Introduction
Coffee is widely consumed as a beverage globally,
and a moderate intake of coffee has been linked to a reduced risk
of chronic diseases, including type 2 diabetes (1), Parkinson's disease (2) and liver disease (3). Multiple epidemiological studies have
demonstrated an inverse association between coffee consumption and
risk of colorectal cancer (4–6). However, the molecular basis of this
effect remains to be fully understood (7).
Colorectal cancer is one of the most common
malignancies in the westernized world. An important step in the
progression of colorectal cancer is the induction of activating
mutations in KRAS proto-oncogene, GTPase (KRAS). Mutations
in KRAS appear in the intermediate adenoma stage, early
during tumorigenesis, and it is thus possible to use them as a
biomarker for early detection of ~40% of colorectal tumors
(8).
Activated KRAS regulates multiple downstream
pathways involved in cancer development, including the
mitogen-activated protein kinase (MAPK) and phosphatidylinositol
3-kinase (PI3K) signaling pathways (9), via the action of epidermal growth factor
(EGF). The EGF receptor is involved in regulating normal growth and
contributes to the malignant growth of several tumor types,
including colon cancer (10) by
phosphorylating tyrosine residues in trans within the
cytoplasmic domain of the receptor to activate Ras and other
downstream effectors. Therefore, the present study investigated the
effects of coffee on KRAS signaling via EGF in human colon cancer
Caco-2 cells.
Materials and methods
Materials
Caco-2 human colon carcinoma cells were obtained
from the RIKEN BioResource Center (Tsukuba, Japan). Colombian
Arabica coffee beans were purchased from Yanaka Coffee Co., Ltd.
(Tokyo, Japan). Reagents for PCR were purchased from Applied
Biosystems; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Reagents for quantification of microRNA (miRNA/miR) were purchased
from Qiagen GmbH (Hilden, Germany). Antibodies for MAPK (cat. no.,
9102), phosphorylated (p-) MAPK (cat. no., 9101), protein kinase B
(Akt; cat. no., 9272) and p-Akt (cat. no., 9271) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies
for K-ras (cat. no., sc-30) and β-actin (cat. no., sc-58673) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Caffeine, caffeic acid, chlorogenic acid and trigonelline were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Human EGF was purchased from PeproTech (Rocky Hill, NJ, USA).
Coffee preparation
Roasted and ground coffee (Colombian Arabica) was
obtained from Starbucks Corporation (Seattle, WA, USA). Coffee
extracts were prepared by a commonly utilized method, where 8 g of
ground coffee was extracted with 140 ml hot water (95°C). The
extract was then filtered through a paper filter (Mellita Group,
Minden, Germany), divided into small aliquots and stored at −80°C
until used. Undiluted extract, with a dry weight of 8.4 mg/ml, was
assigned a concentration of 100% (v/v). For roasting experiments,
green Colombian Arabica coffee beans were purchased from Yanaka
Coffee Co., Ltd. Green coffee beans were roasted at 200–220°C for
up to 20 min. Coffee extracts were prepared and stored as described
for roasted and ground coffee. Optical densities at 500 nm of 10%
brewed coffee made from roasted beans were 0.05 (green beans),
0.153 (medium roasted beans) and 0.269 (dark roasted beans),
respectively.
Cell culture and coffee treatment
Caco-2 cells were grown in 6-well plates (Iwaki Co.,
Ltd., Tokyo, Japan) in 2 ml Dulbecco's modified Eagle's medium
(Nacalai Tesque, Inc., Kyoto, Japan) supplemented with 10% fetal
bovine serum (Biological Industries USA, Cromwell, CT, USA), 2 mM
glutamine, 10 U/ml penicillin, 10 U/ml streptomycin and additional
non-essential amino acids (Sigma-Aldrich; Merck KGaA). Cells were
seeded at a concentration of 1×105 cells/ml and grown to
80–90% confluence (2–3 days) in an incubator at 37°C in a
humidified atmosphere containing 5% CO2. The medium was changed
every 4–5 days. Cultured cells were exposed to coffee extracts at
0, 0.31, 0.63, 1.25, 2.5, 3.75 and 5.0% (v/v) or caffeine, caffeic
acid, chlorogenic acid, and trigonelline at 100 µM. Control cells
were treated with 0.1% DMSO. Cell numbers and viability were
analyzed using a Vi-Cell counter (Beckman Coulter, Inc., Brea, CA,
USA) and a trypan blue exclusion assay using 0.4% trypan blue dye
(Thermo Fisher Scientific KK, Yokohama, Japan).
Analyses of gene expression
Total RNA was isolated from the cultured cells using
the Direct-zol™ RNA MiniPrep kit and TRIzol reagent (Zymo Research,
Irvine, CA, USA). First-strand cDNA was synthesized from 1 µg total
RNA using 100 units/ml of reverse transcriptase and random primers
using a ReverTra-Plus Kit (TOYOBO, Osaka, Japan) according to the
manufacturer's protocol. The primers used for the amplification of
cDNAs were designed using a web application (Primer3) based on
sequences obtained from the NCBI database. The sequences used were
as follows: KRAS forward, 5′-CCTGCTGTGTCGAGAATATCCA-3′ and reverse,
5′-TTGACGATACAGCTAATTCAGAATCA-3′; 18S RNA forward,
5′-TGGTTGCAAAGCTGAAACTTAAAG-3′ and reverse,
5′-AGTCAAATTAAGCCGCAGGC-3′. Quantitative polymerase chain reaction
(qPCR) analysis was performed in a CFX96 Real Time PCR Detection
System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using the
SYBR Green PCR Core Reagent kit (Roche Diagnostics, Basel,
Switzerland). Samples were denatured at 95°C for 10 min and
amplified by 40 cycles of denaturation at 95°C for 15 sec, followed
by annealing and extension at 60°C for 60 sec. The amount of target
gene relative to the reference gene (18S rRNA) was quantified using
the cycle threshold (Cq) (11).
