured using an inverted microscope
(Olympus, Tokyo, Japan). All immunofluorescence experiments were
repeated three times.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Pairs of data were compared using Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Combined effects of bendamustine and
MK615 on the proliferation of lymphoma and myeloma cells
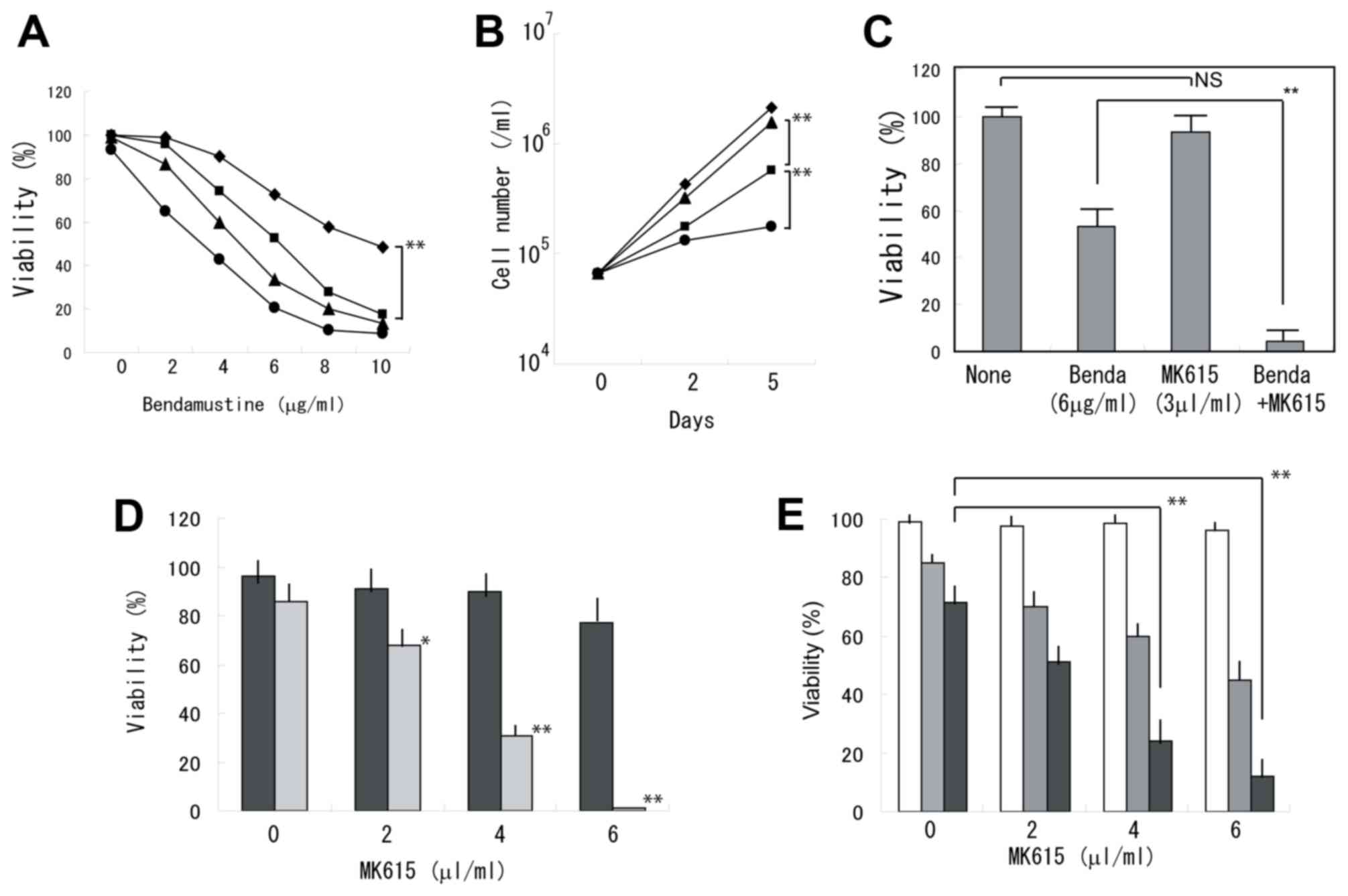
Bendamustine exhibited synergistic effects with
MK615 in inhibiting the viability of BALM3 cells (Fig. 1A). When the cells were treated with 6
µg/ml bendamustine alone, the cells continued to proliferate,
although the viability was markedly decreased. Whereas MK615 at 3
µl/ml exhibited a limited effect on cell viability, proliferation
was almost completely prevented by the combined treatment of MK615
and bendamustine (Fig. 1B). At 5
days, viability was significantly decreased following treatment
with bendamustine plus MK615 (Fig.
