Introduction
Bufalin (B) and cinobufotalin (CB) are two members
of the cardiotonic steroids known as the bufadienolides and
constituents of the traditional Chinese medicine Chan Su. The drugs
are also known to be found in toad venom, which is extracted from
the skin secretions of giant toads, including Bufo
gargarizans (1). In a previous
study, we found that CB was able to significantly induce apoptotic
cell death in the human lymphoma U937 cell line at concentrations
>0.5 µM (2). In addition, B was
demonstrated to significantly induce apoptotic cell death in
various cancer cell types at concentrations >0.1 µM (3–6). The
preferential killing of tumor cells by bufadienolides has made them
potential candidates for chemotherapeutic research in the last 50
years; however, owing to their structural resemblance to digitalis
glycosides, they also act as potent cardiotonic steroids, which
increase the contractile force of the heart muscle. This effect is
a result of the action of bufadienolides on the
Na+/K+-ATPase pump, an essential membrane
protein of animal cells, which results in an increase in the
cytoplasmic Ca2+ concentration (1). The application of bufadienolides in
cancer treatment is, therefore, restricted by their cardiotoxic
side-effects.
Previous studies have revealed that hyperthermia
(HT) and radiation (Rad) are able to enhance the cell killing
effect of cytotoxic drugs, termed thermal- and
radio-chemosensitization, either directly through affecting drug
activity or indirectly through affecting the cancer cell itself
(7–12). The adjuvant effect of this combination
allows for the lowering of the chemotherapeutic dose required, thus
lowering the unfavorable side-effects. Overall, the benefits of
their concomitant use evidently overweigh the possible
aforementioned disadvantages. For this reason, the scope of the
present study was to investigate the possibility of lowering the
chemotherapeutic dose of bufadienolides in combination with HT or
Rad, while maintaining the chemotherapeutic efficacy.
Materials and methods
Drug and drug concentration
CB was purchased from Sigma-Aldrich (Merck KGgA,
Darmstadt, Germany), while B was purchased from Enzo Life Sciences,
Inc. (Farmingdale, NY, USA). Stock solutions were prepared using
DMSO as a solvent, and further dissolved to make the desired
concentrations for experimental use. The minimum toxic doses of CB
and B were then determined using DNA fragmentation and cell
viability assays, and were identified as 0.2 and 0.1 µM,
respectively (data not shown). Accordingly, further experiments
were conducted using these concentrations for 6-h incubation
periods.
Cell culture
The human lymphoma U937 cell line, obtained from the
Health Sciences Research Resource Bank (Japan Health Sciences
Foundation, Tokyo, Japan) was maintained in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
both Sigma-Aldrich; Merck KGaA). The cell line was maintained at
37°C in a humidified atmosphere with 5% CO2. Cells were
harvested when they reached 80% confluence and subcultures of
1×106 cells/ml were used in all assays.
Heat stress exposure
HT is known to cause cell membrane damage and
impairment to the ion transport mechanisms (7). Thus, treating the cells with HT prior to
B and CB administration diminishes the cytotoxic activity of the
drugs that principally target the Na+/K+
pump. The drugs (0.2 µM CB and 0.1 µM B) were therefore
administered for 1 h prior to HT treatment. Cells were transferred
into test tubes and placed in a hot water bath at 44°C for 20 min.
Subsequently, the cells were transferred into plates and incubated
for 6 h at 37°C, prior to the required assay.
X-irradiation
U937 cells (1×106 cells/ml) were treated
with B and CB in a 6-cm culture dish for 1 h at room temperature
prior to Rad. X-irradiation was performed at room temperature using
X-ray apparatus (MBR-1520R-3; Hitachi, Ltd., Tokyo, Japan)
operating at 150 kV and 20 mA, at a dose rate of 5 Gy/min, 10 Gy
total as determined using Fricke dosimetry. Cells were subsequently
incubated for 6 h at 37°C with 5% CO2 for further
assessment.
Assessment of apoptosis
Morphological examination was performed using Giemsa
staining under a light microscope (magnification, ×400), and a
quantitative assay of DNA fragmentation was performed according to
the modified Sellins and Cohen method (13,14).