The cDNAs of miRNAs were synthesized from 250 ng
total RNA using the miScript II RT kit (Qiagen GmbH) according to
the manufacturer's protocol. Quantification of miRNAs was performed
using the miScript SYBR Green PCR kit (Hs_miR-96_1 miScript Primer
Assay and Hs_miR-30c_2 miScript Primer Assay; Qiagen GmbH) using
the aforementioned thermocycler conditions according to the
manufacturer's protocol, and quantified using the cycle threshold
(Cq) (11). The Hs_RNU6-2_1 miScript
Primer Assay (Qiagen GmbH) was used for normalization.
Western blotting
Cells were lysed in Nonidet P-40 lysis buffer (50 mM
Tris-HCl pH 8.0, 120 mM NaCl, 1 mM NaCl, 1 mM EDTA pH 8.0, 0.5%
Nonidet P-40), phosphatase inhibitor cocktail (Nacalai Tesque,
Inc., Kyoto, Japan), and protease inhibitor cocktail (Nacalai
Tesque, Inc.). Proteins (20 µg) were resolved by 10% sodium dodecyl
sulfate (SDS) polyacrylamide gel electrophoresis and then
electrotransferred to a polyvinylidene difluoride membrane (Merck
KGaA). The membrane was incubated for 1 h at room temperature in
blocking buffer consisting of TBS (20 mM Tris-HCl pH 7.4, 137 mM
NaCl) containing 5% skim milk. The membrane was incubated overnight
at 4°C with primary antibody. Primary antibodies were mouse
monoclonal anti-K-ras (1:500), rat monoclonal anti-H-ras (1:500)
and goat polyclonal anti-β-actin (1:2,000; Santa Cruz
Biotechnology, Inc.); rabbit monoclonal anti-p44/42 MAPK
[(extracellular signal related kinase 1/2 (ERK1/2; 1:1,000), rabbit
monoclonal anti-p-p44/42 MAPK (ERK1/2; Thr202/Tyr204; 1:1,000),
rabbit monoclonal anti-Akt (1:500) and anti p-Akt (1:1,000; Cell
Signaling Technology, Danvers, MA, USA). The next day, the membrane
was incubated with either anti-rabbit (cat. no., RPN4301),
anti-mouse (cat. no., RPN4201) or anti-rat (cat. no., NA934)
immunoglobulin G horseradish peroxidase-linked secondary antibodies
(all 1:2,000; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) at
room temperature for 60 min. Immunoreactive proteins were
visualized using an enhanced chemiluminescence western blotting
detection system (GE Healthcare Bio-Sciences).
Analysis of effects of coffee on EGF
signaling
Caco-2 cells were treated with 5% coffee for 24 h,
and then EGF (50 ng/ml) was added to the medium. Cells were
harvested at 0, 5, 10 and 15 min following the addition of EGF and
the cell lysates (20 µg protein) were analyzed by western
blotting.
Statistical analysis
The Student's t-test was used for statistical
analysis using a software (SPSS station, v. 23; IBM SPSS, Armonk,
NY, USA), and P<0.05 was considered to indicate a statistically
significant difference.