1C). Similar results were obtained in the other lymphoma cell
lines (Fig. 1D and E). RPMI8226
myeloma cells were less sensitive to bendamustine and the
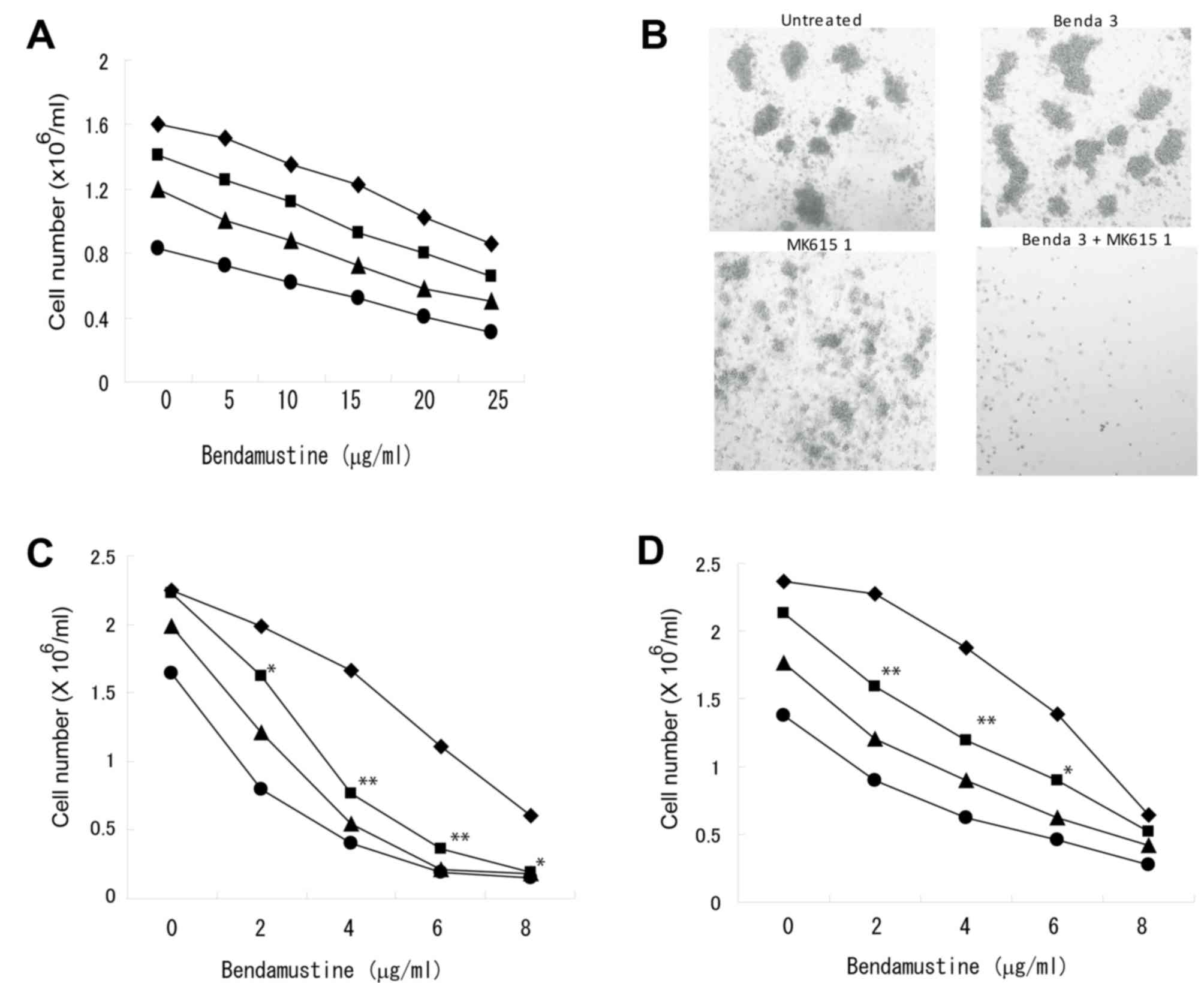
combination with MK615 was less effective (Fig. 2A). A colony-forming assay indicated
that the combination of bendamustine and MK615 completely
suppressed colony formation by BALM3 cells, although bendamustine
at 3 mg/ml had only a limited effect on colony formation (Fig. 2B). As MK615 contains a number of
triterpenoids that exhibit antitumor activities (15), the effect of chemically defined
triterpene on the proliferation of lymphoma cells was examined. The
combination of bendamustine and ursolic acid, one of the major
components of MK615, inhibited the proliferation of BALM3 cells
(Fig. 2D), but was slightly less
effective than the combined effects of bendamustine and MK615
(Fig. 2E).
