Introduction
At present, resection is an important treatment
option for liver cancer. However, intraoperative liver damage is
often inevitable. Ischemia-reperfusion injury in liver surgery is a
common pathophysiological consequence in patients (1). Patients with liver cancer may already
experience a certain degree of liver dysfunction before surgery.
Therefore, choosing an appropriate anesthetic is key for minimizing
further damage to the liver during this type of surgery.
On the surface of hepatocytes, hepatic sinusoidal
endothelial cells and vascular endothelial cells express various
adhesion molecules, among which intercellular adhesion molecule-1
(ICAM-1) is primarily expressed in liver tissue. The expression of
ICAM-1 can serve as a biomarker of the extent of liver damage,
where higher expression indicates more severe liver damage
(2). In the present study,
remifentanil was used for general anesthesia of patients undergoing
liver cancer surgery, to investigate the effects of remifentanil on
liver function and ICAM-1 expression, as well as blood pressure
variation in patients. The findings may serve as a reference for
selecting appropriate anesthesia during surgery.
Patients and methods
Patients
A total of 60 patients who underwent liver cancer
resection in The First People's Hospital of Xiangyang, Hubei
University of Medicine (Xiangyang, China) were randomly selected
from January 2014 to January 2016, including 33 males and 27
females with average age of 54.12±4.77 years. All selected patients
were designated as class I or class II according to the American
Society of Anesthesiologists Physical Status classification system,
and Class A according to the Child-Pugh classification of liver
disease. The patients were randomly divided into the control group
and experimental group, with 30 cases in each group. There were 17
males and 13 females in the control group, with average age of
54.87±3.76 years, and 15 males and 15 females in the experimental
group, with an average age of 53.43±4.12 years. There were no
significant differences in preoperative liver function tests
between patients, and surgery was completed by the same group of
physicians in The First People's Hospital of Xiangyang, Hubei
University of Medicine. Patients had no history of mental disease,
neurological disease, or serious cardiovascular disease; no recent
administration of narcotic analgesics and sedative hypnotics; and
no history of using drugs that can cause liver damage, such as
benzodiazepines and opioids. This study was approved by the Ethics
Committee of Xiangyang No. 1 People's Hospital. Signed written
informed consents were obtained from all participants before the
study.
Anesthesia
Patients in the control group were anesthetized with
propofol/isoflurane, whereas patients in the experimental group
were administered remifentanil/propofol (Yangze Pharma, Taizhou,
China). Patients were connected to the electrocardiogram monitor,
and received oxygen flow through a face mask after entering the
operating room. Physiological indicators such as blood pressure,
heart rate (HR), and finger oxygen saturation were monitored. After
establishing the upper extremity venous access, a catheter was
placed in the right internal jugular vein for measuring the central
venous pressure while keeping the venous access open. Anesthetics
such as remifentanil, propofol, and midazolam (Yangze Pharma) were
administered to patients at the dose of 4 µg/kg, 2.5 mg/kg, and
0.05 mg/kg, respectively, for anesthesia induction before
anesthesia to facilitate intubation. Patients in the control group
were anesthetized with propofol/isoflurane. Following the protocol,
propofol was administered intravenously at a dose of 6.5–9.0
mg/kg/h, and 2–3% isoflurane was simultaneously administered by
inhalation. The administration of isoflurane and propofol was
stopped 15 and 5 min, respectively, before surgery was finished.
During surgery, the concentration of isoflurane was adjusted in
time in accordance with the depth of anesthesia and changes of
patient hemodynamic parameters. Patients in the experimental group
were anesthetized with remifentanil/propofol at a dose of 0.3–0.5
µg/kg/min and 7–10 mg/kg/h, respectively. The doses of remifentanil
and propofol were adjusted according to hemodynamic changes during
surgery. Infusion of remifentanil and propofol was stopped 5 min
before surgery was finished. Patients in the two groups were
extubated after wake-up, recovery of spontaneous breathing, and
activation of cough and swallowing reflexes. Finally, patients were
sent to the intensive care unit.
ICAM-1 measurement
Hepatic blood flow was controlled during surgery in
patients in the two groups when liver tissues were harvested. The
obtained liver tissues were marked and stored in liquid nitrogen.