Assessment of cell viability
Cell viability was evaluated by WST-1 cell viability
assay using a WST-1 Cell Counting kit (Dojindo Molecular
Technologies Inc., Kumamoto, Japan) according to the manufacturer's
protocol. Briefly, cell suspensions were subjected to drug (CB or
B) treatment, HT or Rad, then equal volumes of the cell suspension
were seeded into 96-well plate followed by a further incubation
period for 6 h at 37°C. Subsequently, cells were subjected to the
WST-1 assay. The absorbance was measured at 450 nm using a
microplate reader.
Assessment of mitochondrial membrane
potential (MMP)
To measure the changes in the MMP, U937 cells were
harvested by centrifugation at 350 × g at 4°C for 4 mins and
stained with 10 nM tetramethylrhodamine methyl ester (TMRM;
Molecular Probes; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
for 15 min at 37°C in PBS containing 1% FBS. The fluorescence of
TMRM was then analyzed using flow cytometry (excitation wavelength,
488 nm; emission wavelength, 575 nm) (15).
Western blot analysis of proteins
Western blot analysis was performed as described
previously (2). Cells were collected
and washed with cold PBS, then lysed using lysis buffer for 20 min
at 4°C (1 M Tris-HCl, 5 M NaCl, 1% Nonidet P-40 (v/v), 1% sodium
deoxycholate, 0.05% SDS, 1 mM phenylmethylsulfonyl fluoride).
Following brief sonication, the lysates were centrifuged at 13,362
× g for 10 min at 4°C and the protein content in the supernatant
was measured using a Bio-Rad protein system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Total protein (30 µg) was heated at 96°C
for 5 min following mixing with 5 µl of SDS-loading buffer. Samples
were then subjected to 12% SDS-PAGE (Daiichi Pure Chemicals Co.,
Ltd., Tokyo, Japan) and transferred to nitrocellulose membranes (GE
Healthcare Life Sciences, Chalfont, UK). Membranes were then
blocked using 1X Tris-buffered saline-Tween 20 with 5% w/v non-fat
dry milk for 1 h at room temperature. Western blot analysis was
performed using anti-BH3 interacting domain death agonist (Bid,),
anti-caspase-3 and anti-heat shock 70-kDa protein (HSP-70; 2002S,
9661S and 4872S, respectively; Cell Signaling Technology, Inc.,
Danvers, MA, USA) polyclonal antibodies, and anti-β-actin
monoclonal antibody (A2228, Sigma-Aldrich; Merck KGgA). The
membranes and the primary antibodies (all at 1:1,000 dilution) were
incubated overnight with gentle agitation at 4°C. Following their
incubation with horseradish peroxidase-conjugated anti-rabbit and
anti-mouse immunoglobulin G secondary antibodies for 1 h at room
temperature, band signals were visualized on X-ray film using a
chemiluminescence EspspCL system (NA934 and NA931, respectively; GE
Healthcare Life Sciences, Chalfont, UK) (15).
Statistical analysis
The statistical analysis was performed using JMP
software (version 10; SAS Institute, Inc., Cary, NC, USA). Data are
presented as the mean ± standard deviation. Statistical
significance was evaluated using one-way analysis of variance
followed by the Bonferroni post hoc test. All experiments were
performed in triplicate. P<0.01 was considered to indicate a
statistically significant difference.