Induction of apoptosis in BALM3 cells
treated with bendamustine and MK615
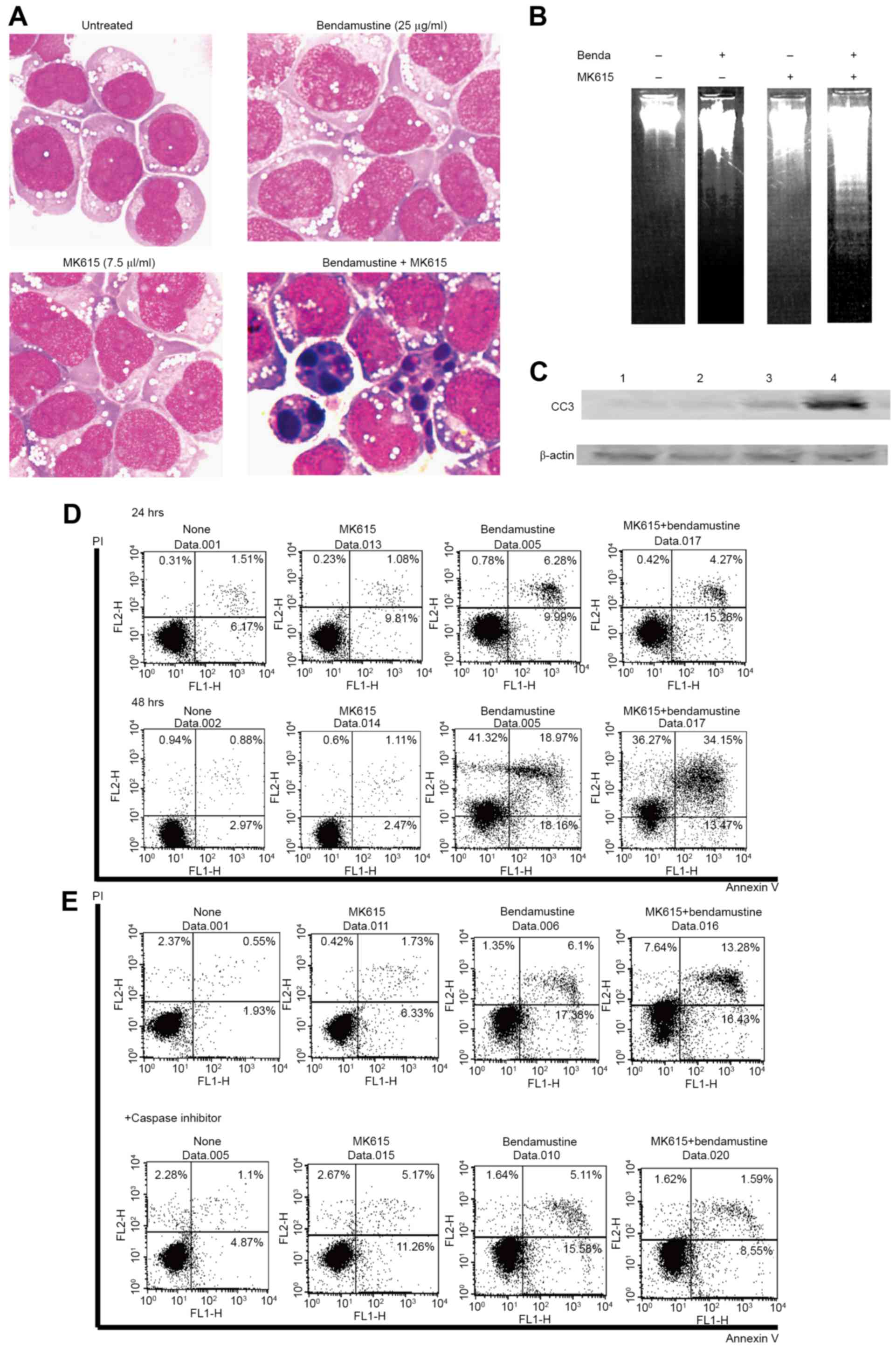
When BALM3 cells were exposed to 25 µg/ml
bendamustine and 7.5 µl/ml MK615 for 24 h, morphological analysis
revealed shriveled cells, chromatin condensation, nuclear
fragmentation and cytoplasmic blebbing, whereas these morphological
changes were rarely observed in cells treated with 25 µg/ml
bendamustine alone (Fig. 3A). The
induction of apoptosis was confirmed by gel electrophoresis of DNA
from cells exposed to bendamustine and MK615 (Fig. 3B), induction of cleaved caspase-3
(Fig. 3C) and the expression of
Annexin V (Fig. 3D). Annexin
V-positive cells were induced by 6 µg/ml bendamustine, but MK615
had limited effect on Annexin V expression. MK615 markedly
increased the bendamustine-induced Annexin V expression. After 48
h, PI+ Annexin V+ cells were 0.88, 1.11, 18.9
and 34.15% in untreated, MK615-treated, bendamustine-treated and
MK615 plus bendamustine-treated cells, respectively (Fig. 3D). The induction of Annexin V
expression by bendamustine plus MK615 was significantly inhibited
by the general caspase inhibitor Z-VAD-FMK (Fig. 3E). These results indicate that
combined treatment with bendamustine and MK615 effectively induced
caspase-dependent apoptosis in BALM3 cells.
