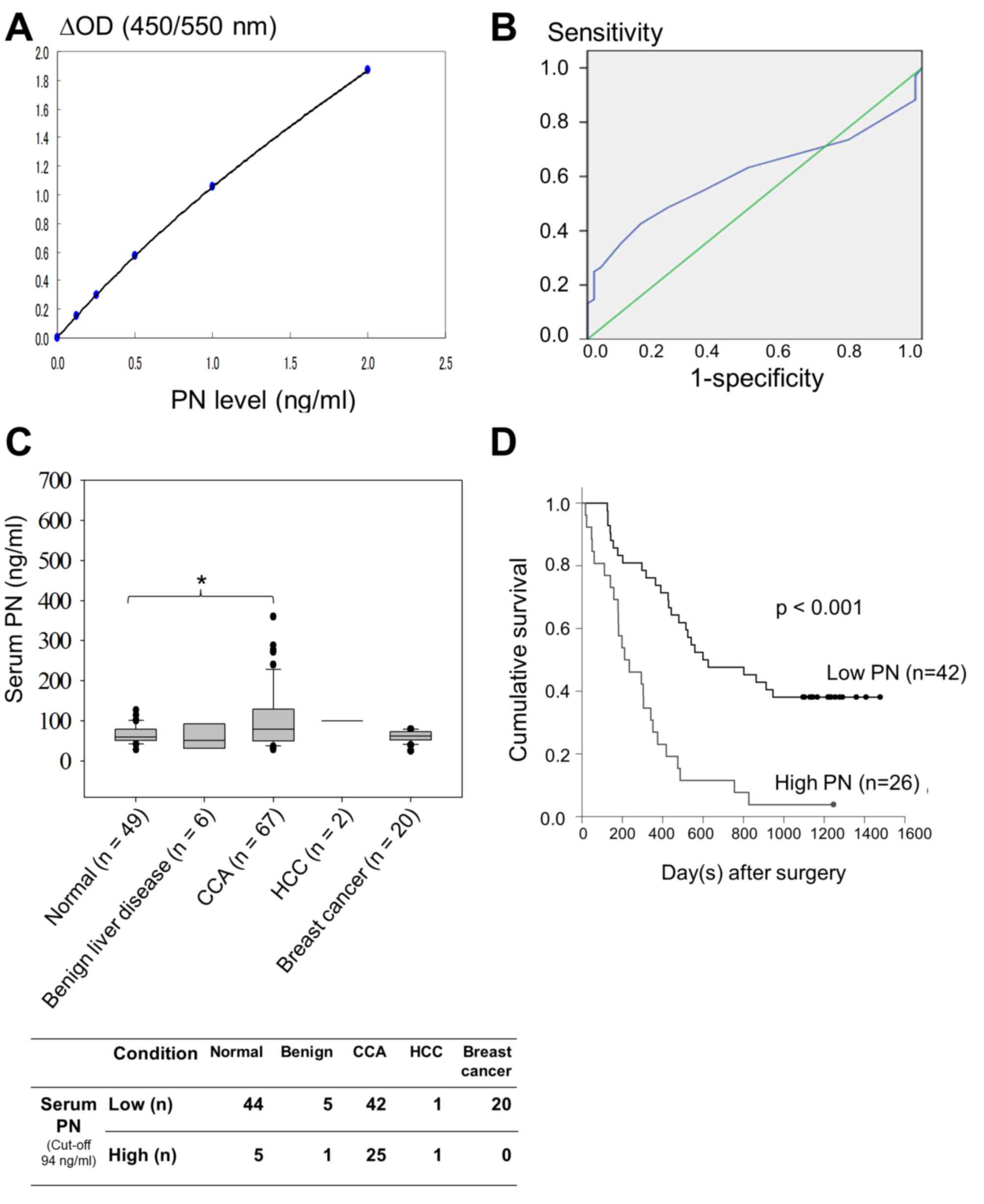
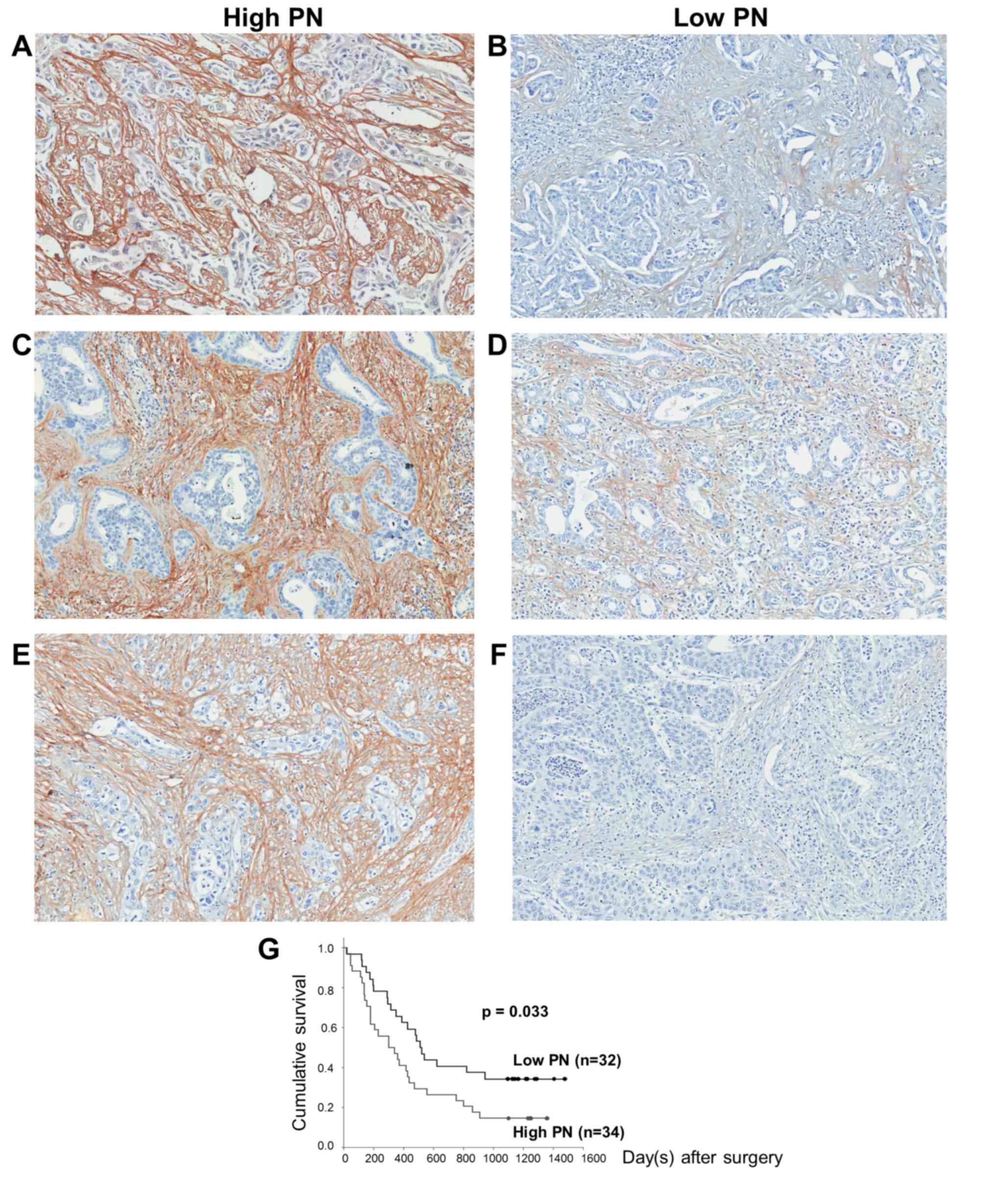
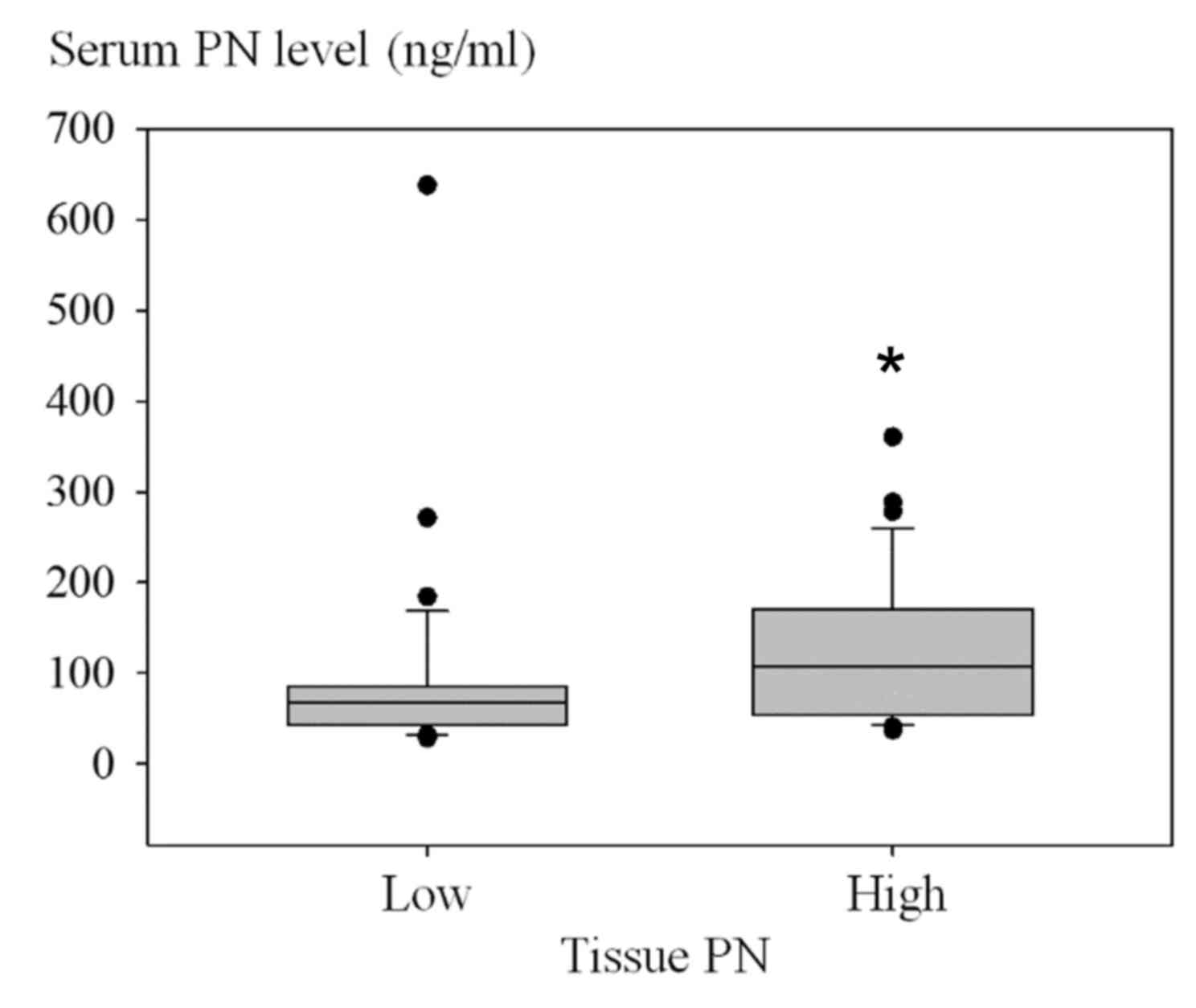
|
1
|
Sithithaworn P, Andrews RH, Nguyen VD,
Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ and
Sripa B: The current status of opisthorchiasis and clonorchiasis in
the Mekong Basin. Parasitol Int. 61:10–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shin HR, Oh JK, Masuyer E, Curado MP,
Bouvard V, Fang YY, Wiangnon S, Sripa B and Hong ST: Epidemiology
of cholangiocarcinoma: An update focusing on risk factors. Cancer
Sci. 101:579–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vatanasapt V, Uttaravichien T, Mairiang
EO, Pairojkul C, Chartbanchachai W and Haswell-Elkins M:
Cholangiocarcinoma in north-east Thailand. Lancet. 335:116–117.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sripa B, Brindley PJ, Mulvenna J, Laha T,
Smout MJ, Mairiang E, Bethony JM and Loukas A: The tumorigenic
liver fluke Opisthorchis viverrini-multiple pathways to cancer.
