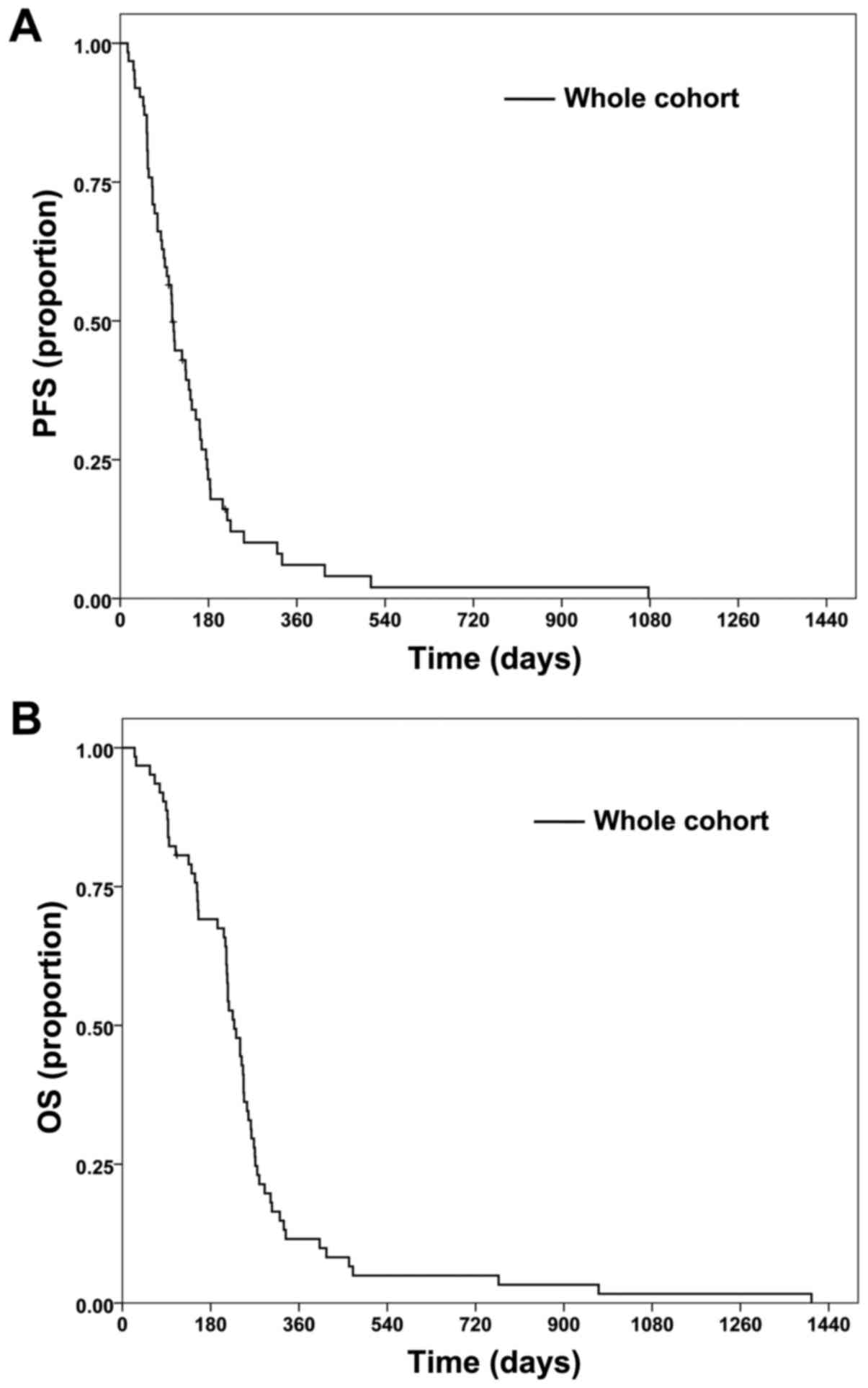
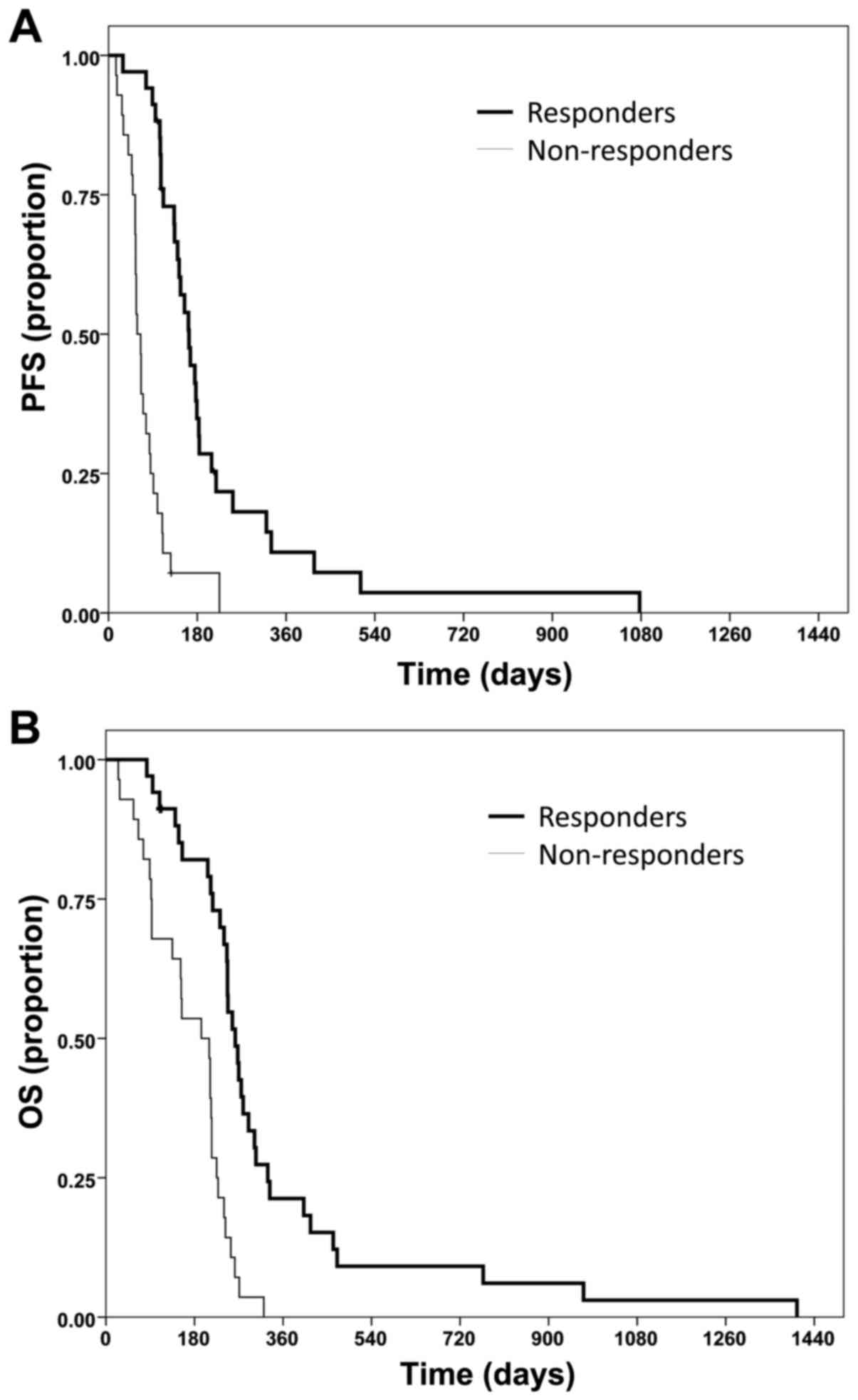
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weller M, van den Bent M, Hopkins K, Tonn
JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D,
Henriksson R, Balana C, et al: EANO guideline for the diagnosis and
treatment of anaplastic gliomas and glioblastoma. Lancet Oncol.
15:e395–e403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wirsching HG, Galanis E and Weller M:
Glioblastoma. Handb Clin Neurol. 134:381–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keime-Guibert F, Chinot O, Taillandier L,
Cartalat-Carel S, Frenay M, Kantor G, Guillamo JS, Jadaud E, Colin
P, Bondiau PY, et al: Radiotherapy for glioblastoma in the elderly.