Effect of MK615 on the
bendamustine-induced DNA damage response
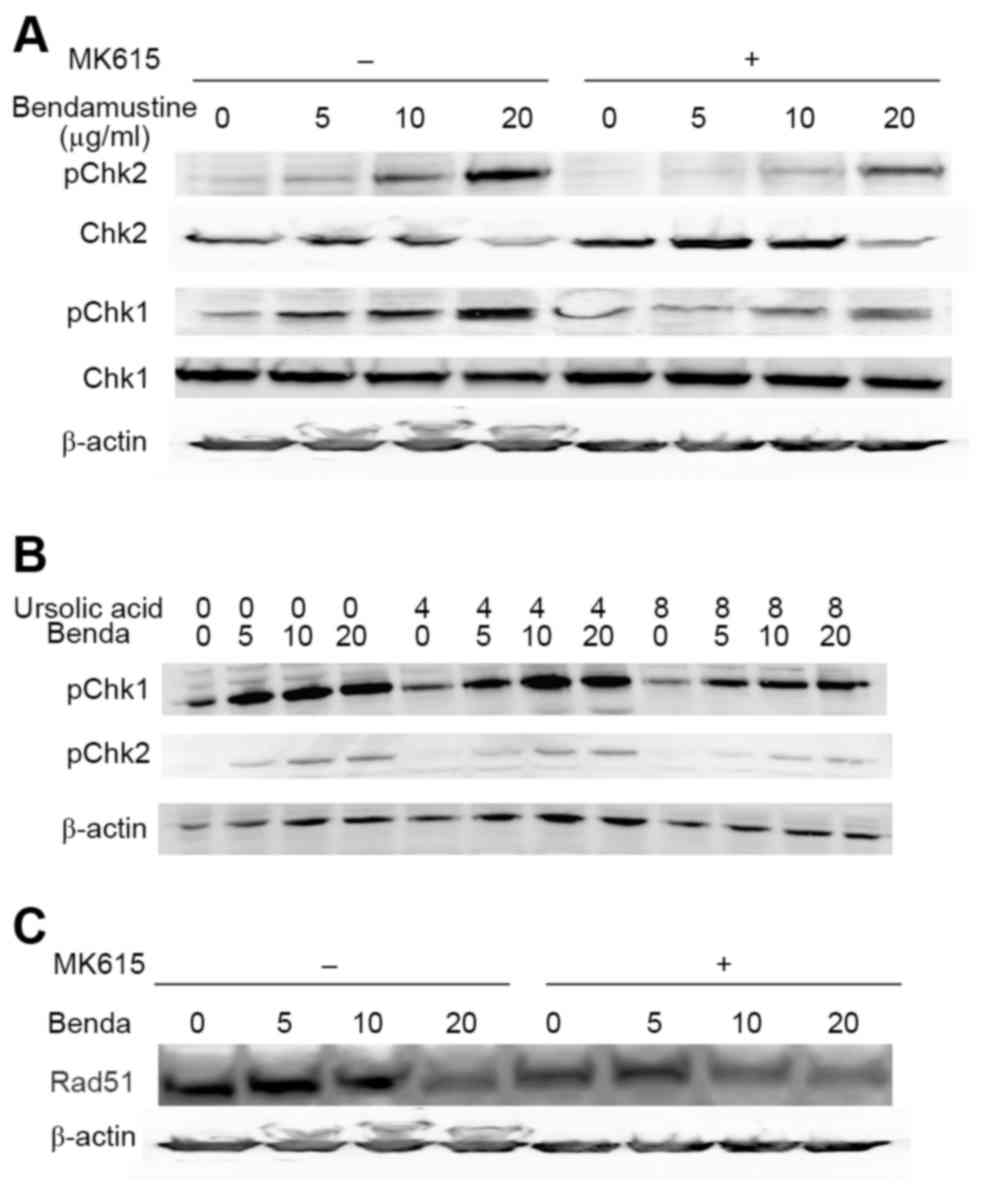
Alkylating agents including bendamustine activate a
DNA damage response (10). ATM and
ATR are central to the entire DNA damage response and directly
regulate at least two effector kinases: Chk1 and Chk2 (18). We examined phosphorylation of the
kinases in BALM3 cells treated with bendamustine for 6, 12, and 24
h. Bendamustine induced marked phosphorylation of Chk1 and Chk2 at
24 h, whereas MK615 did not (Fig.
4A). MK615 substantially inhibited the phosphorylation of Chk1
and Chk2 between 12 and 24 h, although this inhibition by MK615 was
not observed at 6 h, suggesting that bendamustine rapidly induced
phosphorylation of the checkpoint kinases, and phosphorylation was
gradually suppressed by MK615. Ursolic acid, one of the major
components of MK615, also markedly inhibited phosphorylation of the
kinases, as did MK615 (Fig. 4B).
Effects of ATR/ATM inhibitors on
bendamustine-induced proliferation inhibition of BALM3 cells
As Chk1 and Chk2 are phosphorylated by ATM and ATR
(18), the aforementioned results
suggest that MK615 effectively inhibited the activities of ATM
and/or ATR. Therefore, the effects of ATR and ATM inhibitors on
bendamustine-induced proliferation inhibition were examined
(Fig. 5). VE-821 (an ATR inhibitor)
and KU-60019 (an ATM inhibitor) significantly enhanced the
bendamustine-induced proliferation inhibition of BALM3 cells
(P<0.01), whereas these inhibitors exhibited a limited effect on
MK615-induced proliferation inhibition (Fig. 5A), suggesting that these inhibitors
have the same mechanism of action as that of MK615. The combination
of VE-821 and KU-60019 significantly increased the inhibition of
bendamustine-induced proliferation compared with VE-821 or KU-60019
alone (Fig. 5B). Similar results were
obtained in other lymphoid cells, although the effects differed
among the cell lines (Fig. 5C).