Results
Effect of combining HT or Rad on the
cytotoxic activity of B and CB
Based on our previous studies, the minimum toxic
doses for B and CB in U937 cells were identified as 0.01 and 0.2
µM, respectively (2,5,6). Upon
further analysis, administration of B (0.1 µM) and CB (0.2 µM) in
combination with HT (44°C for 20 min) or Rad (10 Gy) was
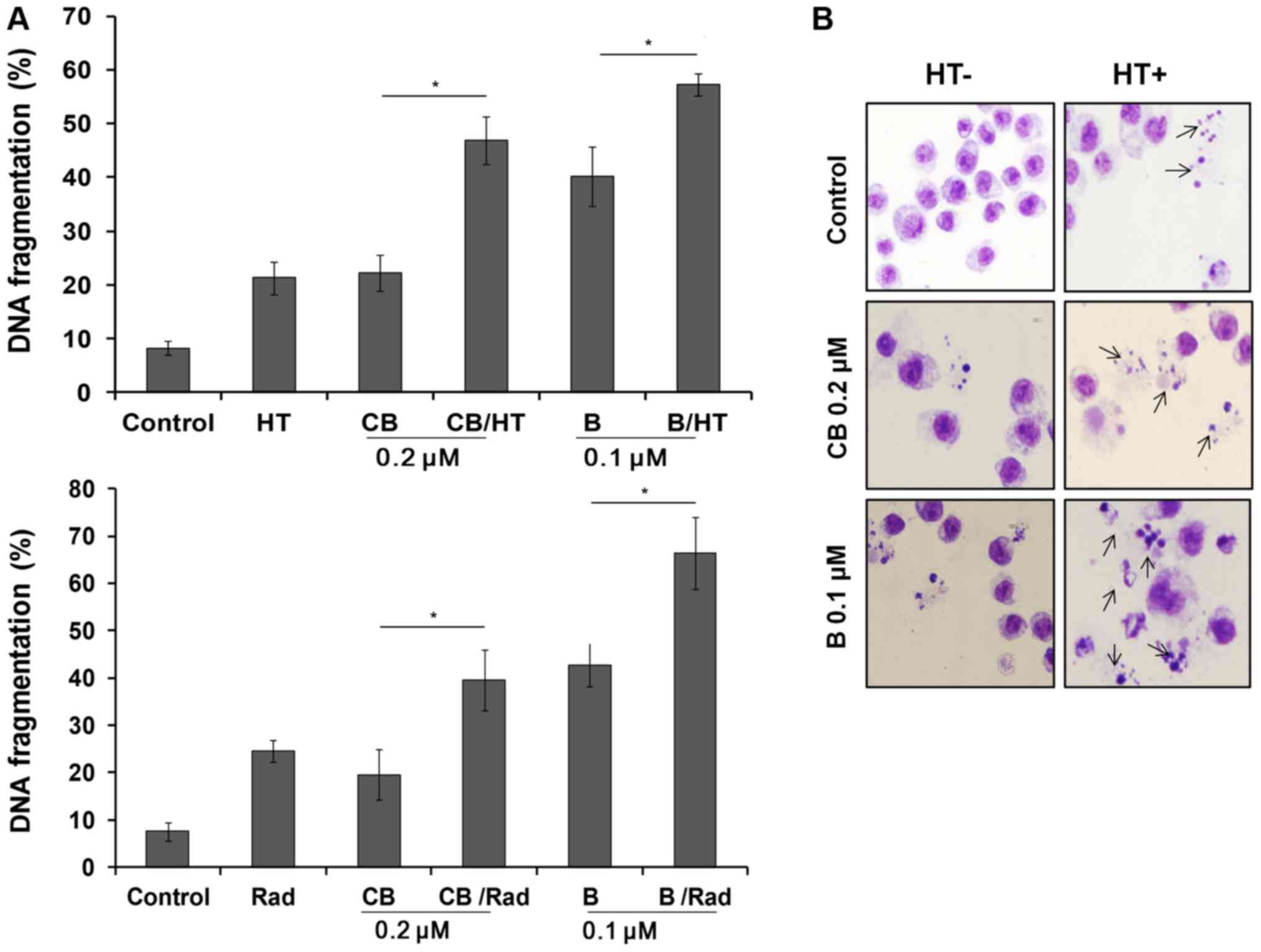
demonstrated to significantly increase the DNA fragmentation
compared with single-drug treatment groups (P<0.01; Fig. 1A). Treated cells were then examined
using Giemsa staining under a light microscope. Untreated cells
exhibited intact nuclear structure, whereas B and CB in combination
with HT-treated cells showed typical apoptotic morphological
changes, as indicated by chromosomal condensation and nuclear
fragmentation (Fig. 1B).