Results
Coffee reduced proliferation of Caco-2
cells
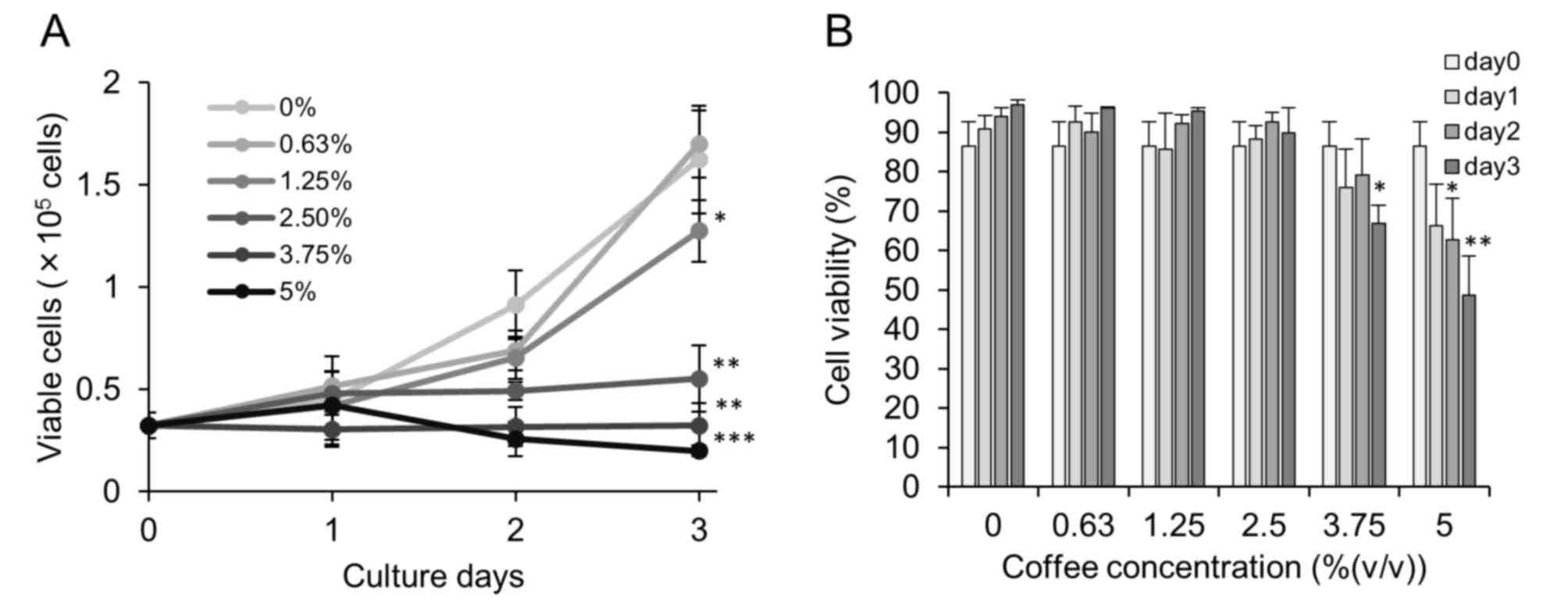
Human colon carcinoma Caco-2 cells were treated with
coffee extract (0–5%) for up to 3 days. An inhibitory effect of
coffee extract on cell proliferation was detected at concentrations
of 1.25% and above, with complete inhibition observed at a
concentration of 3.75% (Fig. 1A). No
significant cytotoxicity was observed for coffee extract
concentrations up to 3.75%, even following incubation for 3 days
(Fig. 1B).
Coffee reduced KRAS expression in
Caco-2 cells
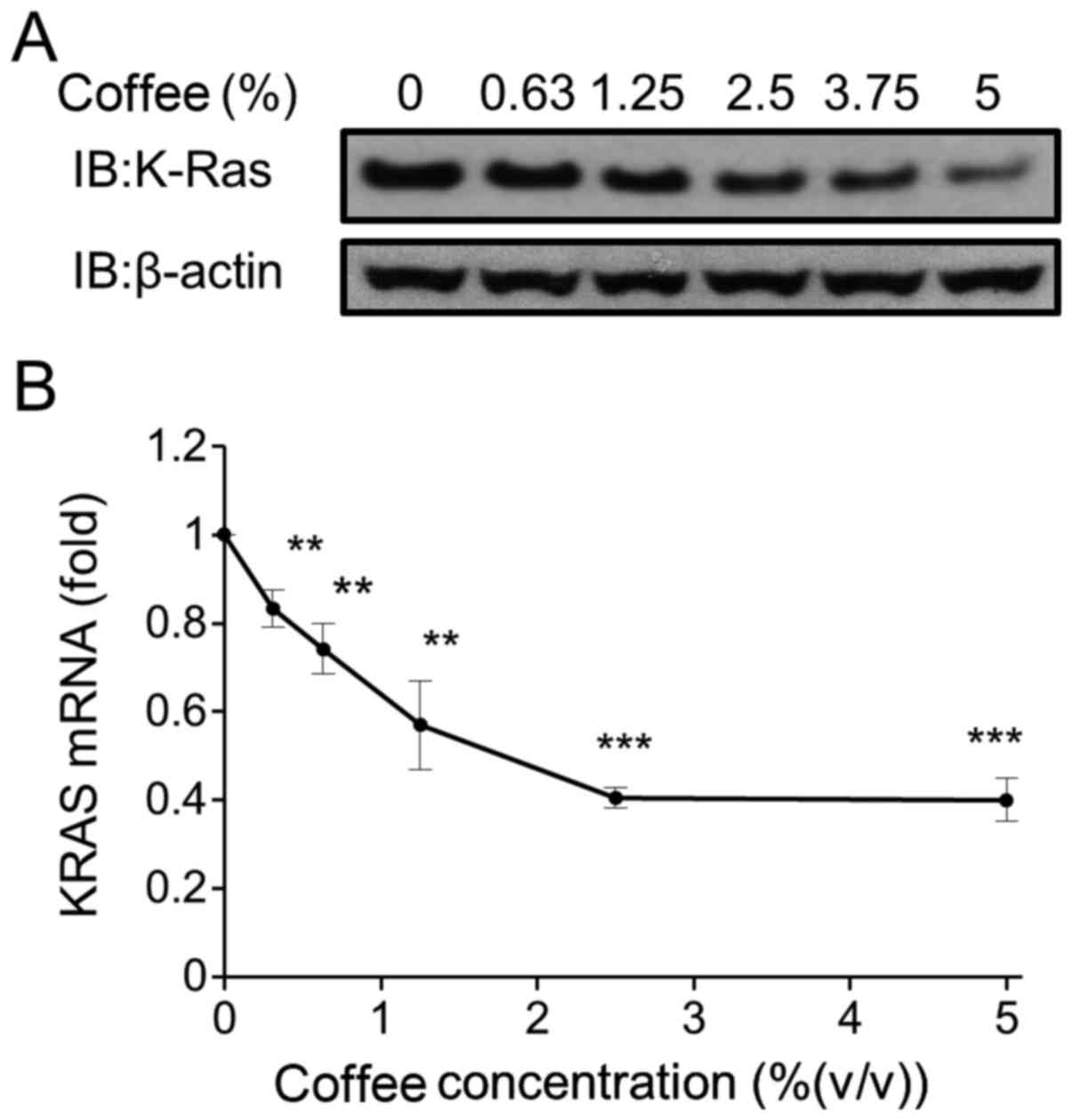
As the development and growth of colon cancer is
associated with abnormal activation of the KRAS signaling pathway
(12), KRAS expression in the coffee
extract-treated Caco-2 cells was measured. Coffee extract reduced
the expression of the KRAS protein and KRAS mRNA in a
dose-dependent manner (Fig. 2A and B,
respectively). KRAS mRNA expression decreased to <50% of the
control level following treatment with 2.5% coffee extract for 24
h.