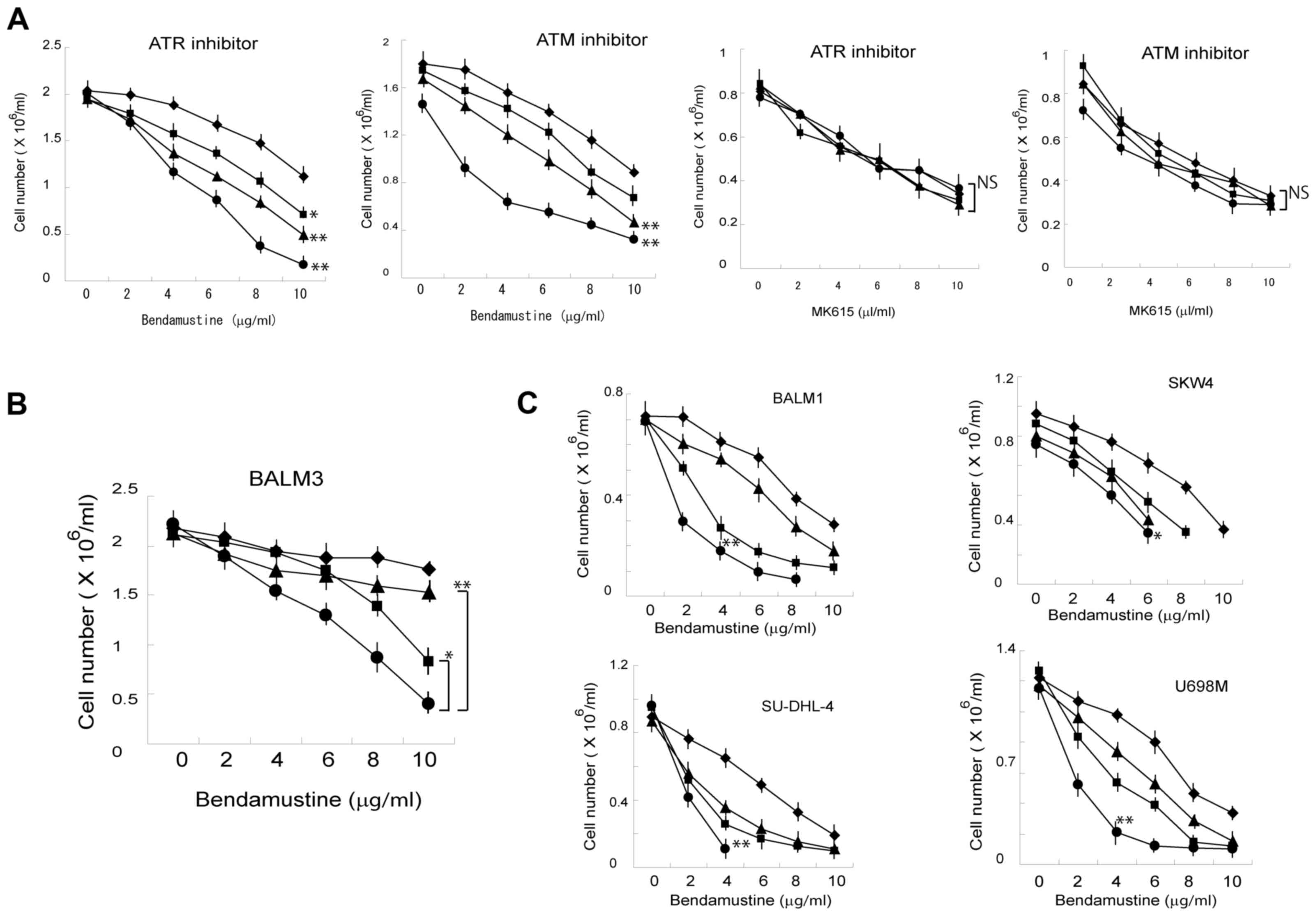 | Figure 5.(A) Effects of ATM/ATR inhibitors on
the proliferation of BALM3 cells treated with bendamustine or
MK615. ATR inhibitor: Cells were treated with various
concentrations of bendamustine or MK615 in the presence of 0 (◆),
10 (■), 30 (▲) or 100 (•) ng/ml VE-821 for 4 days. ATM inhibitor:
Cells were treated with various concentrations of bendamustine or
MK615 in the presence of 0 (◆), 10 (■), 100 (▲) or 1,000 (•) ng/ml
KU-60019 for 4 days. The values are means of three separate
experiments. (B) Combined effects of VE-821 and KU-60019 on the
proliferation of BALM3 lymphoid cells treated with bendamustine.