The SABC staining technique was used to determine the expression of
ICAM-1 in liver tissue. Briefly, samples were first prepared and
cut into 5 µm paraffin-embedded sections. The sections were then
affixed on microscope glass slides using an adhesive. After drying,
the sections were dewaxed and hydrated. After endogenous enzymes
were inactivated at 20°C for 10 min, the sections were washed three
times with distilled water, and then immersed in citrate buffer.
The buffer was heated until it boiled, and was then left to stand
for 10 min without heating. After repeating the heating/cooling
cycle two times, the sections were rinsed two times with PBS,
followed by the addition of antigen retrieval buffer to expose the
antigen. The sections were then rinsed two times with PBS, followed
by addition of BSA blocking buffer, and incubation for 20 min.
Rabbit anti-rat ICAM-1 monoclonal antibody was added and incubated
at 4°C. After warming the sections to room temperature, they were
rinsed with PBS, followed by the addition of SABC reagent, and
incubation. The sections were placed in a kit and developed at
20°C, followed by washing, counterstaining, dehydration, clearing,
and mounting. The sections were then observed under a light
microscope (BX-42; Olympus, Tokyo, Japan). ICAM-1 antibody, DAB
chromogenic reagent, and other immunohistochemistry reagents were
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China).
Observational indicators
The general parameters of patients (preoperative)
were measured and recorded, such as sex, age, mean HR, mean
arterial pressure (MAP), and liver function markers including
aspartate aminotransferase (AST), alanine aminotransferase (ALT),
and total bilirubin (TBIL). The mean systolic blood pressure (SBP)
and mean diastolic blood pressure (DBP) were obtained before
treatment, during anesthesia induction and intubation, during
blockade of traction reflexes in surgery, and before extubation at
the end of surgery. The recovery time from anesthesia withdrawal to
spontaneous breathing, time of eye opening, time of extubation, as
well as the level of consciousness were recorded. The level of
consciousness was graded according to the Observer's Assessment of
Alertness/Sedation Scale. Patients received a score of 5 if they
replied readily to name call and were fully awake, a score of 4 if
they replied sluggishly to name call with a slow speech rate, a
score of 3 if they replied only to loud name call with blurred
speech and dull eyes, a score of 2 if they did not reply to name
call and only responded to mild prodding or shaking, and a score of
1 if they were in a lethargic state and did not respond to mild
prodding and shaking. In addition, the levels of liver function
markers, AST, ALT, and TBIL were measured in all patients at the
first (D1), third (D3), fifth (D5), and seventh (D7) day after
surgery.
Statistical analysis
SPSS 19.0 statistical software was used for data
analysis (IBM SPSS, Armonk, NY, USA). Numerical data are presented
as mean ± standard deviation. A Chi-squared (χ2) test
was used for categorical data. The t-test for two independent
samples was used for intergroup comparisons. Repeated measures
analysis of variance was employed for intragroup comparisons.
P<0.05 was considered to be statistically significant.
Results
General parameters (preoperative)
There were no significant differences in sex, age,
HR, MAP, AST, ALT, or TBIL between the control group and
experimental group (P>0.05; Table
I).
 | Table I.General parameters of patients
(preoperative). |
Table I.
General parameters of patients
(preoperative).
| Group | Sex
(male/female) | Age (years) | HR (per min) | MAP (mm Hg) | AST (µmol/l) | ALT (µmol/l) | TBIL (µmol/l) |
|---|
| Control | 17/13 | 54.87±3.76 | 82.95±7.28 |
87.54±10.29 | 41.28±11.39 | 47.13±12.84 | 15.24±7.93 |
| Experimental | 15/15 | 53.43±4.12 | 84.26±7.15 | 88.78±9.53 | 39.52±12.63 | 45.21±11.83 | 15.87±8.44 |
| P-Value | P>0.05 | P>0.05 | P>0.05 | P>0.05 | P>0.05 | P>0.05 | P>0.05 |
Hemodynamic data
In the control group, SBP and DBP were significantly
higher during blockade of traction reflexes in surgery and before
extubation at the end of surgery compared with the values during
anesthesia induction and intubation (P<0.05). However, in the
experimental group, SBP and DBP were comparable at these time
points (P>0.05). Comparing the two groups, SBP and DBP during
blockade of traction reflexes in surgery and before extubation at
the end of surgery were lower in the experimental group than in the
control group (P<0.05; Table
II).
 | Table II.Hemodynamic data of the two groups at
different time points. |
Table II.