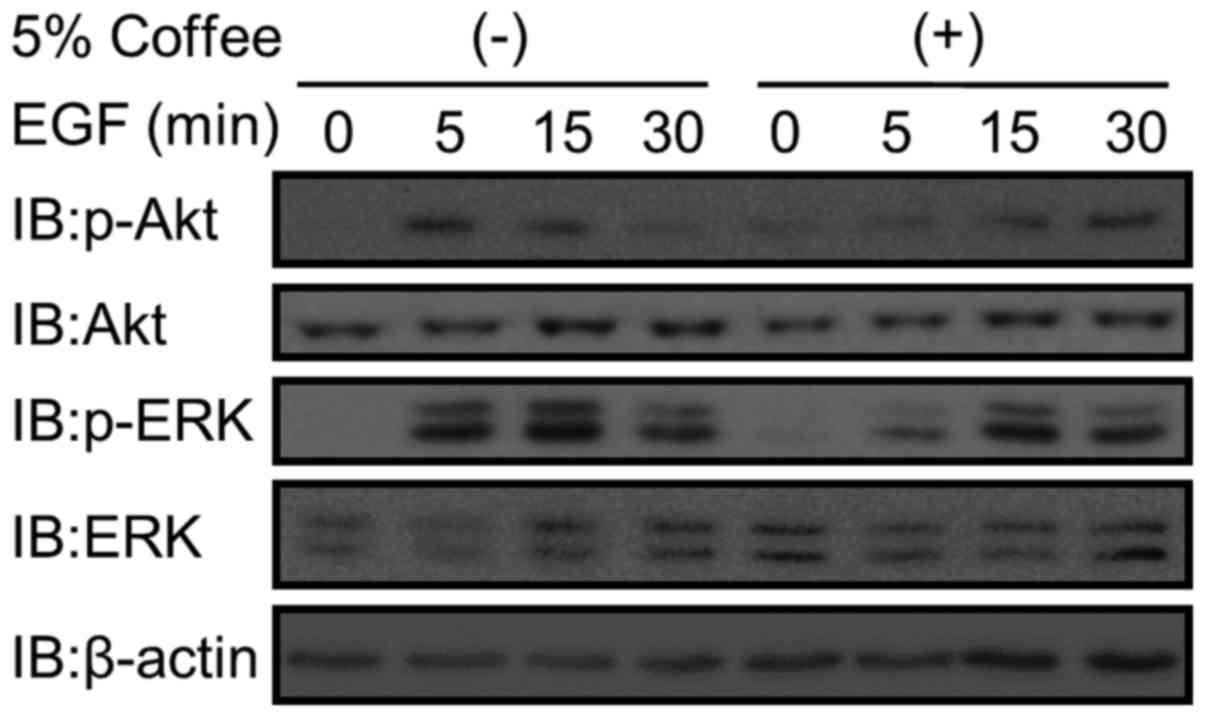
In order to elucidate the effect of coffee on KRAS
signaling pathways, the activation of Akt (PI3K pathway) and ERK
(MAPK pathway) elicited by 50 ng/ml EGF in coffee extract-treated
Caco-2 cells was analyzed. Phosphorylation of Akt and ERK was
observed 5 min following addition of 50 ng/ml EGF in cells
untreated with coffee extract (Fig.
3). In contrast, phosphorylation of Akt and ERK occurred
relatively slowly (at 15–30 min) in the coffee extract-treated
cells (Fig. 3).