(C) Combined effects of VE-821 and KU-60019 on the proliferation of
BALM1, SKW4, SU-DHL-4 and U698M lymphoid cells treated with
bendamustine. All five cell lines were treated without (◆) or with
100 ng/ml VE-821 (■), 100 ng/ml KU-60019 (▲), or the two drugs in
combination (•) for 4 days. Results are presented as the mean ±
standard deviation of three separate experiments. *P<0.05,
**P<0.01 and NS, not significant vs. cells without inhibitors.
ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and
Rad3-related. |
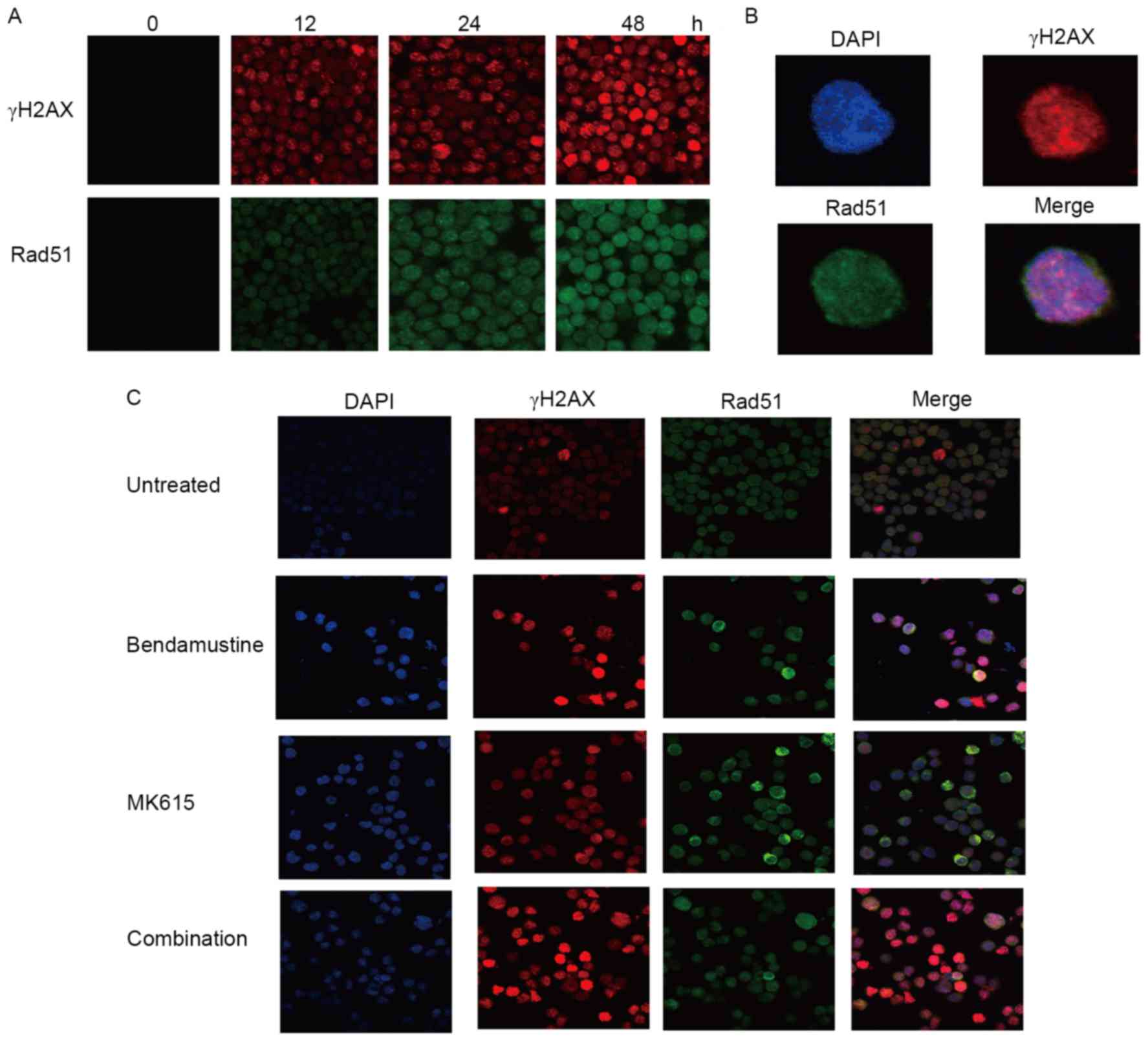
Suppression of bendamustine-induced
formation of Rad51 foci by MK615
BALM3 cells treated with bendamustine exhibited an
early increase in the number of γH2AX, a marker of DNA damage, and
of Rad51 nuclear foci, which are the sites of repair of DNA damage
(Fig. 6A and B). MK615 did not
exhibit any marked effect on the number of γH2AX and Rad51 foci in
the absence of bendamustine, but markedly increased the number of
bendamustine-induced γH2AX foci. However, the number of
bendamustine-induced Rad51 foci was not increased by MK615
(Fig. 6C). As presented in Fig. 4C, bendamustine decreased the amount of
Rad51 protein in BALM3 cells in the presence or absence of MK615.
These results suggest that MK615 suppresses Rad51 assembly and
stimulates its degradation, independent of DNA damage.
Discussion
Previous studies have investigated the combined
effects of bendamustine and various agents on the activation of
cell-death pathways in malignant cells. These agents have included
navitoclax (an inhibitor of B cell lymphoma 2), everolimus (an
inhibitor of mammalian target of rapamycin), SGI-1776 (an inhibitor
of Pim kinase), entinostat (an inhibitor of histone deacetylase)
and YM155 (an inhibitor of survivin) (19–23).