Hemodynamic data of the two groups at
different time points.
| Group | Blood pressure | Before surgery | During anesthesia
induction and intubation | During blockade of
traction reflexes in surgery | Before extubation at
the end of surgery |
|---|
| Control | SBP (mm Hg) | 132.25±15.43 | 119.95±14.52 |
150.37±17.27a,c |
145.63±12.54a,c |
|
| DBP (per min) | 78.47±10.52 | 72.54±7.28 |
88.76±11.75a,c |
86.49±10.17a,c |
| Experimental | SBP (mm Hg) | 133.78±14.53 | 122.14±13.43 |
124.07±13.12b,c |
123.82±12.89b,c |
|
| DBP (per min) | 77.68±11.17 | 72.88±8.15 |
73.96±9.82b,c |
72.19±9.48b,c |
Recovery time
In the comparison of recovery time of spontaneous
breathing, time of eye opening, extubation time, and level of
consciousness (Table III), the
experimental group was better than the control group, and the
differences were statistically significant (P<0.05).
 | Table III.Recovery data of the two groups. |
Table III.
Recovery data of the two groups.
| Group | Recovery time of
spontaneous breathing | Time of eye
opening | Extubation time | Level of
consciousness |
|---|
| Control |
8.81±3.51a |
19.53±4.69a |
21.27±5.83a |
3.96±0.67a |
| Experimental | 5.42±2.64 | 11.66±3.92 | 13.73±4.38 | 4.48±0.31 |
Liver function
The levels of the liver function markers (AST, ALT,
and TBIL) of the two groups of patients were significantly higher
after surgery compared with those before surgery (P<0.05). The
levels of AST, ALT, and TBIL on the first, third, and fifth day
after surgery were higher in the control group than in the
experimental group, and the differences were statistically
significant (P<0.05). On the seventh day, the levels of the
three markers returned to normal in both groups, and there were no
significant differences between the two groups (Table IV).
 | Table IV.The levels of liver function
markers. |
Table IV.
The levels of liver function
markers.
| Group | Marker | D0 | D1 | D3 | D5 | D7 |
|---|
| Control | AST | 41.28±11.39 |
188.46±29.19a,c |
152.93±34.62a,c |
90.63±23.64a,c | 47.41±13.64 |
|
| ALT | 47.13±12.84 |
196.11±32.75a,c |
160.37±34.51a,c |
99.82±25.77a,c | 49.63±14.96 |
|
| TBIL | 15.24±7.93 |
19.81±8.1a,c |
24.14±8.69a,c |
27.59±10.67a,c | 16.17±7.81 |
| Experimental | AST | 39.52±12.63 |
154.82±26.39b,c |
129.86±25.19b,c |
72.47±19.78b,c | 40.42±10.56 |
|
| ALT | 45.21±11.83 |
161.14±31.46b,c |
131.43±30.31b,c |
75.97±20.73b,c | 42.55±11.87 |
|
| TBIL | 15.87±8.44 |
17.15±7.88b,c |
22.97±8.13b,c |
18.77±9.64b,c | 15.93±8.11 |
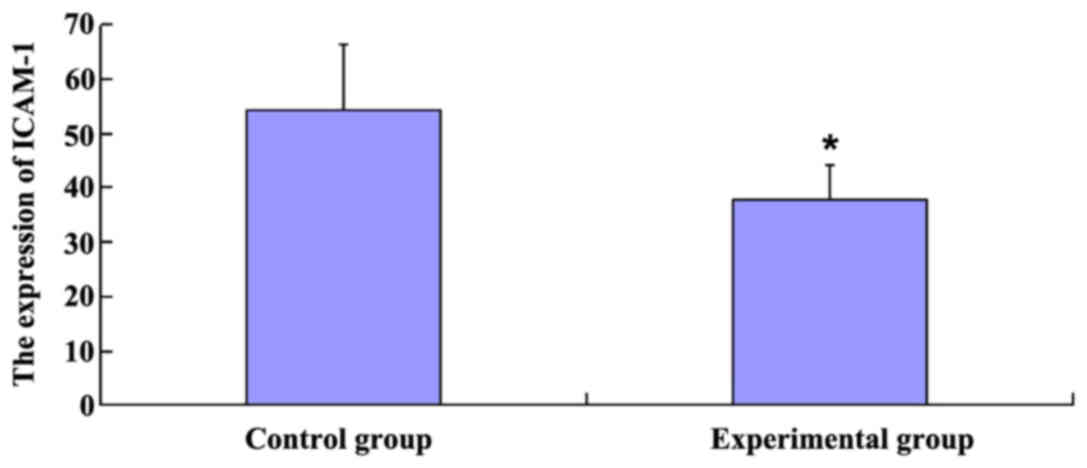
ICAM-1 expression
The expression of ICAM-1 was 54.31% in the control
group, which was significantly higher than in the experimental
group (37.66%) (P<0.05; Fig.