Characterization of active coffee
constituents in coffee extracts
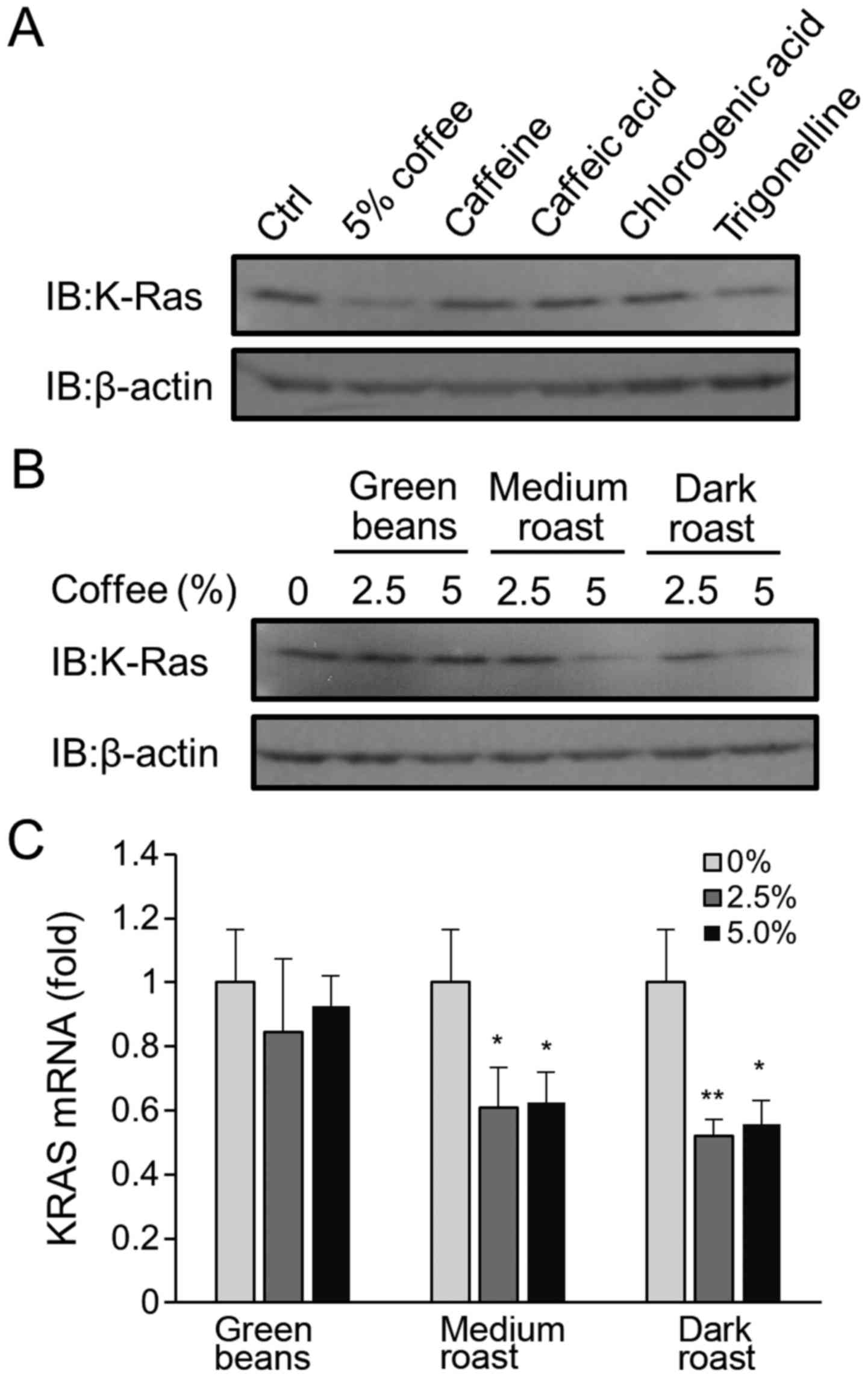
In order to elucidate which constituents were
responsible for coffee extract-mediated reduction in KRAS
expression, KRAS protein expression was measured following
treatment of cells with 100 µM caffeine, chlorogenic acid, caffeic
acid or trigonelline. All these compounds are major constituents of
coffee. None of these coffee constituents, except for trigonelline,
altered KRAS expression (Fig. 4A),
even though the concentration used was roughly equivalent to that
present in in 10–50% coffee extracts (13). Weak reduction with trigonelline was
detected.
In order to examine the possibility that
constituents with KRAS expression-altering capabilities are formed
during the roasting process, the activity of extracts of green
coffee beans that had undergone varying degrees of roasting prior
to brewing was assessed. Increasing the duration of roasting
resulted in greater reduction in KRAS protein and mRNA expression
(Fig. 4B and C, respectively).