Trends Parasitol. 28:395–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sriputtha S, Khuntikeo N, Promthet S and
Kamsa-Ard S: Survival rate of intrahepatic cholangiocarcinoma
patients after surgical treatment in Thailand. Asian Pac J Cancer
Prev. 14:1107–1110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan SA, Miras A, Pelling M and
Taylor-Robinson SD: Cholangiocarcinoma and its management. Gut.
56:1755–1756. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan SA, Davidson BR, Goldin RD, Heaton N,
Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD,
Thillainayagam AV, et al: Guidelines for the diagnosis and
treatment of cholangiocarcinoma: An update. Gut. 61:1657–1669.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Franco OE and Hayward SW: Targeting the
tumor stroma as a novel therapeutic approach for prostate cancer.
Adv Pharmacol. 65:267–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hasebe T: Tumor-stromal interactions in
breast tumor progression-significance of histological heterogeneity
of tumor-stromal fibroblasts. Expert Opin Ther Targets. 17:449–460.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bremnes RM, Donnem T, Al-Saad S, Al-Shibli
K, Andersen S, Sirera R, Camps C, Marinez I and Busund LT: The role
of tumor stroma in cancer progression and prognosis: Emphasis on
carcinoma-associated fibroblasts and non-small cell lung cancer. J
Thorac Oncol. 6:209–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spector I, Zilberstein Y, Lavy A, Nagler
A, Genin O and Pines M: Involvement of host stroma cells and tissue
fibrosis in pancreatic tumor development in transgenic mice. PLoS
One. 7:e418332012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chuaysri C, Thuwajit P, Paupairoj A,
Chau-In S, Suthiphongchai T and Thuwajit C: Alpha-smooth muscle
actin-positive fibroblasts promote biliary cell proliferation and
correlate with poor survival in cholangiocarcinoma. Oncol Rep.
21:957–969. 2009.PubMed/NCBI
|
|
13
|
Utispan K, Thuwajit P, Abiko Y, Charngkaew
K, Paupairoj A, Chau-in S and Thuwajit C: Gene expression profiling
of cholangiocarcinoma-derived fibroblast reveals alterations
related to tumor progression and indicates periostin as a poor
prognostic marker. Mol Cancer. 9:132010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ratajczak-Wielgomas K and Dziegiel P: The
role of periostin in neoplastic processes. Folia Histochem
Cytobiol. 53:120–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Utispan K, Sonongbua J, Thuwajit P,
Chau-In S, Pairojkul C, Wongkham S and Thuwajit C: Periostin
activates integrin α5β1 through a PI3K/AKT-dependent pathway in
invasion of cholangiocarcinoma. Int J Oncol. 41:1110–1118.