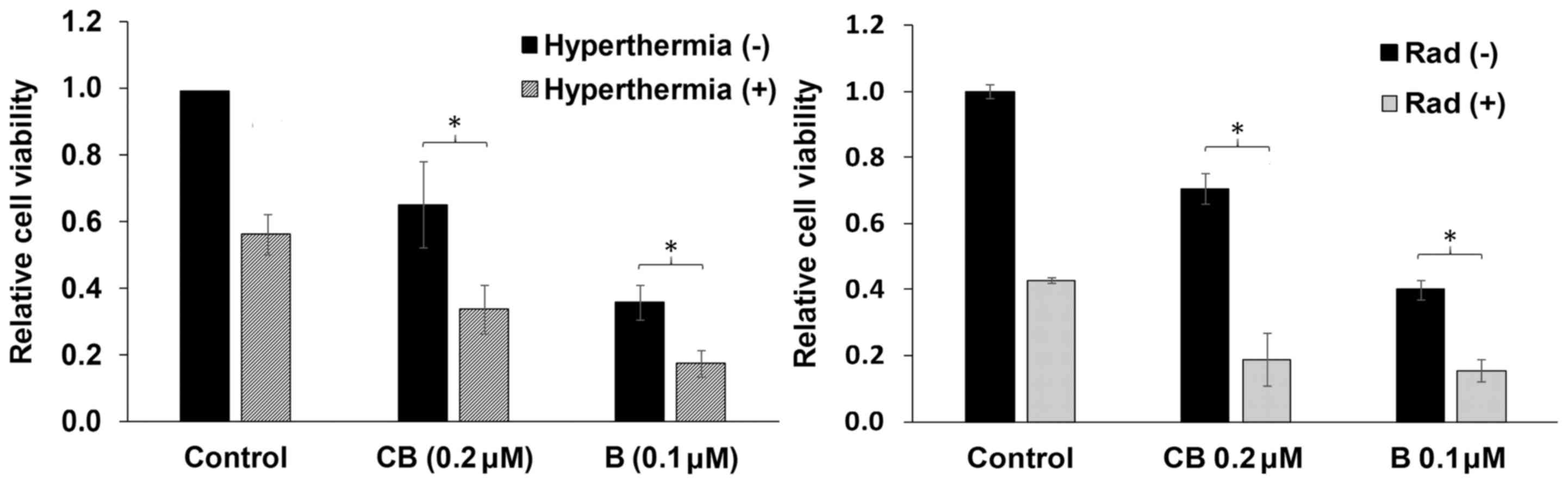
Decrease in cell viability
U937 cells were treated as aforementioned, followed
by use of the WST-1 cell viability assay. In coherence with the
previous assays, B/HT- and CB/HT-treated cells showed a significant
decrease in cell viability, reaching 40.0±4.0 and 35.0±3.0%,
respectively, following 6 h of incubation (Fig. 2). Similarly, B/Rad- and CB/Rad-treated
cells showed a further decrease in cell viability, reaching
20.0±4.0 and 15.0±3.0%, respectively, following 6 h of incubation
(Fig. 2).
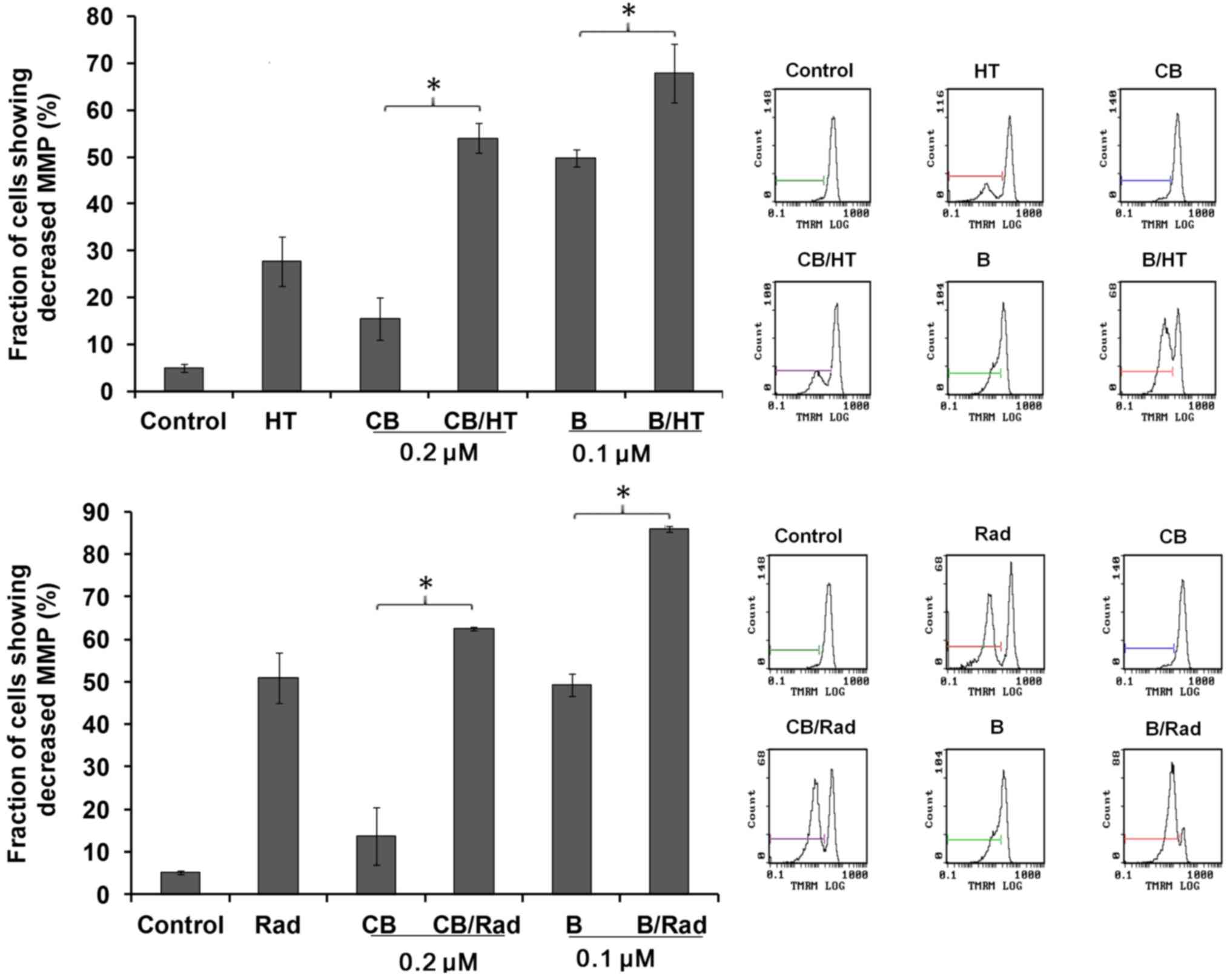
Induction of MMP
To assess the effect of these combinations on the
MMP of U937 cells, treated cells were assessed for MMP decline
using TMRM staining. The results revealed significant reductions in
MMP in the combination-treatment groups compared with treatment
with the drugs alone, as shown in Fig.
3.
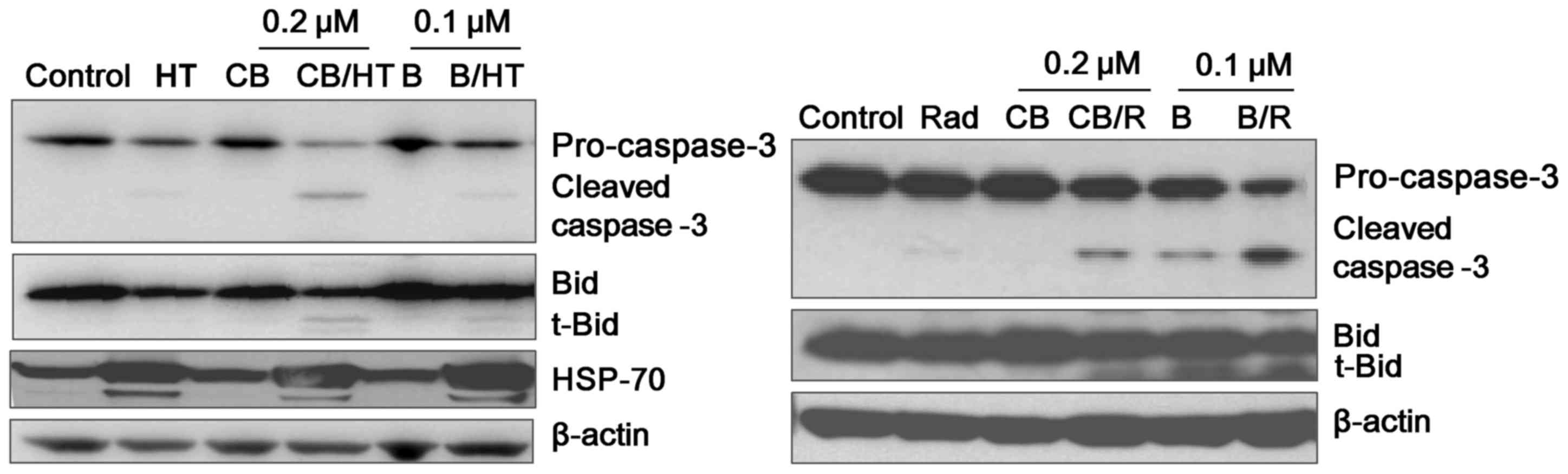
Effect on apoptosis-associated protein
expression
Caspases serve an important role in the apoptotic
signaling pathway and are considered as the main executioners of
cell death (16). To examine the
involvement of the caspases in the effect of HT or Rad on the two
drugs, western blot analysis was performed using caspase-3 antibody
following 6 h of treatment of U937 cells using the treatment
combinations. Given that the activation of a specific caspase
results in the reduction of its pro-enzyme form, it was
demonstrated that the protein levels of procaspase-3 were markedly
downregulated in the HT- or Rad-treated groups (Fig. 4). Simultaneously, the cleaved form of
caspase-3 was also markedly detectable in the groups treated in
combination with HT or Rad. Expression of activated pro-apoptotic