Coffee induced miRNAs that target KRAS
gene expression
Previous studies have revealed that several miRNAs
are involved in the regulation of KRAS gene expression in
colorectal cancers (14,15). A preliminary microarray analysis of
Caco-2 cell miRNAs following treatment with 2.5% coffee extract
indicated that coffee induced expression of miR-30c and miR-96,
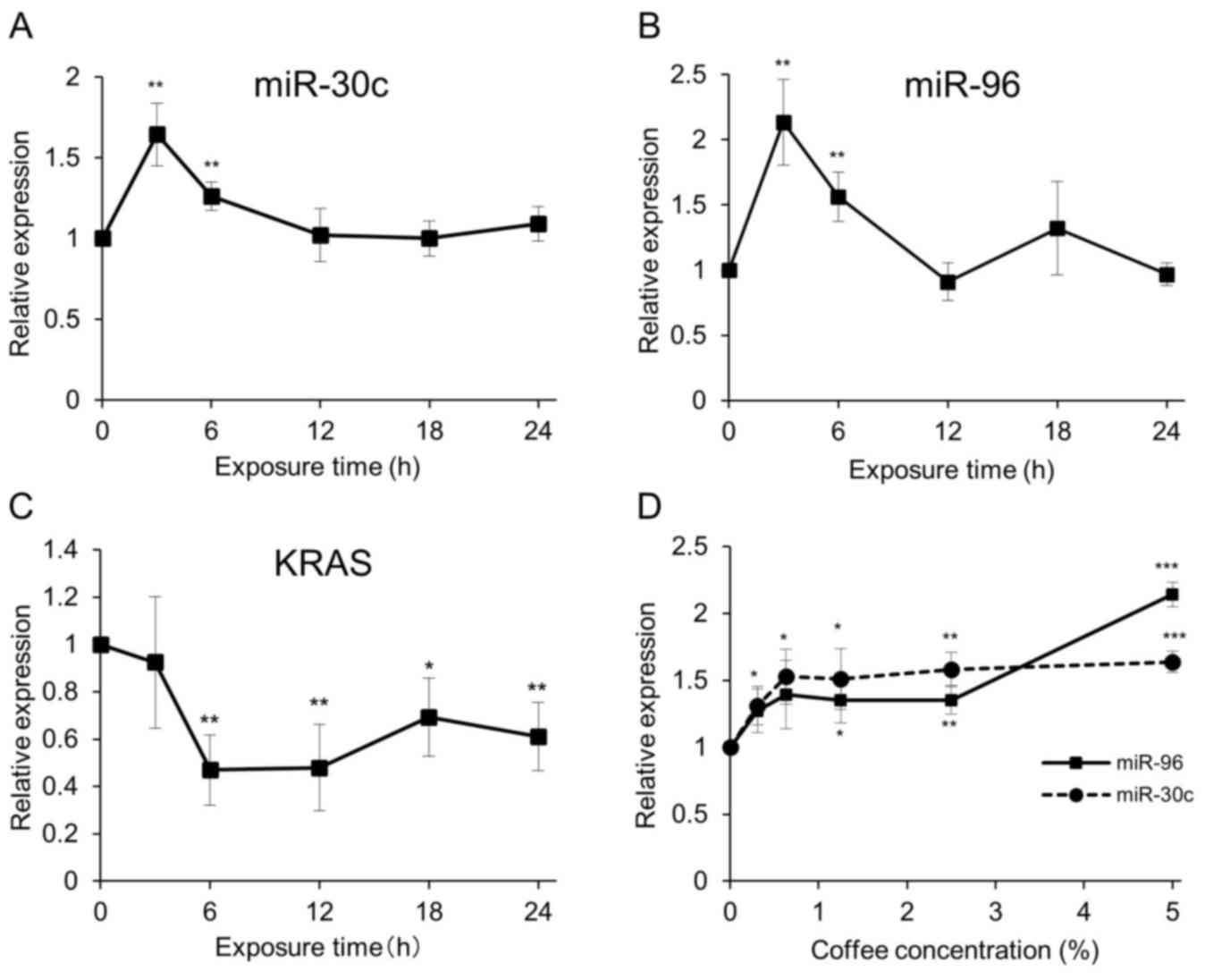
which are known to target the KRAS gene (data not shown).
Therefore, the expression of these miRNAs in coffee extract-treated
Caco-2 cells was examined. The expression of miR-30c and miR-96 in
Caco-2 cells was induced 3 h following the addition of 5% coffee
extract and gradually decreased to basal levels over time (Fig. 5A and B, respectively). Concomitantly,
KRAS mRNA expression levels decreased 6 h following coffee extract
treatment (Fig. 5C). The induction of
miRNAs occurred in a dose-dependent manner (Fig. 5D).
Discussion
The present study reported that coffee extract
reduced KRAS expression in Caco-2 human colon carcinoma cells.
Coffee extract mediated these effects by activating two miRNAs,
miR-30c and miR-96, which are known to target the KRAS gene
(Fig. 5). Coffee inhibited the
proliferation of Caco-2 cells. As KRAS is a key molecule for cell
growth and proliferation mediated by EGF, the inhibition of cell
proliferation following coffee extract may be due to the reduction
of KRAS expression.
Previous studies have reported regulation of KRAS
expression by miRNAs. For example, resveratrol prevents
tumorigenesis of colorectal cancers by suppressing KRAS expression,
which occurs by increasing miR-96 expression (14). Let-7a miRNA inhibits cell
proliferation in the lung cancer cell line, 95D, by regulating the
translation of KRAS mRNA (16). The
miRNA miR-30c suppresses breast cancer cell growth, potentially
through the inhibition of KRAS signaling (17). Similarly, in coffee extract-treated
Caco-2 cells, elevated miR-30c and miR-96 may suppress KRAS
expression, potentially by affecting KRAS mRNA stability (17). Further studies are needed to clarify
the mechanisms underlying regulation of miRNA expression by coffee
extract.
The induction of miRNAs occurred 3 h following the
addition of coffee extract and decreased following this. However,
the reduction of KRAS expression started at 6 h and continued to 24
h (Fig. 5C). The initial reduction in
KRAS expression by miRNAs may cause other cellular reactions to
suppress the expression of KRAS gene. Further studies are necessary
to clarify this issue.