2012.PubMed/NCBI
|
|
17
|
He J, Whelan SA, Lu M, Shen D, Chung DU,
Saxton RE, Faull KF, Whitelegge JP and Chang HR: Proteomic-based
biosignatures in breast cancer classification and prediction of
therapeutic response. Int J Proteomics. 2011:8964762011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dumur CI, Campbell DJ, DeWitt JL, Oyesanya
RA and Sirica AE: Differential gene expression profiling of
cultured neu-transformed versus spontaneously-transformed rat
cholangiocytes and of corresponding cholangiocarcinomas. Exp Mol
Pathol. 89:227–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tischler V, Fritzsche FR, Wild PJ, Stephan
C, Seifert HH, Riener MO, Hermanns T, Mortezavi A, Gerhardt J,
Schraml P, et al: Periostin is up-regulated in high grade and high
stage prostate cancer. BMC Cancer. 10:2732010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riener MO, Fritzsche FR, Soll C,
Pestalozzi BC, Probst-Hensch N, Clavien PA, Jochum W, Soltermann A,
Moch H and Kristiansen G: Expression of the extracellular matrix
protein periostin in liver tumours and bile duct carcinomas.
Histopathology. 56:600–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morra L, Rechsteiner M, Casagrande S, von
Teichman A, Schraml P, Moch H and Soltermann A: Characterization of
periostin isoform pattern in non-small cell lung cancer. Lung
Cancer. 76:183–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Wang Y and Jiang C: Stromal
protein periostin identified as a progression associated and
prognostic biomarker in glioma via inducing an invasive and
proliferative phenotype. Int J Oncol. 42:1716–1724. 2013.PubMed/NCBI
|
|
23
|
Xu X, Chang W, Yuan J, Han X, Tan X, Ding
Y, Luo Y, Cai H, Liu Y, Gao X, et al: Periostin expression in
intra-tumoral stromal cells is prognostic and predictive for
colorectal carcinoma via creating a cancer-supportive niche.
Oncotarget. 7:798–813. 2016.PubMed/NCBI
|
|
24
|
Lv Y, Wang W, Jia WD, Sun QK, Li JS, Ma
JL, Liu WB, Zhou HC, Ge YS, Yu JH, et al: High-level expression of
periostin is closely related to metastatic potential and poor
prognosis of hepatocellular carcinoma. Med Oncol. 30:3852013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jang SY, Park SY, Lee HW, Choi YK, Park
KG, Yoon GS, Tak WY, Kweon YO, Hur K and Lee WK: The Combination of
Periostin Overexpression and Microvascular Invasion Is Related to a
Poor Prognosis for Hepatocellular Carcinoma. Gut Liver. 10:948–954.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sirica AE, Almenara JA and Li C: Periostin
in intrahepatic cholangiocarcinoma: Pathobiological insights and
clinical implications. Exp Mol Pathol. 97:515–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hong L, Sun H, Lv X, Yang D, Zhang J and
Shi Y: Expression of periostin in the serum of NSCLC and its
function on proliferation and migration of human lung
adenocarcinoma cell line (A549) in vitro. Mol Biol Rep.
37:2285–2293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ben QW, Zhao Z, Ge SF, Zhou J, Yuan F and
Yuan YZ: Circulating levels of periostin may help identify patients
with more aggressive colorectal cancer. Int J Oncol. 34:821–828.
2009.PubMed/NCBI
|
|
29
|
Nuzzo PV, Rubagotti A, Argellati F, Di
Meglio A, Zanardi E, Zinoli L, Comite P, Mussap M and Boccardo F:
Prognostic value of preoperative serum levels of periostin (PN) in
early breast cancer (BCa). Int J Mol Sci. 16:17181–17192. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Contie S, Voorzanger-Rousselot N, Litvin
J, Clézardin P and Garnero P: Increased expression and serum levels
of the stromal cell-secreted protein periostin in breast cancer
bone metastases. Int J Cancer. 128:352–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abbott KL, Lim JM, Wells L, Benigno BB,
McDonald JF and Pierce M: Identification of candidate biomarkers
with cancer-specific glycosylation in the tissue and serum of
endometrioid ovarian cancer patients by glycoproteomic analysis.