proteins, including Bid, were also demonstrated to be markedly
decreased with regard to cytosolic levels, as shown in Fig. 4. However, HSP70 expression was
upregulated in the HT-treated groups.
Discussion
Cardiac glycosides (CG) belong to the most commonly
used group of drugs in medicine. The two main subgroups of CG are
the cardenolides, where ouabin and its derivative digoxin are the
most typical examples, and the bufadienolides, which the present
study was focused upon. There are ~250 substances in the
bufadienolide group, including B and CB (17). The bufadienolides have been previously
investigated in several studies for their cytotoxic activity, as
well as the underlying signaling pathway involved (3–6).
Bufadienolides act primarily through inhibiting the transport
enzyme Na+/K+-adenosine triphosphatase
(18), thus acting as their signal
transducer. Conversely, despite the cytotoxic nature of
bufadienolides, a previous study reported their use in
cardiovascular and kidney diseases (19). Introducing the bufadienolides as
chemotherapeutic agents also depends on the extent of their
cardiovascular side-effects. However, their cell type specificity
towards cancer cells and the use of specific bufadienolides at low
doses and for long periods without severe side-effects have been
reported (20,21). Thus, in the present study, the use of
B and CB was investigated at the lowest cytotoxic dose in
combination with HT or Rad in order to attain a significant
cytotoxicity with limited possible side-effects.
HT efficacy is not sufficient to replace any of the
therapeutic modalities when used alone, yet it has shown
significant results in enhancing the cell killing effect of other
cytotoxic drugs. HT induces several changes in the cellular