Active constituents responsible for the reduction in
KRAS expression in coffee extract-treated Caco-2 cells were
demonstrated to emerge during the roasting of coffee beans
(Fig. 4B and C). A slight reduction
in KRAS expression occurred following exposure to 100 µM
trigonelline, however, trigonelline is known to be decomposed by
the roasting process. Therefore, trigonelline may not be
responsible for the KRAS reduction observed following exposure to
coffee extract. Multiple phenolic constituents and Maillard
reaction products are known to form during the coffee bean roasting
process (18–20). These compounds have been reported to
possess various types of antioxidant and pro-oxidant activity
(21,22) and often modulate antioxidant
transcription factors, including nuclear factor-κB (NF-κB) and
nuclear factor-E2-related factor 2. Furthermore, these
compounds escape digestion and pass through the upper
gastrointestinal tract into the colon (23). Thus, these compounds may be able to
interact with colon epithelial cells.
Previous evidence has suggested that NF-κB is
involved in the upregulation of miR-30c in several biological
responses (24,25). In addition, resveratrol, an
antioxidant, activates miR-96 in sporadic colorectal cancer cells
as mentioned above (14). Taken
together with the results of the present study, coffee antioxidants
that emerge during the roasting process may upregulate miR-30c in
Caco-2 cells by activating NF-κB. Activated miR-30c may
subsequently reduce KRAS expression in coffee extract-treated
Caco-2 cells. Coffee extract-induced NF-κB-mediated activation of
the ATP binding cassette subfamily G member 2 (Junior blood group)
gene has been observed in Caco-2 cells (26). Further studies are required to clarify
the identities of the active constituents in order to validate this
mechanism.
The results of the present study demonstrated that
KRAS inhibition by coffee resulted in reduced proliferation of
Caco-2 human colon carcinoma cells through the regulation of
miRNAs. Previous epidemiological studies indicated that coffee
consumption is associated with a protective effect for colorectal
cancer with a relative risk of 0.83 (95% confidence interval:
0.75–0.92) in a previous meta-analysis (27,28). The
inhibitory effect of coffee extract on KRAS expression may be a key
factor underlying the protective effects of coffee against
colorectal cancer.
Mutational activation of KRAS at residues 12, 13 and
61 is known to result in constitutive activation of downstream
effector pathways and deregulation of cell growth, survival and
differentiation. These are characteristics of a cancerous state
(29). Since KRAS in Caco-2 cells is
wild-type, a critical point for consideration is whether inhibition
of mutated KRAS by coffee consumption is protective against
colorectal cancer. Preliminary data indicated that the growth of
HCT116 cells, which have a mutated KRAS at codon 13, is also
suppressed by coffee extract, although the inhibition was
relatively weak (unpublished data).
Epidemiological studies have indicated that coffee
also confers protective effects against other cancers, including
liver cancers (5,28,30). KRAS
is frequently mutated in multiple cancers and is therefore an
attractive target for cancer therapy (31). Based on the results of the present,
coffee constituents that inhibit KRAS expression may be promising
candidates for cancer therapy, not only for colorectal cancer but
also for other cancers. Further investigations in other cell lines
and animals are necessary to identify these active
constituents.
Acknowledgements
The authors would like to thank Dr Kitaro Oka
(Emeritus professor of Tokyo University of Pharmacy and Life
Sciences, Tokyo, Japan), for his encouragement. The present study
was supported in part by a Grant-in-Aid from the Ministry of
Education, Culture, Sports, Science, and Technology (MEXT) of Japan
(Grant no., 15K00883) and by a grant from the MEXT-Supported
Program for the Strategic Research Foundation at Private
Universities. The abstract was presented at the 38th ESPEN
Congress, Copenhagen, Denmark, 17–20 September 2016, and published
as abstract no. SUN-P083 in Clinical Nutrition 35, (Suppl 1):
2016.
References
|
1
|
van Dam RM and Feskens EJ: Coffee
consumption and risk of type 2 diabetes mellitus. Lancet.
360:1477–1478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ascherio A, Weisskopf MG, O'Reilly EJ,
McCullough ML, Calle EE, Rodriguez C and Thun MJ: Coffee
consumption, gender, and Parkinson's disease mortality in the
cancer prevention study II cohort: The modifying effects of
estrogen. Am J Epidemiol. 160:977–984. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruhl CE and Everhart JE: Coffee and tea
consumption are associated with a lower incidence of chronic liver
disease in the United States. Gastroenterology. 129:1928–1936.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Je Y, Liu W and Giovannucci E: Coffee
consumption and risk of colorectal cancer: A systematic review and
meta-analysis of prospective cohort studies. Int J Cancer.
124:1662–1668. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu X, Bao Z, Zou J and Dong J: Coffee
consumption and risk of cancers: A meta-analysis of cohort studies.
BMC Cancer. 11:962011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian C, Wang W, Hong Z and Zhang X: Coffee
consumption and risk of colorectal cancer: A dose-response analysis
of observational studies. Cancer Causes Control. 24:1265–1268.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang NJ, Lee KW, Kim BH, Bode AM, Lee HJ,
Heo YS, Boardman L, Limburg P, Lee HJ and Dong Z: Coffee phenolic
phytochemicals suppress colon cancer metastasis by targeting MEK
and TOPK. Carcinogenesis. 32:921–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lev Z, Kislitsin D, Rennert G and Lerner
A: Utilization of K-ras mutations identified in stool DNA for the
early detection of colorectal cancer. J Cell Biochem Suppl.