N Engl J Med. 356:1527–1535. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kreth FW, Thon N, Simon M, Westphal M,
Schackert G, Nikkhah G, Hentschel B, Reifenberger G, Pietsch T,
Weller M, et al: Gross total but not incomplete resection of
glioblastoma prolongs survival in the era of radiochemotherapy. Ann
Oncol. 24:3117–3123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laperriere N, Zuraw L and Cairncross G;
Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology
Disease Site Group, : Radiotherapy for newly diagnosed malignant
glioma in adults: A systematic review. Radiother Oncol. 64:259–273.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Senft C, Bink A, Franz K, Vatter H, Gasser
T and Seifert V: Intraoperative MRI guidance and extent of
resection in glioma surgery: A randomised, controlled trial. Lancet
Oncol. 12:997–1003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stummer W, Pichlmeier U, Meinel T,
Wiestler OD, Zanella F and Reulen HJ; ALA-Glioma Study Group, :
Fluorescence-guided surgery with 5-aminolevulinic acid for
resection of malignant glioma: A randomised controlled multicentre
phase III trial. Lancet Oncol. 7:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vuorinen V, Hinkka S, Färkkilä M and
Jääskeläinen J: Debulking or biopsy of malignant glioma in elderly
people-a randomised study. Acta Neurochir (Wien). 145:5–10. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
De Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seystahl K, Wick W and Weller M:
Therapeutic options in recurrent glioblastoma-An update. Crit Rev
Oncol Hematol. 99:389–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Montemurro N, Perrini P, Blanco MO and
Vannozzi R: Second surgery for recurrent glioblastoma: A concise
overview of the current literature. Clin Neurol Neurosurg.
142:60–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nieder C, Andratschke NH and Grosu AL:
Re-irradiation for recurrent primary brain tumors. Anticancer Res.
36:4985–4995. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brandes AA, Bartolotti M, Tosoni A and
Franceschi E: Nitrosoureas in the management of malignant gliomas.
Curr Neurol Neurosci Rep. 16:132016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedman HS, Prados MD, Wen PY, Mikkelsen
T, Schiff D, Abrey LE, Yung WKA, Paleologos N, Nicholas MK, Jensen
R, et al: Bevacizumab alone and in combination with irinotecan in
recurrent glioblastoma. J Clin Oncol. 27:4733–4740. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wick W, Weller M, van den Bent M and Stupp
R: Bevacizumab and recurrent malignant gliomas: A European
perspective. J Clin Oncol. 28:e188–e192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chinot OL, Wick W, Mason W, Henriksson R,
Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea
D, et al: Bevacizumab plus radiotherapy-temozolomide for newly
diagnosed glioblastoma. N Engl J Med. 370:709–722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taal W, Oosterkamp HM, Walenkamp AM,
Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D,
De Vos FY, et al: Single-agent bevacizumab or lomustine versus a
combination of bevacizumab plus lomustine in patients with
recurrent glioblastoma (BELOB trial): A randomised controlled phase
2 trial. Lancet Oncol. 15:943–953. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wick W, Brandes A, Gorlia T, Bendszus M,
Sahm F, Taal W, Taphoorn M, Domont J, Idbaih A, Campone M, et al:
Lb-05 phase iii trial exploring the combination of bevacizumab and
lomustine in patients with first recurrence of a glioblastoma: The
eortc 26101 trial. Neuro Oncol. 17 Suppl 5:v12015. View Article : Google Scholar
|
|
22
|
Wen PY, Macdonald DR, Reardon DA,
Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert
MR, Lassman AB, et al: Updated response assessment criteria for
high-grade gliomas: Response assessment in neuro-oncology working
group. J Clin Oncol. 28:1963–1972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bähr O, Hattingen E, Rieger J and
Steinbach JP: Bevacizumab-induced tumor calcifications as a
surrogate marker of outcome in patients with glioblastoma. Neuro
Oncol. 13:1020–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bähr O, Harter PN, Weise LM, You SJ,
Mittelbronn M, Ronellenfitsch MW, Rieger J, Steinbach JP and
Hattingen E: Sustained focal antitumor activity of bevacizumab in
recurrent glioblastoma. Neurology. 83:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schaub C, Tichy J, Schäfer N, Franz K,
Mack F, Mittelbronn M, Kebir S, Thiepold AL, Waha A, Filmann N, et
al: Prognostic factors in recurrent glioblastoma patients treated
with bevacizumab. J Neurooncol. 129:93–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herrlinger U, Schäfer N, Steinbach JP,
Weyerbrock A, Hau P, Goldbrunner R, Friedrich F, Rohde V, Ringel F,
Schlegel U, et al: Bevacizumab plus irinotecan versus temozolomide
in newly diagnosed O6-methylguanine-DNA methyltransferase
nonmethylated glioblastoma: The randomized GLARIUS trial. J Clin
Oncol. 34:1611–1619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chamberlain MC and Johnston SK: Salvage
therapy with single agent bevacizumab for recurrent glioblastoma. J
Neurooncol. 96:259–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kreisl TN, Kim L, Moore K, Duic P, Royce
C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al:
Phase II trial of single-agent bevacizumab followed by bevacizumab
plus irinotecan at tumor progression in recurrent glioblastoma. J
Clin Oncol. 27:740–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Møller S, Grunnet K, Hansen S, Schultz H,
Holmberg M, Sorensen M, Poulsen HS and Lassen U: A phase II trial
with bevacizumab and irinotecan for patients with primary brain
tumors and progression after standard therapy. Acta Oncol.