physiology, including at the level of the cell membrane of the
cancer cell and on a nucleic acid level (7). Generally, HT cannot cause severe damage
by itself, but instead, independently hinders the repair of cell
damage (22,23). Furthermore, HT affects the fluidity
and stability of cellular membranes, and hinders the function of
the transmembrane transport proteins and cell surface receptors
(24–28). In addition, X-irradiation is known to
induce apoptosis by acting directly on the plasma membrane and
nuclear DNA, or both (14).
Simultaneously, mitochondrial-dependent generation of reactive
oxygen species serves an essential role in G2/M arrest
and X-irradiation-induced apoptosis (29,30).
Accordingly, the present study reported promising results when B
and CB were administered 1 h prior to HT or Rad treatment avoiding
its destructive effect on the cell membrane, which is the primary
target of B and CB.
In all the assays in the current study, B was
identified to be generally more cytotoxic when compared with CB,
and HT significantly enhanced the cytotoxic effect of low doses of
the two drugs. Notably, HT was able to potentiate the cytotoxic
activity of CB to be almost equal to that of B alone. The apoptotic
nature of the drugs appeared to be unhindered yet augmented.
Firstly, using Giemsa staining, the presence of apoptotic-like
bodies in all treatment groups was demonstrated and was more
evident in the CB/HT and B/HT combinations compared with the groups
with drug treatment alone. To assess the viability of the cells, a
WST-1 cell viability assay was used. The results demonstrated that
~50% of the cells died within 6 h in the CB/HT and B/HT combination
groups vs. 20–30% of cells when the drugs were used alone.