34:35–39. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Campbell SL, Khosravi-Far R, Rossman KL,
Clark GJ and Der CJ: Increasing complexity of Ras signaling.
Oncogene. 17(11 Reviews): 1395–1413. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hackel PO, Zwick E, Prenzel N and Ullrich
A: Epidermal growth factor receptors: Critical mediators of
multiple receptor pathways. Curr Opin Cell Biol. 11:184–189. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feinberg AP, Vogelstein B, Droller MJ,
Baylin SB and Nelkin BD: Mutation affecting the 12th amino acid of
the c-Ha-ras oncogene product occurs infrequently in human cancer.
Science. 220:1175–1177. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oka K: Pharmacological bases of coffee
nutrients for diabetes prevention. Yakugaku Zasshi. 127:1825–1836.
2007.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saud SM, Li W, Morris NL, Matter MS,
Colburn NH, Kim YS and Young MR: Resveratrol prevents tumorigenesis
in mouse model of Kras activated sporadic colorectal cancer by
suppressing oncogenic Kras expression. Carcinogenesis.
35:2778–2786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luu C, Heinrich EL, Duldulao M, Arrington
AK, Fakih M, Garcia-Aguilar J and Kim J: TP53 and let-7a micro-RNA
regulate K-Ras activity in HCT116 colorectal cancer cells. PLoS
One. 8:e706042013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YY, Ren T, Cai YY and He XY: MicroRNA
let-7a inhibits the proliferation and invasion of nonsmall cell
lung cancer cell line 95D by regulating K-Ras and HMGA2 gene
expression. Cancer Biother Radiopharm. 28:131–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanic M, Yanowsky K, Rodriguez-Antona C,
Andrés R, Márquez-Rodas I, Osorio A, Benitez J and Martinez-Delgado
B: Deregulated miRNAs in hereditary breast cancer revealed a role
for miR-30c in regulating KRAS oncogene. PLoS One. 7:e388472012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
del Castillo MD, Ames JM and Gordon MH:
Effect of roasting on the antioxidant activity of coffee brews. J
Agric Food Chem. 50:3698–3703. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paur I, Balstad TR, Kolberg M, Pedersen
MK, Austenaa LM, Jacobs DR Jr and Blomhoff R: Extract of oregano,
coffee, thyme, clove, and walnuts inhibits NF-kappaB in monocytes
and in transgenic reporter mice. Cancer Prev Res (Phila).
3:653–663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paur I, Balstad TR and Blomhoff R: Degree
of roasting is the main determinant of the effects of coffee on
NF-kappaB and EpRE. Free Radic Biol Med. 48:1218–1227. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nicoli MC, Anese M, Parpinel MT,
Franceschi S and Lerici CR: Loss and/or formation of antioxidants
during food processing and storage. Cancer Lett. 114:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Daglia M, Papetti A, Gregotti C, Bertè F
and Gazzani G: In vitro antioxidant and ex vivo protective
activities of green and roasted coffee. J Agric Food Chem.
48:1449–1454. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fogliano V and Morales FJ: Estimation of
dietary intake of melanoidins from coffee and bread. Food Funct.
2:117–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nguyen HT, Dalmasso G, Müller S, Carrière
J, Seibold F and Darfeuille-Michaud A: Crohn's disease-associated
adherent invasive Escherichia coli modulate levels of microRNAs in
intestinal epithelial cells to reduce autophagy. Gastroenterology.
146:508–519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goparaju CM, Blasberg JD, Volinia S,
Palatini J, Ivanov S, Donington JS, Croce C, Carbone M, Yang H and
Pass HI: Onconase mediated NFKβ downregulation in malignant pleural
mesothelioma. Oncogene. 30:2767–2777. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Isshiki M, Umezawa K and Tamura H: Coffee
induces breast cancer resistance protein expression in Caco-2
cells. Biol Pharm Bull. 34:1624–1627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galeone C, Turati F, La Vecchia C and
Tavani A: Coffee consumption and risk of colorectal cancer: A
meta-analysis of case-control studies. Cancer Causes Control.
21:1949–1959. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bøhn SK, Blomhoff R and Paur I: Coffee and
cancer risk, epidemiological evidence, and molecular mechanisms.
Mol Nutr Food Res. 58:915–930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Roock W, Claes B, Bernasconi D, De
Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V,
Papamichael D, Laurent-Puig P, et al: Effects of KRAS, BRAF, NRAS,
and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy
in chemotherapy-refractory metastatic colorectal cancer: A
retrospective consortium analysis. Lancet Oncol. 11:753–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bravi F, Bosetti C, Tavani A, Bagnardi V,
Gallus S, Negri E, Franceschi S and La Vecchia C: Coffee drinking
and hepatocellular carcinoma risk: A meta-analysis. Hepatology.
46:430–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Friday BB and Adjei AA: K-ras as a target
for cancer therapy. Biochim Biophys Acta. 1756:127–144.
2005.PubMed/NCBI
|