Proteomics. 10:470–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Capoun O, Soukup V, Kalousová M, Sobotka
R, Pešl M, Zima T and Hanuš T: Diagnostic importance of selected
protein serum markers in the primary diagnostics of prostate
cancer. Urol Int. 95:429–435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y and Du L: Role of pancreatic
stellate cells and periostin in pancreatic cancer progression.
Tumour Biol. 36:3171–3177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kudo Y, Iizuka S, Yoshida M, Nguyen PT,
Siriwardena SB, Tsunematsu T, Ohbayashi M, Ando T, Hatakeyama D and
Shibata T: Periostin directly and indirectly promotes tumor
lymphangiogenesis of head and neck cancer. PLoS One. 7:e444882012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujimoto K, Kawaguchi T, Nakashima O, Ono
J, Ohta S, Kawaguchi A, Tonan T, Ohshima K, Yano H, Hayabuchi N, et
al: Periostin, a matrix protein, has potential as a novel
serodiagnostic marker for cholangiocarcinoma. Oncol Rep.
25:1211–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okamoto M, Hoshino T, Kitasato Y, Sakazaki
Y, Kawayama T, Fujimoto K, Ohshima K, Shiraishi H, Uchida M, Ono J,
et al: Periostin, a matrix protein, is a novel biomarker for
idiopathic interstitial pneumonias. Eur Respir J. 37:1119–1127.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masuoka M, Shiraishi H, Ohta S, Suzuki S,
Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, et al:
Periostin promotes chronic allergic inflammation in response to Th2
cytokines. J Clin Invest. 122:2590–2600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kanemitsu Y, Matsumoto H, Izuhara K, Tohda
Y, Kita H, Horiguchi T, Kuwabara K, Tomii K, Otsuka K, Fujimura M,
et al: Increased periostin associates with greater airflow
limitation in patients receiving inhaled corticosteroids. J Allergy
Clin Immunol. 132:305–12 e3. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamaguchi Y, Ono J, Masuoka M, Ohta S,
Izuhara K, Ikezawa Z, Aihara M and Takahashi K: Serum periostin
levels are correlated with progressive skin sclerosis in patients
with systemic sclerosis. Br J Dermatol. 168:717–725. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takayama G, Arima K, Kanaji T, Toda S,
Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T and Izuhara
K: Periostin: A novel component of subepithelial fibrosis of
bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy
Clin Immunol. 118:98–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Horiuchi K, Amizuka N, Takeshita S,
Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A:
Identification and characterization of a novel protein, periostin,
with restricted expression to periosteum and periodontal ligament
and increased expression by transforming growth factor beta. J Bone
Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Inai K, Norris RA, Hoffman S, Markwald RR
and Sugi Y: BMP-2 induces cell migration and periostin expression
during atrioventricular valvulogenesis. Dev Biol. 315:383–396.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ohira S, Itatsu K, Sasaki M, Harada K,
Sato Y, Zen Y, Ishikawa A, Oda K, Nagasaka T, Nimura Y and Nakanuma
Y: Local balance of transforming growth factor-beta1 secreted from
cholangiocarcinoma cells and stromal-derived factor-1 secreted from
stromal fibroblasts is a factor involved in invasion of
cholangiocarcinoma. Pathol Int. 56:381–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu JP, Mao JQ, Li MS, Lu SL, Hu XQ, Zhu SN
and Nomura S: In situ detection of TGF betas, TGF beta receptor II
mRNA and telomerase activity in rat cholangiocarcinogenesis. World
J Gastroenterol. 9:590–594. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Puppin C, Passon N, Frasca F, Vigneri R,
Tomay F, Tomaciello S and Damante G: In thyroid cancer cell lines
expression of periostin gene is controlled by p73 and is not
related to epigenetic marks of active transcription. Cell Oncol
(Dordr). 34:131–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oshima A, Tanabe H, Yan T, Lowe GN,
Glackin CA and Kudo A: A novel mechanism for the regulation of
osteoblast differentiation: Transcription of periostin, a member of
the fasciclin I family, is regulated by the bHLH transcription
factor, twist. J Cell Biochem. 86:792–804. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Romeo F, Falbo L, Di Sanzo M, Misaggi R,
Faniello MC, Barni T, Cuda G, Viglietto G, Santoro C, Quaresima B
and Costanzo F: Negative transcriptional regulation of the human
periostin gene by YingYang-1 transcription factor. Gene.