Additionally, the DNA fragmentation assay revealed a significant
increase in the DNA fragments in the combination groups.
Furthermore, the effects of the combinations were greater when
assessing the MMP, since there was doubling in the MMP percentile
loss in the two drugs. MMP is the preliminary step towards the
initiation of programmed cell death (31,32). This
eventually leads to the activation of caspase-3, followed by DNA
fragmentation and cell death (33,34). HT
and Rad enhanced the expression of caspase-3 and Bid proteins when
combined with the drugs. However, the upregulation of HSP70,
acknowledged as an anti-apoptotic protein, remains a question to be
assessed. Nevertheless, few previous studies have demonstrated the
apoptotic nature of the HSP70 protein. This could stand as an
explanation for the lack of a synergistic effect of this
combination (35).
In conclusion, HT and Rad, in combinations with low
doses of B and CB appear to enhance the apoptotic cell death of
U937 cells through the intrinsic apoptotic signaling pathway.
Notably, when combined with CB, chemotherapeutic effects were
demonstrated equivalent to the relatively more toxic B used alone.
The present study has provided a direction for the introduction of
B and CB into the chemotherapeutic field, ensuring similar efficacy
while reducing the anticipated side-effects of high doses.
References
|
1
|
Xu Y, Liu X, Schwarz S, Hu L, Guo D, Gu Q
and Schwarz W: Inhibitory efficacy of bufadienolides on Na+,
K+-pump activity versus cell proliferation. Biochem Biophys Rep.
6:158–164. 2016.
|
|
2
|
Emam H, Zhao QL, Furusawa Y, Refaat A,
Ahmed K, Kadowaki M and Kondo T: Apoptotic cell death by the novel
natural compound, cinobufotalin. Chem Biol Interact. 199:154–160.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takai N, Kira N, Ishii T, Yoshida T,
Nishida M, Nishida Y, Nasu K and Narahara H: Bufalin, a traditional
oriental medicine, induces apoptosis in human cancer cells. Asian
Pac J Cancer Prev. 13:399–402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watabe M, Ito K, Masuda Y, Nakajo S and
Nakaya K: Activation of AP-1 is required for bufalin-induced
apoptosis in human leukemia U937 cells. Oncogene. 16:779–787. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watabe M, Masuda Y, Nakajo S, Yoshida T,
Kuroiwa Y and Nakaya K: The cooperative interaction of two
different signaling pathways in response to bufalin induces
apoptosis in human leukemia U937 cells. J Biol Chem.
271:14067–14072. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hildebrandt B, Wust P, Ahlers O, Dieing A,
Sreenivasa G, Kerner T, Felix R and Riess H: The cellular and
molecular basis of hyperthermia. Crit Rev Oncol Hematol. 43:33–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engin K, Tupchong L, Moylan DJ, Alexander
GA, Waterman FM, Komarnicky L, Nerlinger RE and Leeper DB:
Radomized trial of one versus two adjuvant hyperthermia treatments
per week in patients with superficial tumors. Int J Hyperthermia.
9:327–340. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Urano M, Kuroda M and Nishimura Y: For the
clinical application of themochemotherapy given at mild
temperatures. Int J Hyperthermia. 15:79–107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Falk MH and Issels RD: Hyperthermia in
oncology. Int J Hyperthermia. 17:1–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dewey WC, Thrall D and Gilette EL:
Hyperthermia and radiation-a selective thermal effect on
chronically hypoxic tumor cells in vivo. Int J Radiat Oncol Biol
Phys. 2:99–103. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dewey WC, Hopwood LE, Sapareto SA and
Gerweck LE: Cellular responses to combinations of hyperthermia and
radiation. Radiology. 123:463–474. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sellins KS and Cohen JJ: Gene induction by
gamma-irradiation leads to DNA fragmentation in lymphocytes. J
Immunol. 139:3199–3206. 1987.PubMed/NCBI
|
|
14
|
Hopcia KL, McCarey YL, Sylvester FC and
Held KD: Radiation- induced apoptosis in HL60 cells: Oxygen effect,
relationship between apoptosis and loss of clonogenicity, and
dependence of time to apoptosis on radiation dose. Radiat Res.