Entinostat was identified to enhance the bendamustine-induced
phosphorylation of Chk2 (22),
whereas YM155 inhibited the bendamustine-induced activation of the
ATM signaling pathway (23). The
results of the present study indicate that MK615 inhibited the
bendamustine-induced activation of the ATM and ATR signaling
pathways. The formation of nuclear foci of Rad51 induced by
bendamustine was effectively inhibited by MK615, suggesting that
MK615 suppresses the DNA damage repair induced by bendamustine. A
previous study indicated that MK615 markedly suppressed cutaneous
in-transit metastasis in a patient with advanced malignant melanoma
(16). M615 significantly inhibited
the proliferation of human pancreatic cancer cells as xenografts
without apparent adverse effects and exhibited synergistic effects
with gemcitabine (12). In advanced
cases and recurrence, the use of supplements is expected to augment
the antineoplastic effects of cancer drugs. Various standardized
extracts or fractions with anticancer effects or with adjuvant
therapy in cancer treatment obtained from single or mixed herbs are
accepted as dietary supplements and botanical drug products in the
USA on the basis of current statutory regulations (24). Certain supplements may enhance the
inhibitory effects of anticancer agents on many cancers. Japanese
apricot has been used for centuries as a traditional medicine and
food in Japanese culture. MK615 is a supplement produced from
Japanese apricot that may be a useful for treating human
malignancies, and further studies are warranted to evaluate its
clinical effectiveness and to elucidate its precise mechanism of
action.
It is hypothesized that targeting ATR and ATM may
selectively sensitize cancer cells, but not normal cells, to DNA
damage, therefore selective inhibitors of ATM and ATR are currently
in preclinical and clinical development (18,25). As
these inhibitors and bendamustine synergistically inhibited the
proliferation of lymphoma cells, combination therapy with
bendamustine and ATM/ATR inhibitors may be useful in the treatment
of malignant lymphoma. Further preclinical and clinical studies may
lead to new possibilities in the therapy of lymphoid
malignancies.
B lymphoma cells are sensitive to bendamustine, and
the combined treatment with MK615 was more marked in B lymphoma
cells. RPMI18226 myeloma cells were less sensitive to bendamustine
and the combination with MK615 was less effective. Similar results
were obtained in other myeloma cell lines and certain myeloid
leukemia cell lines. These results suggest that the combined
therapy may be useful in the treatment of B lymphoma.
Acknowledgements
The present study was supported by the SUIGAN
project, Shimane University, and Japan Blood Products Organization,
Japan. J.S. received research funding from Chugai Pharmaceutical
Co., Ltd.; Kyowa Hakko Kirin Co., Ltd.; Eisai Co., Ltd.; Takeda
Pharmaceutical Co., Ltd.; Astellas Pharma Inc.; and Toyama Chemical
Co., Ltd.
References
|
1
|
Tageja N and Nagi J: Bendamustine:
Something old, something new. Cancer Chemother Pharmacol.
66:413–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartmann M and Zimmer C: Investigation of
cross-link formation in DNA by the alkylating cytostatic IMET 3106,
3393 and 3943. Biochim Biophys Acta. 287:386–389. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strumberg D, Harstrick A, Doll K, Hoffmann
B and Seeber S: Bendamustine hydrochloride activity against
doxorubicin-resistant human breast carcinoma cell lines. Anticancer
Drugs. 7:415–421. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leoni LM, Bailey B, Reifert J, Bendall HH,
Zeller RW, Corbeil J, Elliott G and Niemeyer CC: Bendamustine
(Treanda) displays a distinct pattern of cytotoxicity and unique
mechanistic features compared with other alkylating agents. Clin
Cancer Res. 14:309–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Knauf WU, Lissichkov T, Aldaoud A,
Liberati A, Loscertales J, Herbrecht R, Juliusson G, Postner G,
Gercheva L, Goranov S, et al: Phase III randomized study of
bendamustine compared with chlorambucil in previously untreated
patients with chronic lymphocytic leukemia. J Clin Oncol.
27:4378–4384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friedberg JW, Cohen P, Chen L, Robinson
KS, Forero-Torres A, La Casce AS, Fayad LE, Bessudo A, Camacho ES,
Williams ME, et al: Bendamustine in patients with
rituximab-refractory indolent and transformed non-Hodgkin's
lymphoma: Results from a phase II multicenter, single-agent study.
J Clin Oncol. 26:204–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohmachi K, Niitsu N, Uchida T, Kim SJ,
Ando K, Takahashi N, Takahashi N, Uike N, Eom HS, Chae YS, et al:
Multicenter phase II study of bendamustine plus rituximab in
patients relapsed or refractory diffuse large B-cell lymphoma. J
Clin Oncol. 31:2103–2109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCloskey JK, Broome CM and Cheson BD:
Safe and effective treatment of aggressive non-Hodgkin lymphoma
with rituximab and bendamustine in patients with severe liver
impairment. Clin Adv Hematol Oncol. 11:184–188. 2013.PubMed/NCBI
|
|
9
|
Rummel MJ, Niederle N, Maschmeyer G, Banat
GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M,
Balser C, et al: Bendamustine plus rituximab versus CHOP plus
rituximab as first-line treatment for patients with indolent and
mantle-cell lymphomas: An open-label, multicentre, randomized,
phase 3 non-inferiority trial. Lancet. 381:1203–1210. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiraoka N, Kikuchi J, Yamauchi T, Koyama
D, Wada T, Uesawa M, Akutsu M, Mori S, Nakamura Y, Ueda T, et al:
Purine analog-like properties of bendamustine underlie rapid
activation of DNA damage response and synergistic effects with
pyrimidine analogues in lymphoid malignancies. PLoS One.