487:129–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zinn PO, Mahajan B, Sathyan P, Singh SK,
Majumder S, Jolesz FA and Colen RR: Radiogenomic mapping of
edema/cellular invasion MRI-phenotypes in glioblastoma multiforme.
PLoS One. 6:e254512011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Niu Z, Iyer D, Conway SJ, Martin JF, Ivey
K, Srivastava D, Nordheim A and Schwartz RJ: Serum response factor
orchestrates nascent sarcomerogenesis and silences the
biomineralization gene program in the heart. Proc Natl Acad Sci
USA. 105:pp. 17824–17829. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Honsawek S, Udomsinprasert W, Vejchapipat
P, Chongsrisawat V, Phavichitr N and Poovorawan Y: Elevated serum
periostin is associated with liver stiffness and clinical outcome
in biliary atresia. Biomarkers. 20:157–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tsukahara T, Shimoyama Y, Ebata T,
Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nakamura S
and Nagino M: Cholangiocarcinoma with intraductal tubular growth
pattern versus intraductal papillary growth pattern. Mod Pathol.
29:293–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kharaishvili G, Cizkova M, Bouchalova K,
Mgebrishvili G, Kolar Z and Bouchal J: Collagen triple helix repeat
containing 1 protein, periostin and versican in primary and
metastatic breast cancer: An immunohistochemical study. J Clin
Pathol. 64:977–982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Clezardin P and Teti A: Bone metastasis:
Pathogenesis and therapeutic implications. Clin Exp Metastasis.
24:599–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lv Y, Wang W, Jia WD, Sun QK, Huang M,
Zhou HC, Xia HH, Liu WB, Chen H, Sun SN and Xu GL: High
preoparative levels of serum periostin are associated with poor
prognosis in patients with hepatocellular carcinoma after
hepatectomy. Eur J Surg Oncol. 39:1129–1135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Riener MO: Diagnosis of tumours of the
liver and the biliary tract: New tissue and serum markers.
Pathologe. 32 Suppl 2:S304–S309. 2011. View Article : Google Scholar
|
|
56
|
Bridgewater J, Galle PR, Khan SA, Llovet
JM, Park JW, Patel T, Pawlik TM and Gores GJ: Guidelines for the
diagnosis and management of intrahepatic cholangiocarcinoma. J
Hepatol. 60:1268–1289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Malaguarnera G, Paladina I, Giordano M,
Malaguarnera M, Bertino G and Berretta M: Serum markers of
intrahepatic cholangiocarcinoma. Dis Markers. 34:219–228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tamandl D, Herberger B, Gruenberger B,
Puhalla H, Klinger M and Gruenberger T: Influence of hepatic
resection margin on recurrence and survival in intrahepatic
cholangiocarcinoma. Ann Surg Oncol. 15:2787–2794. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bjornsson E, Kilander A and Olsson R: CA
19-9 and CEA are unreliable markers for cholangiocarcinoma in
patients with primary sclerosing cholangitis. Liver. 19:501–508.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sulpice L, Rayar M, Desille M, Turlin B,
Fautrel A, Boucher E, Llamas-Gutierrez F, Meunier B, Boudjema K,
Clément B and Coulouarn C: Molecular profiling of stroma identifies
osteopontin as an independent predictor of poor prognosis in
intrahepatic cholangiocarcinoma. Hepatology. 58:1992–2000. 2013.
View Article : Google Scholar : PubMed/NCBI
|