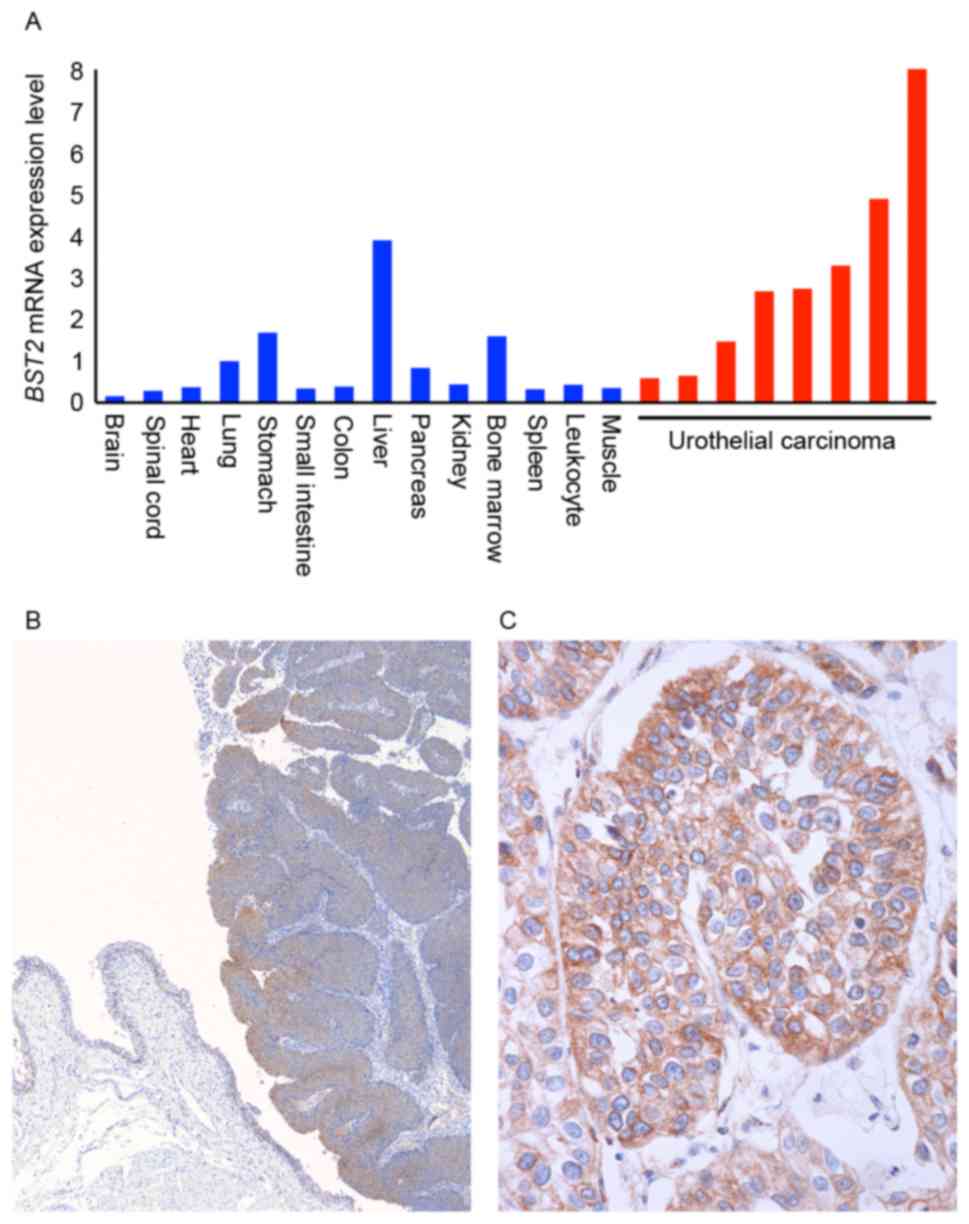
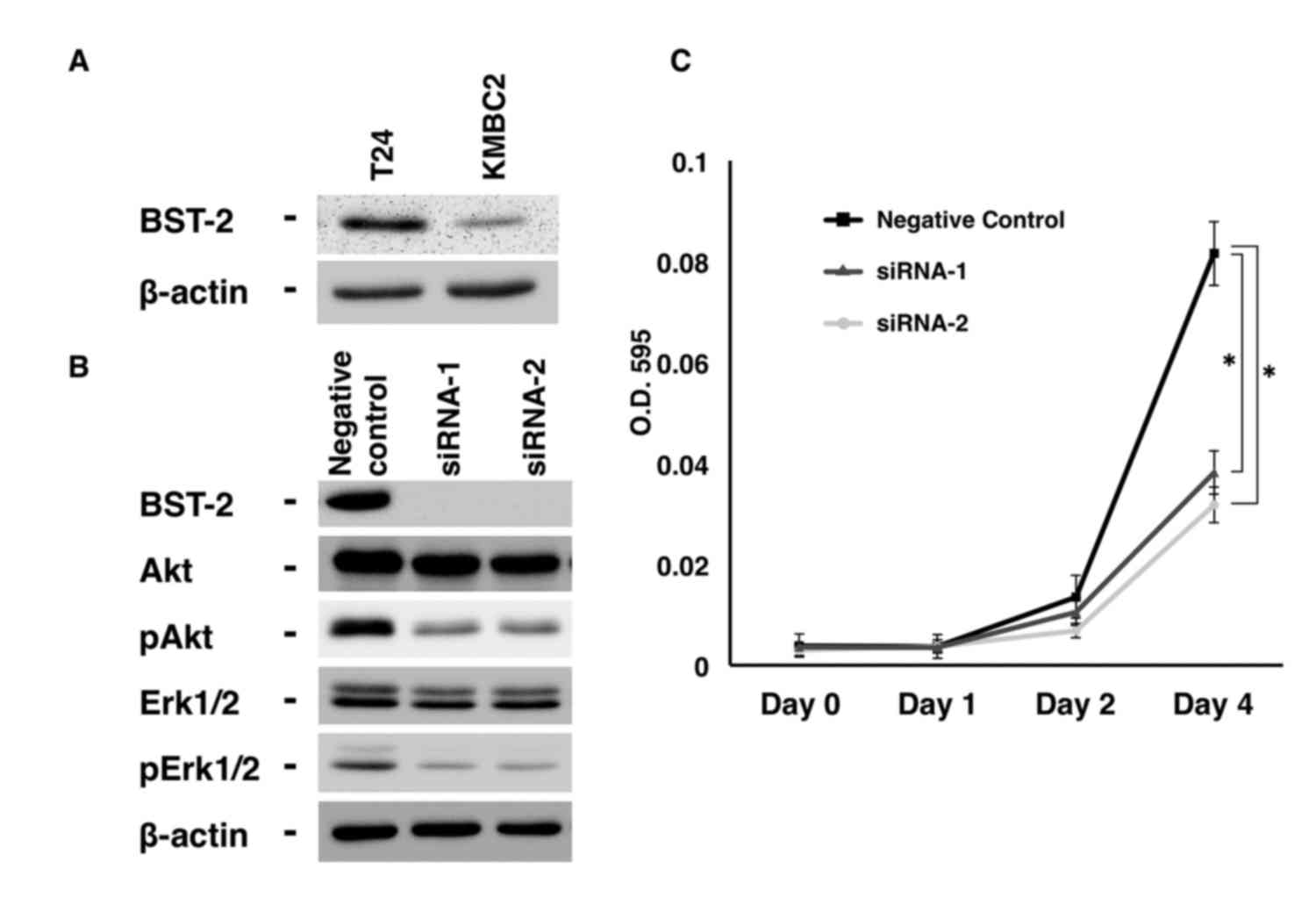
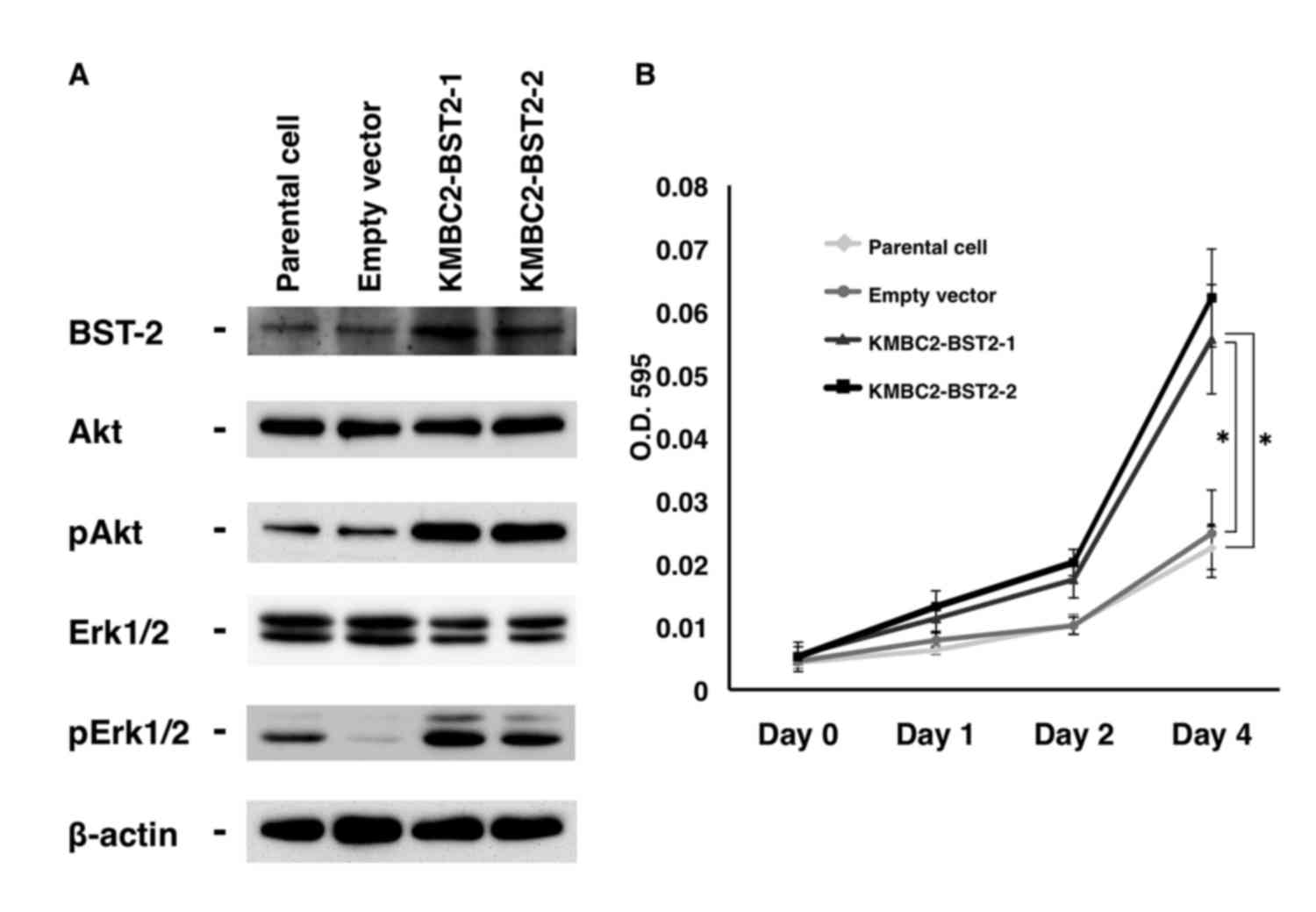
|
1
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: A
combined analysis of 2596 patients from seven EORTC trials. Eur
Urol. 49:466–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Knollman H, Godwin JL, Jain R, Wong YN,
Plimack ER and Geynisman DM: Muscle-invasive urothelial bladder
cancer: An update on systemic therapy. Ther Adv Urol. 7:312–330.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferguson DA, Muenster MR, Zang Q, Spencer
JA, Schageman JJ, Lian Y, Garner HR, Gaynor RB, Huff JW,
Pertsemlidis A, et al: Selective identification of secreted and
transmembrane breast cancer markers using Escherichia coli
ampicillin secretion trap. Cancer Res. 65:8209–8217. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mukai S, Oue N, Oshima T, Mukai R,
Tatsumoto Y, Sakamoto N, Sentani K, Tanabe K, Egi H, Hinoi T, et
al: Overexpression of Transmembrane Protein BST2 is Associated with
Poor Survival of Patients with Esophageal, Gastric, or Colorectal
Cancer. Ann Surg Oncol. 24:594–602. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kupzig S, Korolchuk V, Rollason R, Sugden
A, Wilde A and Banting G: Bst-2/HM1.24 is a raft-associated apical
membrane protein with an unusual topology. Traffic. 4:694–709.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goto T, Kennel SJ, Abe M, Takishita M,
Kosaka M, Solomon A and Saito S: A novel membrane antigen
selectively expressed on terminally differentiated human B cells.