9:e906752014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Visco C, Finotto S, Zambello R, Paolini R,
Menin A, Zanotti R, Zaja F, Semenzato G, Pizzolo G, D'Amore ES and
Rodeghiero F: Combination of rituximab, bendamustine, and
cytarabine for patients with mantle-cell non-Hodgkin lymphoma
ineligible for intensive regimens or autologous transplantation. J
Clin Oncol. 31:1442–1449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hattori M, Kawakami K, Akimoto M, Takenaga
M, Suzumiya J and Honma Y: Antitumor effect of Japanese apricot
extract (MK615) on human cancer cells in vitro and in vivo through
a reactive oxygen species-dependent mechanism. Tumori. 99:239–248.
2013.PubMed/NCBI
|
|
13
|
Adachi M, Suzuki Y, Mizuta T, Osawa T,
Adachi T, Osaka K, Suzuki K, Shiojima K, Arai Y, Masuda K, et al:
The ‘Prunus mume Sieb. et Zucc’ (Ume) is a rich natural source of
novel anti-cancer substance. Int J Food Prop. 10:375–384. 2007.
View Article : Google Scholar
|
|
14
|
Gutterman JU, La HT, Yang P, Haridas V,
Gaikwad A and Marcus S: Effects of the tumor inhibitory
triterpenoid avicin G on cell integrity, cytokinesis, and protein
ubiquitination in fission yeast. Proc Natl Acad Sci USA. 102:pp.
12771–12776. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamai H, Sawada N, Yoshida T, Seike J,
Takizawa H, Kenzaki K, Miyoshi T, Kondo K, Bando Y, Ohnishi Y and
Tangoku A: Triterpenes augment the inhibitory effects of anticancer
drugs on growth of human esophageal carcinoma cells in vitro and
suppress experimental metastasis in vivo. Int J Cancer.
125:952–960. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsushita S, Tada K, Kawahara K, Kawai K,
Hashiguchi T, Maruyama I and Kanekura T: Advanced malignant
melanoma responds to Prunus mume Sieb. Et Zucc (Ume) extract: Case
report and in vitro study. Exp Ther Med. 1:569–574. 2010.PubMed/NCBI
|
|
17
|
Niitsu N, Higashihara M and Honma Y: Human
B-cell lymphoma cell lines are highly sensitive to apoptosis
induced by all-trans retinoic acid and interferon-gamma. Leuk Res.
26:745–755. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weber AM and Ryan AJ: ATM and ATR as
therapeutic targets in cancer. Pharmacol Ther. 149:124–138. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ackler S, Mitten MJ, Chen J, Foster K, Jin
S, Phillips DC, Schlessinger S, Wang B, Leverson JD and Boghaert
ER: Navitoclax (ABT-263) and bendamustine ± rituximab induce
enhanced killing of non-Hodgkin's lymphoma tumours in vivo. Br J
Pharmacol. 167:881–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu B, Li J, Pan J, Huang B, Liu J and
Zheng D: Everolimus enhances the cytotoxicity of bendamustine in
multiple myeloma cells through a network of pro-apoptotic and
cell-cycle-progression regulatory proteins. Acta Biochim Biophys
Sin (Shanghai). 45:683–691. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Q, Chen LS, Neelapu SS and Gandhi V:
Combination of Pim kinase inhibitor SGI-1776 and bendamustine in
B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 13 Suppl 2:S355–S362.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai B, Lyu H, Huang J, Wang S, Lee CK, Gao
C and Liu B: Combination of bendamustine and etinostat
synergistically inhibits proliferation of multiple myeloma cells
via induction of apoptosis and DNA damage response. Cancer Lett.
335:343–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaneko N, Mitsuoka K, Amino N, Yamanaka K,
Kita A, Mori M, Miyoshi S and Kuromitsu S: Combination of YM155, a
surviving suppressant, with bendamustine and rituximab: A new
combination therapy to treat relapse/refractory diffuse large
B-cell lymphoma. Clin Cancer Res. 20:1814–1822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng Y, Wang N, Zhu M, Feng Y, Li H and
Tsao S: Recent progress on anticancer candidates in patents of
herbal medicinal products. Recent Pat Food Nutr Agric. 3:30–48.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fokas E, Prevo R, Hammond EM, Brunner TB,
McKenna WG and Muschel RJ: Targeting ATR in DNA damage response and
cancer therapeutics. Cancer Treat Rev. 40:109–117. 2014. View Article : Google Scholar : PubMed/NCBI
|