145:315–323. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao QL, Fujiwara Y and Kondo T: Mechanism
of cell death induction by nitroxide and hyperthermia. Free Radic
Biol Med. 40:1131–1143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Utz PJ and Anderson P: Life and death
decisions: Regulation of apoptosis by proteolysis of signaling
molecules. Cell Death Differ. 7:589–602. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krenn L and Kopp B: Bufadienolides from
animal and plant sources. Phytochemistry. 48:1–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schatzmann HJ: The role of Na+ and K+ in
the ouabain-inhibition of the Na+ K+-activated membrane adenosine
triphosphatase. Biochim Biophys Acta. 94:89–96. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Puschett JB, Agunanne E and Uddin MN:
Emerging role of the bufadienolides in cardiovascular and kidney
diseases. Am J Kidney Dis. 56:359–370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jing Y, Ohizumi H, Kawazoe N, Hashimoto S,
Masuda Y, Nakajo S, Yoshida T, Kuroiwa Y and Nakaya K: Selective
inhibitory effect of bufalin on growth of human tumor cells in
vitro: Association with the induction of apoptosis in leukemia
HL-60 cells. Jpn J Cancer Res. 85:645–651. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Panesar NS: Bufalin and unidentified
substance(s) in traditional Chinese medicine cross-react in
commercial digoxin assay. Clin Chem. 38:2155–20156. 1992.PubMed/NCBI
|
|
22
|
Dahm-Daphi J, Brammer I and Dikomey E:
Heat effects on the repair of DNA double-strand breaks in CHO
cells. Int J Radiat Biol. 72:171–179. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dikomey E and Franske J: Effect of heat on
induction and repair of DNA strand breaks in X-irradiated CHO
cells. Int J Radiat Biol. 61:221–233. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Calderwood SK and Hahn GM: Thermal
sensitivity and resistance of insulin-receptor binding. Biochim
Biophys Acta. 756:1–8. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stevenson MA, Minton KW and Hahn GM:
Survival and concanavalin- A-induced capping in CHO fibroblasts
after exposure to hyperthermia, ethanol, and X irradiation. Radiat
Res. 86:467–478. 1981. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coss RA and Linnemanns WA: The effects of
hyperthermia on the cytoskeleton: A review. Int J Hyperthermia.
12:173–196. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Konings AW and Ruifrok AC: Role of
membrane lipids and membrane fluidity in thermosensitivity and
thermotolerance of mammalian cells. Radiat Res. 102:86–98. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Majda JA, Gerner EW, Vanlandingham B,
Gehlsen KR and Cress AE: Heat shock-induced shedding of cell
surface integrins in A549 human lung tumor cells in culture. Exp
Cell Res. 210:46–51. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyata H, Doki Y, Yamamoto H, Kishi K,
Takemoto H, Fujiwara Y, Yasuda T, Yano M, Inoue M, Shiozaki H, et
al: Overexpression of CDC25B overrides radiation-induced G2-M
arrest and results in increased apoptosis in esophageal cancer
cells. Cancer Res. 61:3188–3193. 2001.PubMed/NCBI
|
|
30
|
Corbiere C, Liagre B, Terro F and
Beneytout JL: Induction of antiproliferative effect by diosgenin
through activation of p53, release of apoptosis-inducing factor
(AIF) and modulation of caspase-3 activity in different human
cancer cells. Cell Res. 14:188–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gottlieb E, Armour SM, Harris MH and
Thompson CB: Mitochondrial membrane potential regulates matrix
configuration and cytochrome c release during apoptosis. Cell Death
Differ. 10:709–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gottlieb RA: Mitochondria: Execution
central. FEBS Lett. 482:6–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zamzami N, Marchetti P, Castedo M,
Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B and
Kroemer G: Sequential reduction of mitochondrial transmembrane
potential and generation of reactive oxygen species in early
programmed cell death. J Exp Med. 182:367–377. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu X, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extract:
Requirements for dATP and cytochrome C. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ran R, Lu A, Zhang L, Tang Y, Zhu H, Xu H,
Feng Y, Han C, Zhou G, Rigby AC and Sharp FR: Hsp70 promotes
TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa
B survival signaling. Genes Dev. 18:1466–1481. 2004. View Article : Google Scholar : PubMed/NCBI
|