1).
Discussion
According to global statistics, liver cancer ranked
third among cancer deaths in 2002 (3). Primary liver cancer is not only a common
malignant tumor, but also one of the leading causes of death.
According to year 2000 statistics, the number of cases of liver
cancer in China accounted for 55% of the global case number,
indicating that China has become a country with high incidence of
liver cancer. Moreover, the number of cases is increasing. The
number of new cases each year has reached approximately 350,000
(4). Presently, there are effective
ways to treat liver cancer, including surgery, radiotherapy and
chemotherapy, liver transplantation, biological therapy, and
Chinese medicine (5). Surgery with
resection offers the only chance to cure liver cancer, thus partial
hepatectomy is currently the preferred method of treatment for
liver cancer (6). Operative blood
loss is a major risk factor associated with operative mortality
after partial hepatectomy. For effective bleeding control,
temporary occlusion of hepatopetal blood flow is highly necessary.
However, this procedure may result in ischemia-reperfusion injury
of hepatocytes. For patients with poor liver function, reperfusion
injury may directly affect postoperative liver function recovery
and liver regeneration, which are associated with postoperative
liver failure and increased mortality (7,8). Thus,
prevention or reduction of liver ischemia-reperfusion injury during
surgery has important clinical significance for patients undergoing
liver cancer surgery.
Establishing procedures to prevent or reduce hepatic
ischemia-reperfusion injury has become a research focus for
researchers and clinicians over the world. It was reported that
administration of an appropriate anesthetic drug during surgery
reduced liver reperfusion injury to a certain extent by improving
liver tolerance to ischemia (9,10).
Remifentanil is a potent opioid receptor agonist
characterized by fast onset and rapid metabolism. It is rapidly
metabolized to an inactive metabolite by plasma and tissue
esterases, which is independent of the liver and kidney, thus it
has been widely used in liver surgery anesthesia (11). In addition, it was recently reported
that remifentanil plays a role in reducing ischemia-reperfusion
injury (12). In this control study,
it was found that patients receiving remifentanil during liver
surgery experienced a smaller increase in the levels of the liver
function markers on the first, third, and fifth day after surgery,
compared with patients in the control group. Moreover, the
experimental group (administered remifentanil) was better than the
control group (administered isoflurane) in terms of recovery time
of spontaneous breathing, time of eye opening, extubation time, and
level of consciousness. This showed that administration of
remifentanil during surgery reduced liver ischemia-reperfusion
injury, played a protective role in postoperative liver function,
and to some extent promoted recovery of liver function. In
addition, remifentanil was obviously advantageous for postoperative
recovery.
ICAM-1 is a single-chain transmembrane glycoprotein
with a core peptide molecular weight of 55 kDa. It is a member of
the immunoglobulin superfamily. When inflammation occurs,
inflammatory factors are released, which can induce endothelial
cells and other cells to express ICAM-1 on the cell surface, thus
enhancing surface adhesion. In the present study, it was found that
the level of ICAM-1 in the experimental group was lower than that
in the control group, and the difference was statistically
significant. This showed that the administration of remifentanil
reduced the release of certain cytokines and proinflammatory
factors in liver ischemia-reperfusion, resulting in a reduced
inflammatory response of the liver, and protected liver cells to
some extent.