Blood. 84:1922–1930. 1994.PubMed/NCBI
|
|
7
|
Ozaki S, Kosaka M, Wakatsuki S, Abe M,
Koishihara Y and Matsumoto T: Immunotherapy of multiple myeloma
with a monoclonal antibody directed against a plasma cell-specific
antigen, HM1.24. Blood. 90:3179–3186. 1997.PubMed/NCBI
|
|
8
|
Walter-Yohrling J, Cao X, Callahan M,
Weber W, Morgenbesser S, Madden SL, Wang C and Teicher BA:
Identification of genes expressed in malignant cells that promote
invasion. Cancer Res. 63:8939–8947. 2003.PubMed/NCBI
|
|
9
|
Cai D, Cao J, Li Z, Zheng X, Yao Y, Li W
and Yuan Z: Up-regulation of bone marrow stromal protein 2 (BST2)
in breast cancer with bone metastasis. BMC Cancer. 9:1022009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yokoyama T, Enomoto T, Serada S, Morimoto
A, Matsuzaki S, Ueda Y, Yoshino K, Fujita M, Kyo S, Iwahori K, et
al: Plasma membrane proteomics identifies bone marrow stromal
antigen 2 as a potential therapeutic target in endometrial cancer.
Int J Cancer. 132:472–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Nishioka Y, Ozaki S, Jalili A, Abe
S, Kakiuchi S, Kishuku M, Minakuchi K, Matsumoto T and Sone S:
HM1.24 (CD317) is a novel target against lung cancer for
immunotherapy using anti-HM1.24 antibody. Cancer Immunol
Immunother. 58:967–976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: TNM classification of malignant tumours. 7th. New York:
Wiley-Liss; pp. 262–265. 2009
|
|
13
|
Kondo T, Oue N, Yoshida K, Mitani Y, Naka
K, Nakayama H and Yasui W: Expression of POT1 is associated with
tumor stage and telomere length in gastric carcinoma. Cancer Res.
64:523–529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oue N, Naito Y, Hayashi T, Takigahira M,
Kawano-Nagatsuma A, Sentani K, Sakamoto N, Oo H Zarni, Uraoka N,
Yanagihara K, et al: Signal peptidase complex 18, encoded by
SEC11A, contributes to progression via TGF-α secretion in gastric
cancer. Oncogene. 33:3918–3926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakamoto N, Oue N, Sentani K, Anami K,
Uraoka N, Naito Y, Oo HZ, Hinoi T, Ohdan H, Yanagihara K, et al:
Liver-intestine cadherin induction by epidermal growth factor
receptor is associated with intestinal differentiation of gastric
cancer. Cancer Sci. 103:1744–1750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yasui W, Ayhan A, Kitadai Y, Nishimura K,
Yokozaki H, Ito H and Tahara E: Increased expression of p34cdc2 and
its kinase activity in human gastric and colonic carcinomas. Int J
Cancer. 53:36–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ,
Kussie P and Ferguson KM: Structural basis for inhibition of the
epidermal growth factor receptor by cetuximab. Cancer Cell.
7:301–311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
von der Maase H, Sengelov L, Roberts JT,
Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A and Arning
M: Long-term survival results of a randomized trial comparing
gemcitabine plus cisplatin, with methotrexate, vinblastine,
doxorubicin, plus cisplatin in patients with bladder cancer. J Clin
Oncol. 23:4602–4608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenberg JE, Hoffman-Censits J, Powles T,
van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH,
Balmanoukian A, Loriot Y, et al: Atezolizumab in patients with
locally advanced and metastatic urothelial carcinoma who have
progressed following treatment with platinum-based chemotherapy: A
single-arm, multicentre, phase 2 trial. Lancet. 387:1909–1920.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeuchi K and Ito F: EGF receptor in
relation to tumor development: Molecular basis of responsiveness of
cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J.
277:316–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choudhury NJ, Campanile A, Antic T, Yap
KL, Fitzpatrick CA, Wade JL III, Karrison T, Stadler WM, Nakamura Y
and O'Donnell PH: Afatinib activity in platinum-refractory
metastatic urothelial carcinoma in patients With ERBB alterations.
J Clin Oncol. 34:2165–2171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harada T and Ozaki S: Targeted therapy for
HM1.24 (CD317) on multiple myeloma cells. Biomed Res Int.
2014:9653842014. View Article : Google Scholar : PubMed/NCBI
|