51:797–804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagane M, Nishikawa R, Narita Y, Kobayashi
H, Takano S, Shinoura N, Aoki T, Sugiyama K, Kuratsu J, Muragaki Y,
et al: Phase II study of single-agent bevacizumab in Japanese
patients with recurrent malignant glioma. Jpn J Clin Oncol.
42:887–895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Raizer JJ, Grimm S, Chamberlain MC,
Nicholas MK, Chandler JP, Muro K, Dubner S, Rademaker AW, Renfrow J
and Bredel M: A phase 2 trial of single-agent bevacizumab given in
an every-3-week schedule for patients with recurrent high-grade
gliomas. Cancer. 116:5297–5305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vredenburgh JJ, Desjardins A, Herndon JE
II, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S,
Gururangan S, Wagner M, et al: Phase II trial of bevacizumab and
irinotecan in recurrent malignant glioma. Clin Cancer Res.
13:1253–1259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vredenburgh JJ, Desjardins A, Herndon JE
II, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S,
Gururangan S, Sampson J, et al: Bevacizumab plus irinotecan in
recurrent glioblastoma multiforme. J Clin Oncol. 25:4722–4729.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zuniga RM, Torcuator R, Jain R, Anderson
J, Doyle T, Ellika S, Schultz L and Mikkelsen T: Efficacy, safety
and patterns of response and recurrence in patients with recurrent
high-grade gliomas treated with bevacizumab plus irinotecan. J
Neurooncol. 91:329–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wick W, Puduvalli VK, Chamberlain MC, van
den Bent MJ, Carpentier AF, Cher LM, Mason W, Weller M, Hong S,
Musib L, et al: Phase III study of enzastaurin compared with
lomustine in the treatment of recurrent intracranial glioblastoma.
J Clin Oncol. 28:1168–1174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Batchelor TT, Mulholland P, Neyns B,
Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S,
Ashby LS, et al: Phase III randomized trial comparing the efficacy
of cediranib as monotherapy, and in combination with lomustine,
versus lomustine alone in patients with recurrent glioblastoma. J
Clin Oncol. 31:3212–3218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hattingen E, Jurcoane A, Daneshvar K,
Pilatus U, Mittelbronn M, Steinbach JP and Bähr O: Quantitative T2
mapping of recurrent glioblastoma under bevacizumab improves
monitoring for non-enhancing tumor progression and predicts overall
survival. Neuro Oncol. 15:1395–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sandmann T, Bourgon R, Garcia J, Li C,
Cloughesy T, Chinot OL, Wick W, Nishikawa R, Mason W, Henriksson R,
et al: Patients with proneural glioblastoma may derive overall
survival benefit from the addition of bevacizumab to first-line
radiotherapy and temozolomide: Retrospective analysis of the
AVAglio trial. J Clin Oncol. 33:2735–2744. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schnell O, Thorsteinsdottir J, Fleischmann
DF, Lenski M, Abenhardt W, Giese A, Tonn JC, Belka C, Kreth FW and
Niyazi M: Re-irradiation strategies in combination with bevacizumab
for recurrent malignant glioma. J Neurooncol. 130:591–599. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Minniti G, Agolli L, Falco T, Scaringi C,
Lanzetta G, Caporello P, Osti MF, Esposito V and Enrici RM:
Hypofractionated stereotactic radiotherapy in combination with
bevacizumab or fotemustine for patients with progressive malignant
gliomas. J Neurooncol. 122:559–566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fathpour P, Obad N, Espedal H, Stieber D,
Keunen O, Sakariassen PØ, Niclou SP and Bjerkvig R: Bevacizumab
treatment for human glioblastoma. Can it induce cognitive
impairment? Neuro Oncol. 16:754–756. 2014.PubMed/NCBI
|
|
42
|
Zafar SY, Currow DC, Cherny N, Strasser F,
Fowler R and Abernethy AP: Consensus-based standards for best
supportive care in clinical trials in advanced cancer. Lancet
Oncol. 13:e77–e82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zafar SY, Currow D and Abernethy AP:
Defining best supportive care. J Clin Oncol. 26:5139–5140. 2008.
View Article : Google Scholar : PubMed/NCBI
|