Most patients with liver cancer are middle-aged and
older, who show age-related low responses in autonomic reflexes,
and have poor ability to adjust their response to various stimuli
such as cutting, traction, and clipping during surgery. Therefore,
these patients tend to experience large fluctuations in
hemodynamics, and it is difficult to maintain a stable state. For
older patients whose hemodynamics are unstable during surgery,
selecting an appropriate anesthesia is highly important for
reducing anesthesia complications (13). In the present study, it was found that
when using remifentanil anesthesia during surgery, patients
experienced smaller fluctuations in blood pressure than patients in
the control group. This showed that remifentanil can better
maintain blood pressure stability when patients are sedated during
surgery, and thus the stability of the circulatory system.
In conclusion, remifentanil can better maintain
blood pressure stability of older patients during surgery, and the
stability of the circulatory system. In addition, remifentanil can
reduce the release of liver-related cell adhesion molecules and
inflammatory factors, thus reducing liver ischemia-reperfusion
injury to protect liver function.
References
|
1
|
Kato R, Ross S and Foëx P: Fentanyl
protects the heart against ischaemic injury via opioid receptors,
adenosine A1 receptors and KATP channel linked mechanisms in rats.
Br J Anaesth. 84:204–214. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamanouchi K, Yanaga K, Okudaira S, Eguchi
S, Furui J and Kanematsu T: [D-Ala2, D-Leu5] enkephalin (DADLE)
protects liver against ischemia-reperfusion injury in the rat. J
Surg Res. 114:72–77. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thomas MB, O'Beime JP, Furuse J, Chan AT,
Abou-Alfa G and Johnson P: Systemic therapy for hepatocellular
carcinoma: Cytotoxic chemotherapy, targeted therapy and
immunotherapy. Ann Surg Oncol. 15:1008–1014. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Boer MT, Molenaar IQ and Porte RJ:
Impact of blood loss on outcome after liver resection. Dig Surg.
24:259–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clavien PA, Selzner M, Rüdiger HA, Graf R,
Kadry Z, Rousson V and Jochum W: A prospective randomized study in
100 consecutive patients undergoing major liver resection with
versus without ischemic preconditioning. Ann Surg. 238:843–850;
discussion 851–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banga NR, Homer-Vanniasinkam S, Graham A,
Al-Mukhtar A, White SA and Prasad KR: Ischaemic preconditioning in
transplantation and major resection of the liver. Br J Surg.
92:528–538. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Müller C, Dünschede F, Koch E, Vollmar AM
and Kiemer AK: Alpha-lipoic acid preconditioning reduces
ischemia-reperfusion injury of the rat liver via the PI3-kinase/Akt
pathway. Am J Physiol Gastrointest Liver Physiol. 285:G769–G778.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bedirli N, Ofluoglu E, Kerem M, Utebey G,
Alper M, Yilmazer D, Bedirli A, Ozlu O and Pasaoglu H: Hepatic
energy metabolism and the differential protective effects of
sevoflurane and isoflurane anesthesia in a rat hepatic
ischemia-reperfusion injury model. Anesth Analg. 106:830–837. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lötsch J: Pharmacokinetic-pharmacodynamic
modeling of opioids. J Pain Symptom Manag. 29:S90–S103. 2005.
View Article : Google Scholar
|
|
12
|
Kim HS, Cho JE, Hong SW, Kim SO, Shim JK
and Kwak YL: Remifentanil protects myocardium through activation of
anti-apoptotic pathways of survival in ischemia-reperfused rat
heart. Physiol Res. 59:347–356. 2010.PubMed/NCBI
|
|
13
|
Kim TY, Kim DK, Yoon TG, Lim JA, Woo NS,
Chee HK, Shin JK, Song MG and Kim SH: Myocardial injury in
remifentanil-based anaesthesia for off-pump coronary artery bypass
surgery: An equipotent dose of sevoflurane versus propofol. Anaesth
Intensive Care. 39:418–425. 2011.PubMed/NCBI
|